Abstract
This study compared a two‐layer (Coban™ 2 Layer) and a four‐layer (Profore™) compression bandage system in venous leg ulcer patients. Participants (n = 81) were enrolled into an 8‐week, randomised, open‐label, ten‐centre, crossover clinical trial. The primary endpoint was bandage slippage measured at each dressing change. Secondary endpoints included wound healing, health‐related quality of life (HRQoL) and patient preference. Mean slippage estimated from a mixed analysis of variance model (697 visits) was 2·48 cm for the two‐layer system and 4·17 cm for the four‐layer system (P < 0·001). There were no significant differences in percent of wounds that healed (Fisher’s exact test, P = 0·30), in wound area reduction (Wilcoxon rank‐sum test, P = 0·88) or in linear healing rate (Wilcoxon rank‐sum test, P = 0·94). The HRQoL Physical Symptoms and Daily Living scores were significantly higher with the two‐layer system (pooled two‐sample t‐test, P < 0·05). Patients had a strong preference for the two‐layer system (72%) than the four‐layer system (22%), with 6% having no preference. In conclusion, the two‐layer system exhibited significantly less bandage slippage than the four‐layer system. While less bandage slippage did not appear to impact wound healing, there was indication that it may have influenced patient preference in favour of the two‐layer system and potentially impacted patients’ HRQoL.
Keywords: Compression bandage, Coban 2 Layer, Profore, Slippage, Venous leg ulcer
Introduction
Chronic leg ulcers are still a major challenge to clinical practice. The reported prevalence ranges from 0·05% to 3% 1, 2, with an estimated 0·1–0·2% of the general population having active leg ulceration (3). In the year 2000, it was estimated that 2 667 000 people in North America and Europe were treated for leg ulcers (4). However, because of the recurring nature of the disease, prevalence studies probably underestimate the true magnitude of the problem as only about 20% of individuals with this chronic disease exhibit active leg ulceration (3). The incidence is higher in women than in men (3·7 times that of men in the above 70 years age group) and also increases linearly with age 1, 5. Therefore, it is likely that as the age of the population continues to rise, so will the incidence of chronic leg ulcers.
There is little prospective epidemiological research ascertaining risk factors associated with chronic leg ulcers (3). However, venous insufficiency is thought to play a major role. In a large study of 600 patients with chronic leg ulcers, the majority of leg ulcers (76%) were reported to be associated with venous disease (5). Venous leg ulcers have a large impact on the quality of life of patients 6, 7 as individuals are frequently incapacitated and unable to work 8, 9. Depending on the extent of damage and the nature of involvement of the superficial and/or deep veins, an estimated 2–6 million working days are lost each year in the USA as a result of venous disease 10, 11. This is exacerbated by its recurring nature, requiring frequent hospitalisations and prolonged outpatient therapy 8, 12.
The financial costs to the individual and the community are also substantial, with the greatest element of cost being nursing time 1, 13. In the UK, it is reported that 15–40% of district nurse time is spent dressing venous insufficiency ulcers (13) and that leg ulcers are the most prevalent purpose for community nursing visits (3). In an economic model of venous ulcer care, which was constructed using a meta‐analysis of cost drivers reported in the literature, it was found that cost to heal a single venous ulcer ranged from $1873 (USD) to $15 052 (USD), with only 11% to 17% of ulcers expected to heal within 4 weeks of treatment and 39% to 51% within 12 weeks (14).
Venous leg ulcers are typically accompanied by oedema of the surrounding tissues, which can complicate the healing process 15, 16. Compression bandages, combined with limb elevation and exercises, are generally considered the most effective intervention for both prevention and treatment of venous leg ulcers and the accompanying oedema 17, 18, 19. These therapies are often combined with moderately to highly absorptive products such as foam dressings to manage the large amounts of wound drainage typically seen with these ulcers (20). Data from health‐related quality of life (HRQoL) studies show that when compression treatment is delivered effectively and results in improved wound healing, it can also improve quality of life in these patients 21, 22.
Clinicians have a variety of compression products and systems from which to choose, and there is a large body of literature describing studies comparing efficacy of various products and systems 23, 24. A Cochrane review that analysed 22 clinical trials published between 1985 and 1999 concluded that multi‐layered systems are more effective than single‐layered systems and that high compression is more effective than low compression but that there are no clear differences in the effectiveness of different types of high compression (18). High compression has been defined as >25 mmHg (19); however, most experts generally agree that bandage systems providing 35–40 mmHg are most effective.
Over the past decade, multi‐layer compression systems have grown in popularity with an increasing body of clinical evidence supporting their use. A review of the literature indicates that there is no clear evidence supporting one multi‐layer system over another 25, 26, 27, 28, 29. However, there is no doubt that compression therapy, and multi‐layer systems in particular, has revolutionised care for patients with venous insufficiency. Just 20 years ago, there was little hope for healing these patients. They were faced with a lifetime of swollen limbs, poor mobility, chronic and debilitating pain, skin ulcers and an ever declining quality of life. Multi‐layer compression, along with advancements in wound dressings and skin care, changed all that. It is now possible to manage the pain, heal the ulcers and reverse the decline in quality of life.
Until recently, there has been little advancement and few new innovations in compression therapy, and multi‐layer systems are far from being perfect. Clinicians have recognised deficiencies with current multi‐layer compression systems. These well‐known problems include
-
•
Inconsistency in application techniques, resulting in inconsistent pressures and variable results.
-
•
Bulkiness, which can impede patients from wearing normal footwear and clothing, leading to a low level of concordance and the potential risk of falling (30).
-
•
Bandage slippage and bunching, leading to an uneven distribution of compression, which can result in discomfort at night and the potential for skin breakdown.
-
•
Decreased patient quality of life, including a decrease in social activities.
