Abstract
Oral phenytoin is an extensively used medicine for the treatment of convulsive disorders. Topical phenytoin has also been used for various types of ulcers. To determine the effectiveness of 2% topical phenytoin sodium solution in treating recalcitrant pyoderma gangrenosum. Six patients with treatment‐resistant pyoderma gangrenosum who attended to Dermatology Unit/Ward were taken to the study and applied topical 2% phenytoin sodium solution to the wounds alone with other systemic therapy. Response to the treatment was assessed weekly. Three patients had idiopathic PG and other three had secondary diseases. At the end of the 4th week four patients showed complete resolution of the ulcers whereas other two patients showed the partial resolution. No adverse effects were noted. Phenytoin sodium 2% solution is beneficial for pyoderma gangrenosum (PG) with various etiologies. It enhanced the healing of the ulcer especially when the patient has treatment resistant disease.
Keywords: pyoderma, Venous ulcers, Wound dressing
INTRODUCTION
Pyoderma gangrenosum (PG) is an uncommon, chronic, painful, ulcerative skin disease. In PG, the initial lesion appears as a red papule or pustule changing into a larger ulcerative lesion with undermined edges (1). The prognosis of PG is generally good, however, recurrences may occur and residual scarring is common. The diagnosis is made by excluding other causes of similar‐appearing cutaneous ulcerations, including infection, malignancy, vasculitis, collagen vascular diseases, diabetes and trauma (2).
The mainstay of therapy for PG has been high‐dose corticosteroids but not all patients have a favourable outcome. Other systemic agents have also been used such as cyclosporine (3), azathioprine (4), cyclophosphamide (5) and tacrolimus. However, all these systemic therapies can be complicated by serious side effects. Topical treatments that have been shown some effects are those with steroids, cyclosporine and tacrolimus (6). Topical phenytoin has proven useful for a wide variety of soft tissue wounds such as trophic ulcers, decubitus ulcers, diabetic foot ulcers, burns, traumatic wounds, war‐related missile wounds, venous stasis ulcers and abscesses 7, 8.
Systemic absorption of topical phenytoin is not significant except one case report which showed significant levels of serum phenytoin after topical phenytoin to a large wound (9).
The best method of delivery of topical phenytoin is not known. Phenytoin powder has been applied directly to wounds in a thin, uniform layer, and then covered with gauze. However, the powder from the capsules is reported to cause a white eschar‐like coating. This can be prevented by mixing phenytoin with NaCl (0·9 %) and applying this with gauze (10). Injectable phenytoin has a high pH (about 12) and should not be used topically because it can damage skin (9). One suggested formula is the use of phenytoin powder (90% to 100%) with Polyox™. Polyox™ is a polymer that can bind with water and help the powder maintain contact with the skin (11). There were researches which were performed with 2% and 4% phynytoin sodium suspention (12). As researches are mostly performed with 2–4% phenytoin sodium, we decided to use phenytoin sodium suspension. In our country, topical tacrolimus is used very rarely because of its high cost and topical cyclosporine is not available at all, which are standard topical treatment for PG. Topical corticosteroids are the only topical drug that is freely available for PG in our country and when there is poor response to it there is no other cheap alternative. As topical phenytoin has been proven to be effective on various types of wounds 13, 14, 15, we started topical 2% phenytoin sodium suspension for patients with treatment‐resistant PG and patients with slow response.
In this paper, we report a group of six patients with PG treated successfully with topical 2% phenytoin sodium solution.
MATERIALS AND METHODS
Patients
The study population included six patients with PG (idiopathic or secondary). Diagnosis was carried out with typical clinical findings, investigations and exclusion of other possible causes (Table 1). The subject had the willingness and the ability to understand and provide informed consent. (study design: a cohort study; study setting: Dermatology Clinic/Ward of Teaching Hospital Kandy Sri Lanka.)
Table 1.