Recently, a new two‐layer compression system has been developed and marketed. The product is packaged in a kit form and contains two latex‐free roll bandages. After application, the two layers bond tightly to form one single, stiff layer with no independent movement. The inner ‘comfort’ layer is polyurethane foam laminated to a cohesive bandage. The foam side provides a cushion over bony prominences and mechanical grip to the skin when compressed. The cohesive outer side provides a surface to which the second compression layer bonds tightly. It is believed that the skin‐gripping properties of the inner comfort layer and the cohesive bonding properties of both layers will allow the bandage to stay in place longer with greater comfort and less slippage. Less slippage may result in longer, more effective compression therapy.
This study was designed to compare this new two‐layer system with an established four‐layer system using bandage slippage as the primary endpoint. Additionally, wear time, quality of life, wound healing, patient mobility and patient preference were measured as secondary endpoints.
Methods
Study design
This was a multisite, prospective, randomised, crossover, open‐label clinical trial conducted at five centres in the USA, three in the UK and two in Canada. The primary objective was to compare slippage of a two‐layer compression bandage system (3M™ Coban™ 2 Layer Compression System; 3M™ Health Care, St Paul, MN, USA) with a four‐layer system (Profore™ Multi‐Layer Bandaging System; Smith & Nephew Medical Wound Management, Hull, UK) in the treatment of venous leg ulcers. Participants were initially randomised to one of the two compression systems and were followed for 4 weeks. Their treatment was then switched to the other compression system, and they were treated for an additional 4 weeks. Participants were followed for a total of 8 weeks, which included a minimum of nine clinic visits. A crossover design was chosen so that the effect of the compression system could more easily be compared by having the same subject wear both devices. The subject would thus act as their own control, helping to reduce subject variables, such as leg circumference and activity level. Also, there was not expected to be any carryover effect with regard to bandage slippage (the primary endpoint).
This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and received appropriate approvals by the central and local ethics committees and institutional review boards used by the respective sites. Prior to study enrolment, written informed consent and authorisation to use and disclose protected health information (USA sites only) was obtained from each participant. The procedures and forms used to obtain consent and authorisation were specific to each site and were consistent with local laws and ethics committee requirements.
Study participants
All participants in this study were recruited from either free‐standing wound clinics or wound clinics associated with community hospitals or trusts. Participants needed to be at least 18 years of age (21 years in the USA), be able to understand and answer questionnaire items and have one or more venous leg ulcers that had been treated with compression therapy for at least 2 weeks prior to enrolment in the study.
The investigators were free to exclude for any reason patients they believed were unsuitable for compression therapy or for enrolment into the study. Patients were specifically excluded if they had an ankle–brachial pressure index (ABPI) less than 0·8 within 4 weeks of the start of the study or if their leg ulcers were circumferential or showed signs of clinical infection.
When wounds were present on both legs, with both legs requiring compression therapy, the subject received the same compression treatment on both legs. When more than one wound was present on the leg(s), the investigator used clinical judgement to choose which wound (and leg) to include in the study. All decisions to exclude patients, or which leg/wound to include in the study, were made prior to treatment randomisation.
Study treatments
Treatment of the venous insufficiency ulcer prior to application of the compression bandage system was per standard procedures at each of the study clinics. Medications and/or additional wound treatments were permitted at the discretion of the study investigator. Tubular support systems underneath the compression bandages were not permitted as they were believed to influence the slippage primary endpoint. Also, changes in treatments with mood‐altering substances (e.g. antidepressants) within 2 weeks prior to enrolment or at any time during the study were not permitted as they could potentially interfere with quality‐of‐life assessments. Ulcers were covered with the same primary dressing (Tegaderm™ Foam Dressing; 3M™ Health Care), and the compression bandage was applied over the primary dressing. If an antimicrobial wound contact dressing or other wound treatment was needed, it was applied underneath the primary dressing. Bandages were expected to be worn for 7 days; however, in many cases, the subject’s visit schedule established by the clinical staff required the subject to return for more frequent bandage changes. All reasons for bandage change were documented.
Bandage application
The compression bandages were typically applied by a trained study coordinator under the supervision of the study investigator overseeing the investigation at each study centre. Investigators were selected for this study based on prior knowledge and successful experience with the four‐layer compression system under evaluation. The four‐layer bandage system was applied using manufacturer instructions provided in the package insert, and additional training with the four‐layer system was deemed unnecessary. However, because of the unique design and different application techniques required for the two‐layer system, all investigators and staff applying the two‐layer bandage were required to undergo in‐service training in application technique by the regional technical expert of the manufacturer. Each of the technical experts provided the same training regimen to the different centres following the basic instructions provided in the product package insert. The centre’s clinical staff receiving the training was required to show proficiency in the wrapping technique prior to enrolment of the first patient. Studies conducted by the manufacturer of the two‐layer compression system previously showed that the method is easy to learn, with reproducible results (31).
Outcomes measures
Typically, leg ulcer studies use wound‐healing and/or surrogate wound‐healing endpoints as the primary outcome measure 3, 25, 26, 27, 32, 33, 34, 35, 36, 37. However, it was anticipated that both of the bandages evaluated in this study would provide effective compression treatment to promote wound healing and that there would be little difference between the systems. Therefore, the primary endpoint of this trial was slippage of the compression bandage measured at each bandage change. This was assessed by measuring the length of the bandage from the top of the bandage to the floor immediately after application and again just prior to changing the bandage. This was accomplished with a flexible tape measure with the subject’s foot resting flat on the floor in a standing or sitting position. The difference in length between those two time points was used to calculate slippage of the bandage. No previous studies were identified showing bandage slippage as a validated measurement tool. However, a previous 7‐day study with healthy volunteers had showed feasibility of the technique (data on file at 3M Company).
There were five secondary endpoints in this clinical trial: (i) bandage wear time, (ii) wound healing, (iii) HRQoL, (iv) patient preference for each of the bandage systems and (v) patient mobility (step metre measurements). Impact of the bandage system on HRQoL was assessed using the Cardiff Wound Impact Schedule (CWIS) (22). HRQoL assessments were made at study enrolment, at the time of bandage crossover and/or at the conclusion or withdrawal from the study. Patient mobility was assessed by step metre (pedometer) readings. Wound healing was measured from wound tracings at the start of the study and at 4 weeks. An additional wound tracing was made at 8 weeks to document the wound status at the conclusion of the trial. Patient preference was assessed at the end of the subject’s participation in the study.
Sample size
The sample size for this study was calculated for the primary outcome, bandage slippage. Previous studies on healthy volunteers found that after 7 days of wear, a mean [standard deviation (SD)] difference in slippage between the two compression systems was 6·74 (3·52) cm in favour of the two‐layer system (data on file at 3M Company). These studies also found a higher degree of variability with the four‐layer system. Therefore, a two‐sample Satterthwaite t‐test of equal means with unequal variances was used to calculate the sample size based on slippage data from healthy volunteers. A 3‐cm slippage difference between the two compression systems was determined to be clinically relevant for the purpose of this calculation. Assuming a SD of 1 cm for the two‐layer system and 4 cm for the four‐layer system and using a 90% power of test with an alpha level 0·05 and a 20% dropout rate, 56 subjects (28 subjects per treatment arm) were calculated to be needed to complete this trial in a parallel study design. A crossover design would require fewer subjects as it considers each subject its own control and reduces the between‐subject variability. However, this study additionally had several secondary outcome measures with potentially higher degrees of variability to consider; therefore, it was planned to have a maximum of 80 subjects (40 per treatment arm) to ensure a powerful statistical analysis for not only the primary endpoint but also the multiple secondary endpoints.
Randomisation and blinding
Subjects were expected to gradually feel better during the course of this study and to see improvement in their condition. To avoid introducing potential order bias in this crossover study, subjects were randomised to determine which compression system was used first. Subjects were randomly assigned to one of the two crossover sequences. Either the two‐layer system was used during the first 4 weeks followed by the four‐layer system or the four‐layer system was used first followed by the two‐layer system. The randomisation was stratified by study site so that treatment order assignment was kept balanced within each site. The randomisation schedule was computer generated by the study biostatistician and provided to the investigators in sealed envelopes and opened only after subject enrolment and selection of the study leg/wound. Subject or investigator blinding was not possible because of the obvious differences between the two‐layer and four‐layer systems; however, the individual conducting the wound‐tracing measurements was blinded to treatment.
Statistical methods
With the exception of a post‐hoc step metre analysis conducted for an individual site, all data analyses were carried out according to a pre‐established statistical analysis plan. Unless otherwise stated, data from all enrolled subjects were analysed on an intent‐to‐treat (ITT) basis. For all analyses, the pre‐crossover period was defined as the time from randomisation to the time the second bandage type was applied. Therefore, once crossover to the second bandage had occurred, the post‐crossover period was regarded as having been started, which typically corresponded to the week 4 visit.
Descriptive summary statistics are provided for baseline demographics and wound characteristics for each treatment group. For age, height, weight, baseline wound area and perimeter, ulcer duration and ABPI, the mean and standard deviation are provided. For gender, race, ulcer location, ulcer status and mobility, the number and percent of subjects in each category are summarised. A Wilcoxon rank‐sum test was used to test for sequence order differences in wound area, and an analysis of variance (ANOVA) model with a term for sequence order was used to test for differences among the remaining continuous variables. Fisher’s exact test was used to test for sequence order differences among all categorical variables.
For the primary outcome variable, absolute slippage was analysed in a mixed ANOVA model appropriate for a crossover design, including baseline bandage height and wear time as covariates in the model.
The percent of wounds that healed were compared with Fisher’s exact test, and the percent change in wound area was compared with the Wilcoxon rank‐sum test (a non parametric equivalent to the t‐test). Because relatively few wounds attained complete healing, linear healing rate (LHR) was calculated as a surrogate endpoint. LHR has the added benefit of normalising wounds of varying size and geometry (38). Data for the percent change in wound area and LHR endpoints were extracted from the wound tracings, which were measured by computer planimetry. LHR was calculated for each wound using the methods proposed by Gilman and used by Margolis 38, 39, 40, 41. Differences in LHR means were assessed with the Wilcoxon rank‐sum test.
For the HRQoL data, differences in treatment group means were analysed using a two‐sample pooled t‐test. Step metre measurements were analysed using an ANOVA model appropriate for a crossover design. A natural log transformation was used because of the non normality of the data.
Patient product preference was determined from a question that the subjects answered at the end of the study. Fisher’s exact test was used to compare responses between treatment groups.
Results
This study was initiated on 7 December 2005 and was completed on 19 April 2007. The flow of subjects into and out of the study and the randomisation to the two sequence order treatment groups are summarised in Figure 1. This figure also summarises the healing status of the wounds as the subjects flowed through the study treatments. A total of 81 leg ulcer patients were enrolled into the study, of which 12 did not crossover because of the ulcer healing prior to the week 4 crossover (n = 9) or because they withdrew from the study (n = 3). There were a total of 721 follow‐up visits, 367 visits for the two‐layer bandage and 354 visits for the four‐layer bandage.
Figure 1.
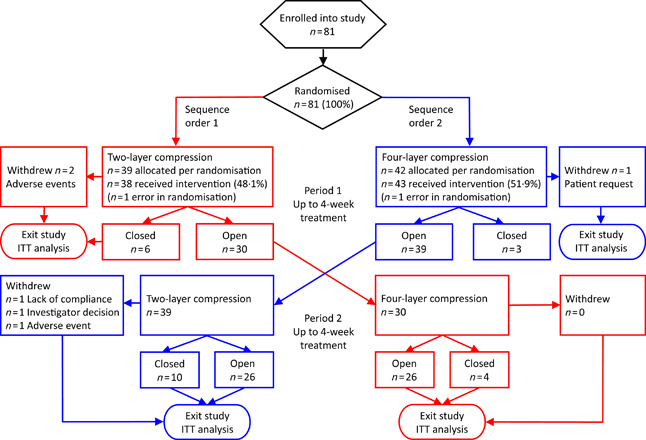
Flow of subjects into different randomised sequential order treatment groups and subsequent wound‐healing outcome. Red lines indicate randomisation to treatment sequence order 1 (two layer followed by four layer) and blue lines to treatment sequence order 2 (four layer followed by two layer).