Clinical features and investigations for diagnosis of PG
| Patient No | Biopsy * | Antibody positive | Antibody negative | Duplex (arterial and venous) | Protein electrophoresis | Specific clinical features |
|---|---|---|---|---|---|---|
| 1 | Not done | ANA, RF | Normal | Normal | ||
| 2 | 4 | RF (weakly), U1RNP | ANA, dsDNA, SCL 70 | Normal | Not done | Scleroderma, RA features † |
| 3 | 1, 2 | ANA, RF | Normal | Not done | Positive pathergy, orogenital ulcers | |
| 4 | Not done | RF (weakly) U1RNP | ANA, SCL 70 | Normal | Not done | Scleroderma † |
| 5 | 3 | ANA, RF | Normal | Normal | ||
| 6 | Not done | ANA, RF | Normal | Normal |
*Biopsy findings: 1 – neutrophilic vascular reaction, 2 – leukocytockasia, 3 – tissue necrosis with mononuclear cell infiltrate, 4 – fibrosing inflammation.
†Not fulfilling criteria for diagnosis.
Primary outcome measures
Wound improvement was measured using photographic documentation, measuring the size using oil papers and asking patients' view about treatment efficacy. Wounds were observed for the presence of healthy granulation tissue, reduction in surface dimensions and time to heal. Adverse events were also noted.
Intervention
Phenytoin sodium solution (2%) was applied directly (in wet gauze) to wounds daily. The solution was made using normal saline. Other interventions such as treating bacterial infection etc. were carried out accordingly (wound swabs were taken for bacterial culture and antibiotic sensitivity test (ABST) when indicated by clinical features).
Evaluation
Patients were followed up weekly at Dermatology Clinic and those who admitted were evaluated weekly at the ward.
RESULTS
Patients' demographic data and their treatments are shown in Table 2.
Table 2.
Demographic data and treatment details of patients
| Patient No | Age | sex | Duration of PG | Cause | Systemic therapy | Duration (month) | Duration of topical Betamethasone |
|---|---|---|---|---|---|---|---|
| 1 | 55 years | Male | 10 years | Idiopathic | Prednisolone, cyclophosphamide, | 12 | 3 months |
| 2 | 50 years | Female | 10 years | MCTD | M. prednisolone pulse, azathioprine | 20 | 6 months |
| 3 | 25 years | Male | 1 years | Behcet's | M. prednisolone pulse, azathioprine | 6 | 6 weeks |
| 4 | 40 years | Female | 2 years | MCTD | Prednisolone, cyclophosphamide, | 6 | 6 weeks |
| 5 | 44 years | Female | 8 years | Idiopathic | None | 6 months | |
| 6 | 57 years | Female | 10 years | Idiopathic | Prednisolone,azathioprine | 8 | 2 months |
MCTD, mixed connective tissue disorder.
All six patients had been treated with betamethasone as the only topical therapy immediately prior to starting topical phenytoin sodium and the duration is mentioned in Table 2. Except patient 5 other patients were on systemic treatments which were continued, and topical betamethasone was discontinued after starting phenytoin sodium dressings. Systemic treatment was discontinued on patient 5 as a result of adverse effects and poor response 7 months prior to starting topical phenytoin sodium. Other topical treatments tried in the past were hypertonic saline dressings, povidone iodine dressings and topical antibiotics.
All six patients showed very mild improvement of wound size while being treated with systemic agents and topical betamethasone.
The addition of topical 2% topical phenytion sodium solution was associated with fast healing of those ulcers and four out of six were completely healed at the end of 4th week (Table 3), whereas other two ulcers also showed a marked improvement (1, 2, 3, 4, 5, 6). The improvement was associated with healthy granulation tissues, reduction of surface diameter, and we did not experience any increase in wound infections (patient 3 had pseudomonas infection before starting topical phenytoin which was treated with systemic ceftazidime for 7 days after culture ABST). Except mild transient burning sensation in two patients, none of the patients developed any side effects.
Table 3.
Details of ulcers and percentage improvement
| Patient No | Site | Size (cm) before treatment | Percentage improvement at end of treatment | ||||
|---|---|---|---|---|---|---|---|
| Length | Width | 1st week | 2nd week | 3rd week | 4th week | ||
| 1 | Lower calf | 11 | 3·5 | 10 | 25 | 40 | 60 |
| 2 | Medial maleolus | 1 | 1 | 15 | 40 | 60 | 80 |
| 1·2 | 1·5 | 20 | 40 | 75 | 100 | ||
| 3 | Lateral calf | 2 | 2 | 25 | 60 | 85 | 100 |
| 4 | Medial calf | 2 | 2 | 30 | 70 | 100 | 100 |
| 5 | Foot‐dorsum | 3 | 2 | 30 | 50 | 80 | 100 |
| 6 | Medial maleolus | 5 | 4·5 | 5 | 10 | 15 | 20 |
Figure 1.