Baseline patient and wound characteristics for the two treatment groups are summarised in Table 1. There were no significant differences in any of the measured parameters, indicating that the two treatment groups were similar in make up.
Table 1.
Baseline demographics and wound characteristics*
| Parameter | Randomised sequence group | |
|---|---|---|
| Coban 2 Layer followed by Profore | Profore followed by Coban 2 Layer | |
| Total number | 39 | 42 |
| Gender: n (%) | ||
| Male | 25 (64) | 22 (52) |
| Female | 14 (36) | 20 (48) |
| Age (years): mean ± SD | 62·5 ± 15·5 | 63·5 ± 12·5 |
| Height (cm): mean ± SD | 176·3 ± 11·9 | 174·0 ± 9·9 |
| Weight (kg): mean ± SD | 109·7 ± 41·7 | 103·1 ± 32·9 |
| Ethnic origin: n (%) | ||
| Caucasian | 31 (79) | 33 (79) |
| Non Caucasian | 8 (21) | 9 (21) |
| Wound area (cm2): mean ± SD | 11·8 ± 19·7 | 5·7 ± 7·9 |
| Wound perimeter (cm): mean ± SD | 12·0 ± 9·9 | 10·0 ± 8·0 |
| Ulcer duration (weeks): mean ± SD | 186·3 ± 438·7 | 195·1 ± 512·1 |
| Ankle–brachial pressure index: mean ± SD | 1·1 ± 0·2 | 1·1 ± 0·2 |
| Ulcer location: n (%) | ||
| Below calf | 28 (72) | 26 (62) |
| Calf | 11 (28) | 15 (36) |
| Above calf | 0 (0) | 1 (2) |
| Ulcer status: n (%) | ||
| Worsening | 6 (15) | 6 (14) |
| Stable | 12 (31) | 23 (55) |
| Improving | 20 (51) | 12 (29) |
| Unknown | 1 (3) | 1 (2) |
| Mobility status: n (%) | ||
| Walks with assistance | 7 (18) | 6 (14) |
| Walks without assistance | 32 (82) | 36 (86) |
There were no significant differences between the sequence order groups in any of the measured parameters.
Bandage slippage
Slippage results for the two compression systems are summarised in Figure 2. Because a large proportion of dressing changes occurred prior to the maximum 7 days of wear, a statistical model was used to estimate slippage at various time points within the study. The ANOVA model was appropriate for a crossover design and adjusted for baseline bandage height and wear time (days between bandage changes). There was a significant difference in slippage between the two‐ and four‐layer systems in favour of the two‐layer system at days 3–7. Any missing bandage height measurements because of bandages being removed prior to the study visits for the reason of ‘slippage’ were imputed as one fourth the baseline bandage height. This was deemed a reasonable value because it was consistent with the worst case observed in change in bandage heights.
Figure 2.
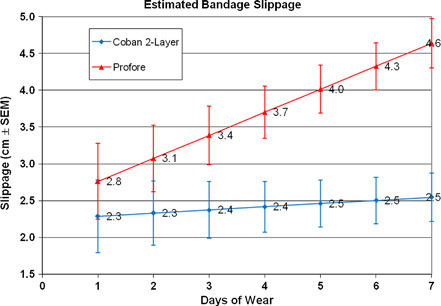
Statistical model estimate of bandage slippage (centimetre ± standard error of the mean) recorded during 697 bandage changes from 81 subjects. Slippage was significantly different between the two compression systems at days 3–7 (P < 0·001).
Bandage wear time
During this study, patient visits were typically scheduled to the maximum allowed 7 days of wear. Patients returned to the clinic prior to these regimented visits if there was a problem with the bandage, if the investigator desired twice‐per‐week visits or if there was another scheduling issue. In both treatment groups, the compression bandage was worn the full time between visits in 98% of the visits. There were only 6/367 (2%) instances in the two‐layer group and 7/354 (2%) instances in the four‐layer group when the patients removed the bandage prior to the scheduled study visit. Reasons for why this occurred included slippage, excessive odour, pain in foot, exudate strike‐through, patient in hospital, excessive tightness and showering with bandage unprotected. There were 36 visits where bandage wear time exceeded the established maximum of 7 days because of forgotten or missed appointments. These long wear time deviations, ranging from 8 to 16 days, were excluded from the wear time analysis. Twenty long wear time deviations occurred in the two‐layer group and 16 in the four‐layer group. Wear time was analysed using an ANOVA model appropriate for a crossover design. The model estimates for wear times were 5·72 and 5·75 days for the two‐ and four‐layer groups, respectively. As expected, because of the regimented nature of the visits, there was no significant difference in wear time between the treatment groups (P = 0·721).
Wound healing
While the wound‐healing endpoints included the percent change in wound area, the log healing rate, the ratio of log areas and the LHR, only percent area change and LHR are reported in this study because of the similarity in endpoint results. Also, only wound‐tracing data prior to the crossover were used in the analysis to eliminate the potential for treatment interactions and because 4‐week healing data have been shown to be predictive of wound‐healing outcomes at 12 and 24 weeks of care 34, 39. There were no significant differences in any of the wound‐healing endpoints. Of the 79 wounds entered into the analysis, the majority (n = 70, 88·6%) did not heal prior to the crossover. Of the nine wounds (11·4%) that healed prior to the crossover, six were in the two‐layer compression group and three were in the four‐layer compression group (P = 0·30). The median (range) percent area change for the two‐layer compression group was 27·8% (−233·3%, 100·0%) compared with 42·2% (−272·1%, 100·0%) for the four‐layer compression group (P = 0·88). The median (range) LHR for the two‐layer compression group was 0·04 cm/week (−0·16, 0·40) compared with 0·04 cm/week (−0·27, 0·19) for the four‐layer compression group (P = 0·94).
Health‐related quality of life
Summary scores for the three domains of the CWIS (Well‐Being, Physical Symptoms and Daily Living, and Social Life) as well as a rating of the subject’s overall HRQoL and the subject’s satisfaction with their overall HRQoL were computed and analysed separately for data collected before and after the crossover. Pre‐crossover scores were calculated relative to data collected at study enrolment, and post‐crossover scores were calculated relative to the data collected at the end of the pre‐crossover period. Data were analysed on a per protocol rather than on an ITT basis. Subjects were excluded if the correct bandage was not applied during at least 75% of the subject’s visits in that period, which resulted in the HRQoL data being excluded for two subjects in the post‐crossover period. Additionally, subjects that completed less than 2 weeks of follow‐up during a period were excluded from the HRQoL analysis. This resulted in the data being excluded for two subjects from post‐crossover period. One subject was excluded from the post‐crossover period as a result of the questionnaire being completed over 2 months after the last study visit.
Summaries of the HRQoL data collected at enrolment and at the end of pre‐ and post‐crossover periods are presented in 2, 3, 4. There was a significant difference in favour of the two‐layer compression system during the pre‐crossover period for the Physical Symptoms and Daily Living scores (P < 0·05). There were no other significant differences found in the HRQoL data in either the pre‐ or the post‐crossover periods.
Table 2.
HRQoL scores at baseline, pre‐crossover and post‐crossover
| HRQoL assessments | Compression system | Baseline: mean ± SD (n) | Pre‐crossover: mean ± SD (n) | Post‐crossover: mean ± SD (n) |
|---|---|---|---|---|
| Well‐Being | Coban 2 Layer | 40·8 ± 19·9 (38) | 44·5 ± 18·1 (33) | 54·6 ± 18·1 (33) |
| Profore | 48·9 ± 16·9 (42) | 52·3 ± 19·1 (42) | 42·9 ± 19·4 (26) | |
| Physical Symptoms and Daily Living | Coban 2 Layer | 56·1 ± 21·7 (37) | 63·7 ± 21·9 (32) | 67·8 ± 21·7 (33) |
| Profore | 64·8 ± 17·1 (43) | 66·1 ± 20·8 (42) | 64·9 ± 20·7 (26) | |
| Social Life | Coban 2 Layer | 68·8 ± 23·3 (38) | 72·9 ± 23·7 (33) | 74·7 ± 24·3 (33) |
| Profore | 75·5 ± 22·7 (43) | 76·7 ± 24·3 (41) | 73·4 ± 25·1 (25) | |
| Overall HRQoL | Coban 2 Layer | 6·0 ± 2·0 (38) | 6·5 ± 2·4 (33) | 7·7 ± 1·6 (32) |
| Profore | 7·1 ± 2·1 (40) | 7·3 ± 2·0 (41) | 6·9 ± 2·0 (26) | |
| Patient satisfaction with overall HRQoL | Coban 2 Layer | 6·6 ± 2·0 (38) | 6·8 ± 2·5 (33) | 7·6 ± 2·0 (32) |
| Profore | 6·8 ± 2·6 (40) | 7·1 ± 2·3 (41) | 6·5 ± 2·5 (26) |
HRQoL, health‐related quality of life.
Table 3.
Changes in HRQoL scores during the pre‐crossover period
| HRQoL assessments | Compression system | Change of within‐patient scores: mean ± SD | Difference between treatment groups (95% CI) | P value |
|---|---|---|---|---|
| Well‐Being | Coban 2 Layer | 4·1 ± 16·0 | 0·74 ± 14·6 (−6·0, 7·5) | 0·827 |
| Profore | 3·4 ± 13·3 | |||
| Physical Symptoms and Daily Living | Coban 2 Layer | 8·7 ± 17·9 | 7·5 ± 15·7 (0·1, 14·9) | 0·046 |
| Profore | 1·2 ± 13·8 | |||
| Social Life | Coban 2 Layer | 6·8 ± 15·2 | 5·6 ± 15·1 (−1·4, 12·6) | 0·118 |
| Profore | 1·3 ± 14·9 | |||
| Overall HRQoL | Coban 2 Layer | 0·6 ± 2·0 | 0·4 ± 1·9 (−0·5, 1·3) | 0·376 |
| Profore | 0·2 ± 1·8 | |||
| Patient satisfaction with overall HRQoL | Coban 2 Layer | 0·4 ± 2·4 | 0·1 ± 2·4 (−1·0, 1·2) | 0·860 |
| Profore | 0·3 ± 2·3 |
HRQoL, health‐related quality of life.
Table 4.
Changes in HRQoL scores during the post‐crossover period
| HRQoL assessments | Compression system | Change of within‐patient scores: mean ± SD | Difference between treatment groups (95% CI) | P value |
|---|---|---|---|---|
| Well‐Being | Coban 2 Layer | 1·5 ± 14·5 | −4·4 ± 14·5 (−12·1, 3·3) | 0·258 |
| Profore | −2·9 ± 14·5 | |||
| Physical Symptoms and Daily Living | Coban 2 Layer | 2·8 ± 12·9 | −5·4 ± 14·5 (−13·2, 2·4) | 0·169 |
| Profore | −2·6 ± 16·4 | |||
| Social Life | Coban 2 Layer | −1·8 ± 13·6 | −3·2 ± 16·4 (−12·1, 5·7) | 0·478 |
| Profore | −5·0 ± 19·7 | |||
| Overall HRQoL | Coban 2 Layer | 0·6 ± 1·2 | −0·3 ± 1·5 (−1·1, 0·5) | 0·494* |
| Profore | 0·3 ± 1·9 | |||
| Patient satisfaction with overall HRQoL | Coban 2 Layer | 0·5 ± 1·5 | −0·9 ± 2·2 (−2·1, 0·3) | 0·172* |
| Profore | −0·4 ± 2·8 |
HRQoL, health‐related quality of life.