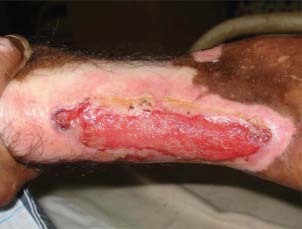
Patient 1 – before treatment.
Figure 2.
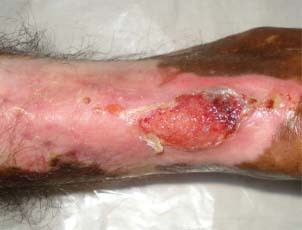
Patient 1 – after 4 weeks.
Figure 3.
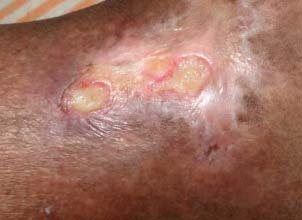
Patient 2 – after 1 week.
Figure 4.
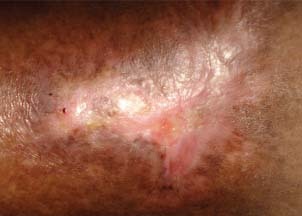
Patient 2 – after 4‐week treatment.
Figure 5.
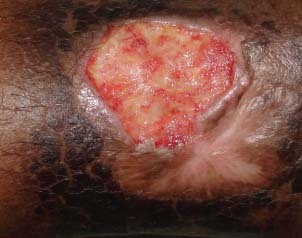
Patient 6 – before treatment.
Figure 6.
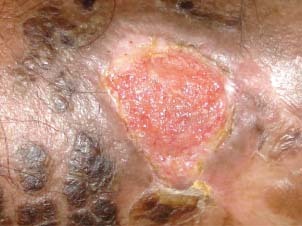
Patient 6 after 4‐week treatment. There is evidence of surface reduction and healthy granulation tissue which was not achieved while using topical betamethasone.
DISCUSSION
These results suggest that topical 2% phenytoin sodium solution is effective in treating patients with PG of various etiologies. The improvement was dramatic. Topical phenytoin may be highly effective when other systemic or topical treatments have been unsuccessful. As PG is an inflammatory disease it can be argued that systemic drugs may have halted the disease process and phenytoin may have simply helped in the healing process via its multiple mechanisms. On the other hand, it can be argued that phenytoin sodium carries its own intrinsic anti‐inflammatory activity, because these patients responded to topical phenytoin therapy when there was poor response to combined systemic drugs with topical corticosteroids. However, there is no current literature at present on topical phenytoin for PG and how it enhances the healing of PG.
Topical phenytoin causes fibroblast proliferation, enhancing the formation of granulation tissue, decreasing collagenase activity (by reducing its production or secretion or both), promoting deposition of collagen and other connective tissue components, decreasing bacterial contamination, and decreasing wound exudate 9, 16, 17.
A number of clinical studies show that phenytoin decreases the bacterial load of wounds 18, 19, 20, 21. Topical phenytoin was reported to eliminate Staphylococcus aureus, Escherichia coli, Klebsiella spp. and Pseudomonas spp. from wounds within 7–9 days (18). It is unknown if phenytoin has intrinsic antibacterial activity, or if the effect of phenytoin on the bacterial load of wounds is mediated indirectly by effects on inflammatory cells and neovascularisation (18). In our cohort of patients, we noted that secondary bacterial infections were less and wound surface looked healthy.
Phenytoin sodium is freely available, cheap and easy to prepare. If this cheap treatment modality is effective it will be a great advantage for doctors and patients. The apparent stimulatory effect of phenytoin on connective tissue suggests an exciting possibility for its use in wound healing which need to be further researched. For countries with limited resources topical phenytoin may be an alternative drug for PG. Although randomised control trials are needed to assess its efficacy with stranded drugs, the low incidence of PG makes it difficult task.