The Satterthwaite method was used assuming unequal variances.
Patient preference
To minimise treatment order bias in the patient preference data, only those subjects who reached the crossover point and had at least one visit in the post‐crossover period were included in the analysis. A total of 68 subjects were included in the analysis, and of these, 49 (72%) preferred the two‐layer compression system, 15 (22%) preferred the four‐layer system and 4 (6%) had no preference. Results were similar regardless of randomisation order (P > 0·99). Of the 31 subjects initially randomised to the two‐layer system that had at least one post‐crossover visit with the four‐layer system, 22 (71%) preferred the two‐layer system. Of the 37 subjects initially randomised to the four‐layer system that had at least one post‐crossover visit with the two‐layer system, 27 (73%) preferred the two‐layer system.
Step metre
During the course of the study, many issues occurred with the step metres that resulted in step data being unrecorded or suspect. Step metres were often lost, not used for one or more days, inadvertently reset by the subject between visits, broken or not affixed properly to the subject. Step values <250 steps/day were judged to be unreliable and were excluded from the analysis. In addition, subject visits where the incorrect bandage was applied were excluded from the analysis. Subjects who had valid step data readings (≥250 steps/day and correct bandage applied) for at least 40% of the days in that period were included in the analysis. Because of the uncertainty in the step data collection, a separate analysis was completed on a single site (n = 13 subjects) in which there was more consistency in the placement of the step metres and step data collection. While there were individual step data reading exclusions at this site, there were no subject period exclusions from the analysis at this site. Analysis of the usable data from all the sites indicated that there was not a significant difference in mobility between the times that the subjects wore the two‐ and four‐layer compression systems (P = 0·82). Analysis of the data from the single, more reliable site confirmed this finding (P = 0·23).
Adverse events
Of 81 patients, 40 (49%) did not experience any adverse event. Forty‐one subjects (51%) had one or more reported adverse events. The majority of the adverse events (92, 68·1%) were determined to be unrelated to the compression bandages, and 43 (32%) were classified as possibly or probably related to the bandages. Two subjects were hospitalised during the study for reasons unrelated to the compression bandages (intestinal bleeding and kidney failure). These two patients had multiple adverse events recorded during their hospitalisation and accounted for 45 (33%) of the 135 reported adverse events. Of the 135 adverse events, 67 (50%) occurred while using the four‐layer system and 68 (50%) occurred while using the two‐layer system. The related adverse events were consistent with what might be expected with the use of compression bandages and included redness, eczema, folliculitis, wound infection and pain at the wound site.
Discussion
The efficacy of compression bandaging to positively impact wound healing is well documented in the literature 3, 25, 26, 27, 32, 33. This study differed from previous studies in that it compared performance of two compression bandage systems using slippage during wear as the primary endpoint. To our knowledge, no other study has attempted to explore this aspect of compression bandage performance. A new two‐layer bandage system, incorporating design elements purported to diminish bandage slippage and increase patient comfort, was compared with a four‐layer system with a long history of use in compression therapy. The study was designed from a patient’s perspective as it additionally explored the potential impact that reduced slippage might have on HRQoL and the patient’s overall preference for a particular compression bandage system. The study results clinically confirmed that the new two‐layer compression system exhibited less slippage during wear than the four‐layer system.
The study also found a difference in improvement of the Physical Symptoms and Daily Living scores from the HRQoL assessments observed during the pre‐crossover period. Both compression systems showed improved scores, but the improvement was much greater for the two‐layer system than the four‐layer system (P < 0·05). This domain of the CWIS focused on the impact of symptoms on daily functioning and comfort (22). It is possible that the decreased slippage observed with the two‐layer system translated into improved comfort to the patient. It is also possible that the ability of patients to wear their normal clothes and shoes over the two‐layer system may have contributed to improved comfort and/or daily functioning; however, measuring quality of life is multifactorial and can be difficult to interpret. Additional research specifically designed to compare and understand the impact of different types of compression bandage systems on HRQoL is needed.
Upon exit from the study, participants were asked to indicate preference for one of the two compression systems. There was a strong preference (72%) for the two‐layer system that held true no matter which product they first received in the randomisation. One explanation for this strong preference was that these bandages slipped less, so they did not bunch up and were more comfortable to wear. Comfort and pain control is a major contributing factor towards preference and concordance (42). A second possible explanation for the strong preference for the two‐layer system is that it is less bulky and has a lower physical profile than the four‐layer system. This is an important advancement in venous leg ulcer treatment as patients want to feel normal again, and not having to wear special clothing or shoes during compression treatment takes them one step closer to this goal. This may also help with patient concordance as patients often remove compression bandages prior to their scheduled clinic visit for social occasions when they desire to wear normal footwear and/or more stylish clothing. Another benefit to wearing normal footwear is that it may promote better ambulation and may help to reduce the number of falls, which may be associated with wearing the specialised footwear (30). Additional research on the impact of different compression bandage systems on patient concordance and ambulation is needed.
This study is limited in the conclusions that can be made regarding differences (or similarities) between the two compression systems with regard to promoting wound healing. In this study, there were no statistical differences in number of wounds that healed after 4 weeks of treatment (P = 0·47), in the mean percent area change (P = 0·87) or in the mean LHRs (P = 0·93). The percentage of ulcers that healed within the 4‐week treatment period (13·2% and 7·3% for the two‐ and four‐layer systems, respectively) was consistent with the anticipated 11–17% range observed by Kerstein et al. in their meta‐analysis of the venous leg ulcer literature (14). It is possible that differences in effectiveness between the two‐ and four‐layer systems in promoting healing of venous leg ulcers do exist but that this study was not sufficiently powered or designed to detect this difference. It can be hypothesised that bandages that stay in place longer provide more consistent and uninterrupted compression therapy, resulting in improved wound healing. This hypothesis is currently being explored in a separate study designed to measure wound healing as a primary endpoint.
A second limitation of the study is that it may have been biased towards the more familiar four‐layer system as investigators were selected who were highly experienced with this system and none were experienced with the two‐layer system. It is well known that the skill of the clinician to use materials correctly is important in achieving effective compression. Learning to apply a new compression system could negatively influence the results if the clinicians are inadequately trained to apply the product correctly. Efforts to mitigate this potential study limitation included on‐site training with each clinician in the application of the two‐layer bandage and allowing adequate practice until skill competency was showed. Previous studies have shown that with proper instruction, the two‐layer system is easy to learn and is more reproducible than the four‐layer system (31).
A third limitation of the study is in drawing conclusions from the step metre data. Empirically, one would expect a higher level of mobility with the two‐layer compression bandage if it is more comfortable and allows patients to wear normal footwear. In this study, there were no detectable differences in mobility of the patients while wearing either of the two compression systems (P = 0·81). However, this data must be interpreted cautiously given the difficulty in obtaining the data and the large number of exclusions from the statistical analysis. Many technical issues occurred with the step metres that resulted in step data being unrecorded or suspect. The step metres were often lost, reset, broken or not used at all by the patients. In other cases, they were not affixed properly, causing them to provide erroneous data. Previous authors have showed success in using patient mobility monitors affixed to the thighs of leg ulcer patients (43). The most notable differences between that study and this study was the method used to adhere the metres to the patients. In the previous study, the step metres were adhered directly to the patient’s skin using adhesive pads designed specifically for their step metres and then covered with an adhesive patch (written communications, March 2008). In this study, the metres were clipped onto the subject’s waistband or belt. Further research is necessary to develop a standardised approach to collecting patient mobility data.
Conclusions
This study shows that there is significantly less bandage slippage with the two‐layer system compared with the four‐layer system. While less bandage slippage did not appear to enhance wound healing, there was indication that it may have influenced patient preference in favour of the two‐layer system and potentially impacted patients’ HRQoL.
Acknowledgements
The authors wish to thank Mr James B Lutz, Lutz Consulting LLC, who provided medical writing services on behalf of 3M Company. This trial was undertaken with financial support from 3M Health Care.
References
- 1. Heit JA, Rooke TW, Silverstein MD, Mohr DN, Lohse CM, Petterson TM, O’Fallon WM, Melton LJ. Trends in the incidence of venous stasis syndrome and venous ulcer: a 25‐year population‐based study. J Vasc Surg 2001;33:1022–7. [DOI] [PubMed] [Google Scholar]
- 2. Jorgensen B, Price P, Andersen KE, Gottrup F, Bech‐Thomsen N, Scanlon E, Kirsner R, Rheinen H, Roed‐Petersen J, Romanelli M, Jemec G, Leaper DJ, Neumann MH, Veraart J, Coerper S, Agerslev RH, Bendz SH, Larsen JR, Sibbald RG. The silver‐releasing foam dressing, Contreet foam, promotes faster healing of critically colonised venous leg ulcers: a randomised, controlled trial. Int Wound J 2005;2:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iglesias C, Nelson EA, Cullum NA, Torgerson DJ. VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers. Health Technol Assess 2004;8:1–105. [DOI] [PubMed] [Google Scholar]
- 4. Viamontes L, Jones AM. Evaluation study of the properties of two adhesive foam dressings. Br J Nurs 2003;12(11 Suppl):S43–4, S6–9. [DOI] [PubMed] [Google Scholar]
- 5. Callam MJ, Harper DR, Dale JJ, Ruckley CV. Chronic ulcer of the leg: clinical history. Br Med J (Clin Res Ed) 1987;294:1389–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Franks PJ, Moffatt CJ. Health related quality of life in patients with venous ulceration: use of the Nottingham health profile. Qual Life Res 2001;10:693–700. [DOI] [PubMed] [Google Scholar]
- 7. Hopkins A. Disrupted lives: investigating coping strategies for non‐healing leg ulcers. Br J Nurs 2004;13:556–63. [DOI] [PubMed] [Google Scholar]
- 8. Rubin JR, Alexander J, Plecha EJ, Marman C. Unna’s boot vs polyurethane foam dressings for the treatment of venous ulceration. A randomized prospective study. Arch Surg 1990;125:489–90. [DOI] [PubMed] [Google Scholar]
- 9. Valencia IC, Falabella A, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol 2001;44:401–21; quiz 22–4. [DOI] [PubMed] [Google Scholar]
- 10. Browse NL, Burnand KG. The postphlebitic syndrome: a new look. In: Bergan JJ, Yao JS, editors. Venous problems. Chicago: Year Book Medical Publishers, Inc, 1978: 395–404. [Google Scholar]
- 11. Weiss RA, Heagle CR, Raymond‐Martimbeau P. The Bulletin of the North American Society of Phlebology. Insurance Advisory Committee Report. J Dermatol Surg Oncol 1992;18:609–16. [DOI] [PubMed] [Google Scholar]
- 12. Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol 2002;46:381–6. [DOI] [PubMed] [Google Scholar]
- 13. Bosanquet N. Costs of venous ulcers: from maintenance therapy to investment programs. Phlebology 1992;7(Suppl):44–6. [Google Scholar]