REFERENCES
- 1. Bolognia JL, Jorizzo JL, Rapini RP. Neutrophilic dermatoses. Dermatology. Vol 2 . St. Louis: Mosby, 2008:383–84. [Google Scholar]
- 2. Bolognia JL, Jorizzo JL, Rapini RP. Neutrophilic dermatoses. Dermatology. Vol 2 . St. Louis: Mosby, 2008:386. [Google Scholar]
- 3. Powell FC, Collins S. Pyoderma gangernosum. Clin Dermatol 2000;18:283–93. [DOI] [PubMed] [Google Scholar]
- 4. Duffill MB. Cyclosporine, azathiorine and local therapy for pyoderma gangrenosum. Australas J Dermatol 2007;35:15–8. [DOI] [PubMed] [Google Scholar]
- 5. Newell LM, Malkinson FD. Pyoderma gangrenosum cyclophosphamide therapy. Arch Dermatol 1983;119:495–7. [DOI] [PubMed] [Google Scholar]
- 6. Lyon CC, Stapleton M, Smith AJ, Mendelsohn S, Beck MH. Management of peristomal pyoderma gangrenosum. Lancet 1998;35:832. [DOI] [PubMed] [Google Scholar]
- 7. Modaghegh S, Salehian B, Tawassoli M, Djamshidi A, Rezai AS. Use of phenytoin in healing of war and non‐war wounds: A pilot study of 25 cases. Int J Dermatol 1989;28:347–50. [DOI] [PubMed] [Google Scholar]
- 8. Shafer WG, Beatty RE, Davis WB. Effect of dilantin sodium on tensile strength of healing wounds. Proc Soc Exp Biol Med 1958;98:348–50. [DOI] [PubMed] [Google Scholar]
- 9. Anstead GM, Hart LM, Sunahara JF, Liter ME. Phenytoin in wound healing. Ann Pharmacol 1996;30: 768–7. [DOI] [PubMed] [Google Scholar]
- 10. Rhodes RS, Heyneman CA, Culbertson VL, Wilson SE, Phatak HM. Topical phenytoin treatment of stage II decubitus ulcers in the elderly. Ann Pharmacother 2001;35:675–81. [DOI] [PubMed] [Google Scholar]
- 11. Allen LV. Phenytoin topical powder for wounds. US Pharmacist 1996;84:86. [Google Scholar]
- 12. Bhatia A, Nanda S, Gupta U, Reddy BS. Topical phenytoin suspension and normal saline in the treatment of leprosy trophic ulcers: a randomized, double‐blind, comparative study. J Dermatolog Treat 2004;15:321–7. [DOI] [PubMed] [Google Scholar]
- 13. Ashima Bhatia MD, Surya Prakash DVD. Topical phenytoin for wound healing. Pharmacology 2010;2:59–62. [PubMed] [Google Scholar]
- 14. Hasamnis AA, Mohanty BK, Muralikrishna SP. Evaluation of wound healing effect of topical phenytoin on excisional wound in albino rats. Dermatol Online J 2004;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shaw J, Hughes CM, Lagan KM, Bell PM. The clinical effect of topical phenytoin on wound healing: a systematic review. Br J Dermatol 2007;157:997–1004. [DOI] [PubMed] [Google Scholar]
- 16. McAnally LE, Thompson D. Use of phenytoin for wound healing. Hosp Pharm 1992;27: 649–50. [Google Scholar]
- 17. Talas G, Brown RA, McGrouther A. Role of phenytoin in wound healing‐a wound pharmacology perspective. Biochem Pharmacol 1999;57: 1085–94. [DOI] [PubMed] [Google Scholar]
- 18. El Zayat SG. Preliminary experience with topical phenytoin in wound healing in a war zone. Mil Med 1989;28:347–50. [PubMed] [Google Scholar]
- 19. Lodha SC, Lohiya ML, Vyas MCR, Sudha Bhandari, Goyal RR, Harsh MK. Role of phenytoin in healing large abscess cavities. Br J Surg 1991;78:105–8. [DOI] [PubMed] [Google Scholar]
- 20. Muthukumarasamy MG, Sivakumar G, Manoharan G. Topical phenytoin in diabetic foot ulcers. Diabetes Care 1991;14:909–11. [DOI] [PubMed] [Google Scholar]
- 21. Pendse AK, Sharma A, Sodani A, Hada S. Topical phenytoin in wound healing. Int J Dermatol 1993;32:214–7. [DOI] [PubMed] [Google Scholar]