- 14. Kerstein MD, Gemmen E, Van Rijswijk L, Lyder CH, Phillips T, Xakellis G, Golden K, Harrington C. Cost and cost effectiveness of venous and pressure ulcer protocols of care. Dis Manage Health Outcomes 2001;9:651–3. [Google Scholar]
- 15. Brem H, Kirsner RS, Falanga V. Protocol for the successful treatment of venous ulcers. Am J Surg 2004;188(1A Suppl):1–8. [DOI] [PubMed] [Google Scholar]
- 16. Mani R. Laboratory evaluation of non‐healing wounds. In: Falanga V, editor. Cutaneous wound healing. London: Martain Dunitz, Ltd, 2001:187–201. [Google Scholar]
- 17. Nelson EA, Bell‐Syer SE, Cullum NA. Compression for preventing recurrence of venous ulcers. Cochrane Database Syst Rev 2000;4:CD002303. [DOI] [PubMed] [Google Scholar]
- 18. Cullum N, Nelson EA, Fletcher AW, Sheldon TA. Compression for venous leg ulcers. Cochrane Database Syst Rev 2001;2:CD000265. [DOI] [PubMed] [Google Scholar]
- 19. Kantor J, Margolis DJ. Management of leg ulcers. Semin Cutan Med Surg 2003;22:212–21. [DOI] [PubMed] [Google Scholar]
- 20. Schulze HJ, Lane C, Charles H, Ballard K, Hampton S, Moll I. Evaluating a superabsorbent hydropolymer dressing for exuding venous leg ulcers. J Wound Care 2001;10:511–8. [DOI] [PubMed] [Google Scholar]
- 21. Franks PJ, Moffatt CJ, Ellison DA, Connolly M, Fielden S, Groarke L, McCollum CN. Quality of life in venous ulceration: a randomized trial of two bandage systems. Phlebology 1999;14:95–9. [Google Scholar]
- 22. Price P, Harding K. Cardiff Wound Impact Schedule: the development of a condition‐specific questionnaire to assess health‐related quality of life in patients with chronic wounds of the lower limb. Int Wound J 2004;1:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rojas AI, Phillips TJ. Venous ulcers and their management. In: Falanga V, editor. Cutaneous wound healing. London, UK: Martin Dunitz Ltd, 2001:263–86. [Google Scholar]
- 24. Meyer FJ, Burnand KG. Compression treatment: methods and controversies. In: Falanga V, editor. Cutaneous wound healing. London, UK: Martin Dunitz Ltd, 2001:287–305. [Google Scholar]
- 25. Ukat A, Konig M, Vanscheidt W, Munter KC. Short‐stretch versus multilayer compression for venous leg ulcers: a comparison of healing rates. J Wound Care 2003;12:139–43. [DOI] [PubMed] [Google Scholar]
- 26. Nelson EA, Iglesias CP, Cullum N, Torgerson DJ. Randomized clinical trial of four‐layer and short‐stretch compression bandages for venous leg ulcers (VenUS I). Br J Surg 2004;91:1292–9. [DOI] [PubMed] [Google Scholar]
- 27. Meyer FJ, McGuinness CL, Lagattolla NR, Eastham D, Burnand KG. Randomized clinical trial of three‐layer paste and four‐layer bandages for venous leg ulcers. Br J Surg 2003;90:934–40. [DOI] [PubMed] [Google Scholar]
- 28. Moffatt CJ, Simon DA, Franks PJ, Connolly M, Fielden S, Groarke L. Randomized trial comparing two four‐layer bandage systems in the management of chronic leg ulceration. Phlebology 1999;14:139–42. [Google Scholar]
- 29. Davis J, Gray M. Is the Unna’s boot bandage as effective as a four‐layer wrap for managing venous leg ulcers? J Wound Ostomy Continence Nurs 2005;32:152–6. [DOI] [PubMed] [Google Scholar]
- 30. Corbett LQ, Kelly C, Magliato B. Fall risk associated with multi‐layer compression wrap therapy in venous leg ulcers. In: Poster, 2007 SAWC/WHS conference in Tampa, FL, 2007 28 April–01 May. 2007. [Google Scholar]
- 31. Moffatt CJ, Glover D, Price P, Clark M, Collier M, Hayes W, Day J. An evolution in compression. J Wound Care 2007;3M (Suppl):1–12. [Google Scholar]
- 32. Polignano R, Bonadeo P, Gasbarro S, Allegra C. A randomised controlled study of four‐layer compression versus Unna’s Boot for venous ulcers. J Wound Care 2004;13:21–4. [DOI] [PubMed] [Google Scholar]
- 33. O’Brien JF, Grace PA, Perry IJ, Hannigan A, Clarke Moloney M, Burke PE. Randomized clinical trial and economic analysis of four‐layer compression bandaging for venous ulcers. Br J Surg 2003;90:794–8. [DOI] [PubMed] [Google Scholar]
- 34. Gelfand JM, Hoffstad O, Margolis DJ. Surrogate endpoints for the treatment of venous leg ulcers. J Invest Dermatol 2002;119:1420–5. [DOI] [PubMed] [Google Scholar]
- 35. Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair Regen 2004;12:163–8. [DOI] [PubMed] [Google Scholar]
- 36. Kantor J, Margolis DJ. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br J Dermatol 2000;142:960–4. [DOI] [PubMed] [Google Scholar]
- 37. O’Donnell TF Jr, Lau J. A systematic review of randomized controlled trials of wound dressings for chronic venous ulcer. J Vasc Surg 2006;44:1118–25. [DOI] [PubMed] [Google Scholar]
- 38. Gilman TH. Parameter for the measurement of wound closure. Wounds 1990;2:95–101. [Google Scholar]
- 39. Margolis DJ, Gross EA, Wood CR, Lazarus GS. Planimetric rate of healing in venous ulcers of the leg treated with pressure bandage and hydrocolloid dressing. J Am Acad Dermatol 1993;28:418–21. [DOI] [PubMed] [Google Scholar]
- 40. Margolis DJ. Wound healing assessment: the clinical utility of wound healing rates. Ostomy Wound Manage 1994;40:20–2, 4, 6–7. [PubMed] [Google Scholar]
- 41. Gilman TH. Comparing healing rates across studies is the vision, but first, a correct equation please! Ostomy Wound Manage 1995;41:6–7. [PubMed] [Google Scholar]
- 42. Moffatt CJ. Factors that affect concordance with compression therapy. J Wound Care 2004;13:291–4. [DOI] [PubMed] [Google Scholar]
- 43. Clarke‐Moloney M, Godfrey A, O’Connor V, Meagher H, Burke PE, Kavanagh EG, Grace PA, Lyons GM. Mobility in patients with venous leg ulceration. Eur J Vasc Endovasc Surg 2007;33:488–93. [DOI] [PubMed] [Google Scholar]


