Abstract
Six out of 10 patients with chronic wounds suffer from persistent wound pain. A multinational and multicentre, randomised, double‐blind clinical investigation of 122 patients compared two moist wound‐healing dressings, a non adhesive foam dressing with ibuprofen (62 patients randomised to Biatain‐Ibu non adhesive, Coloplast A/S) with a non adhesive foam without ibuprofen (60 to Biatain non adhesive).The ibuprofen‐foam was regarded successful, if the pain relief on a 5‐point verbal rating scale was higher than the comparator without compromising safety, including appropriate healing rate. Additional endpoints were change in persistent wound pain between dressing changes and pain at dressing change on days 1–5 and days 43–47. The primary response variable, persistent pain relief, was significantly higher in the ibuprofen‐foam group compared with the comparator on days 1–5, with a quick onset of action (P < 0·05). The patients in the ibuprofen‐foam group had a significant (P < 0·05) higher reduction in the persistent wound pain from baseline (40%) as the comparator (30%). Women reported less pain intensity than men, and pain intensity decreased with increasing age. In addition, pain intensity increased with increasing initial pain intensity and increasing wound size. Wound healing was similar in the ibuprofen‐foam group to that of the comparator group. No difference in adverse events between placebo and local sustained release of low‐dose ibuprofen was observed in this study. This study has demonstrated that the ibuprofen‐foam dressing provided pain relief and reduced pain intensity without compromising healing or other safety parameters. The full report of this study will be published in Wound Repair and Regeneration.
Keywords: Fast wound healing, Topical treatment, Wound pain relief, Wound pain intensity reduction
Introduction
Despite the fact that six out of ten people with chronic wounds suffer from persistent wound pain 1, 2, 3, little is known about the effectiveness of local treatment interventions (4) for persistent pain between dressing changes. Persistent pain associated with non healing wounds is caused by tissue (nociceptive) or nerve (neuropathic) damage and is influenced by dressing changes and chronic inflammation. Chronic wounds take long time to heal, and patients can suffer from chronic wounds for many years (5). Chronic wound healing may be compromised by coexisting underlying conditions, such as peripheral vascular disease, uncontrolled oedema and diabetes mellitus.
The pain from chronic wounds can be extremely severe, causing both physical and mental debilitation. Patients can become immobile, which in turn can lead to social isolation, depression and feelings of hopelessness (5). The pain experienced by persons with chronic wounds has been described as a red raw (feeling), burning and like having acid thrown onto the skin (5).
The application of compression bandaging is generally accepted to improve venous ulcer healing; however, in many cases, patients with chronic venous ulceration find it difficult to tolerate high‐compression bandaging (44%) because of associated pain (6), and this will adversely affect ulcer healing. In general, wound pain is reported to reduce appetite (7), reduce mobility, induce loneliness (8) and disturb sleeping (9). All these factors lead to a general decrease in health, which is a barrier to wound healing.
This investigation compares the performance and safety of a non adhesive foam dressing with ibuprofen (ibuprofen‐foam) with a foam non adhesive dressing alone (comparator). The aim of this clinical investigation was to investigate whether the ibuprofen‐foam dressing relieves venous ulcer pain without compromising the beneficial properties of moist wound healing, with an acceptable safety profile.
Materials and methods
Design
This randomised, controlled, double‐blind, parallel group, multicentre, and multinational clinical investigation was conducted according to the international standards of good clinical practice. The primary outcome was to investigate the pain‐relieving effect of the ibuprofen‐foam, together with monitoring safety. The study compared three investigation periods, days 1–5, days 6–42 and days 43–47 (Figure 1). On days 1–5 and days 6–42, the patients were randomised 1:1 to either the ibuprofen‐foam group or the comparator group using premade, treatment‐coded envelopes. On days 43–47, the treatment was blinded only to the patients, and all patients were crossed over and treated with the comparator dressing. The dressings were changed on a regular 48‐hour interval to ensure a uniform dressing environment (absorption, retention, release).
Figure 1.
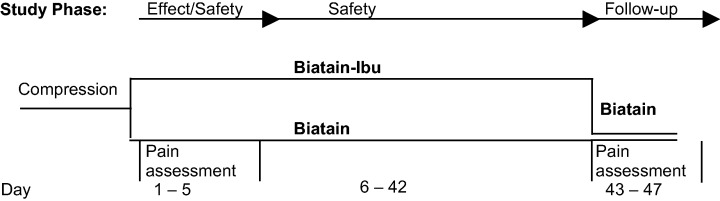
The patients were in two groups. On days 1–5 and on days 43–47, pain intensity was assessed on a numeric box scale and pain relief on a verbal rating scale.
Patient characteristics
Independent ethic boards for each study site have approved this study according to internationally approved standards. The recruited patients had received both oral and written information about the study, and signed consent. The study subjects were older than 18 years of age with painful chronic venous leg ulcer of more than 8‐week duration, and they were required to receive compression therapy for at least 2 weeks before inclusion, and throughout the study period. The ulcer was required to have a minimum length of 1·6 cm in any direction, and a maximum area of 50 cm2. The minimum leg ulcer pain score at inclusion was moderate on a 5‐point verbal rating scale (VRS) (none, slight, moderate, lots, complete). Exclusion criteria included painful ulcers that had been resistant to analgesic treatment over the past 6 months or more, pregnant or lactating women, clinical infection (erysipelas and cellulitis) or critical colonisation (local infection) within or surrounding the study ulcer, vasculitis, allergy or other contraindication to ibuprofen or other related analgesics (including known hypersensitivity to acetylsalicylic acid or related non steroidal anti‐inflammatory agents especially associated with a history of asthma, rhinitis or urticaria), diabetes, the use of unscheduled additional pain medication for 3 days prior to study admission (regular concomitant pain medication was acceptable for inclusion), concomitant treatment with systemic antibiotics other than nitrofurantoin, concomitant treatment with systemic corticosteroids (more than 10 mg/day of prednisolone or equivalent), treatment with other immunosuppressant or cancer chemotherapeutic agents within 1 month prior to inclusion, concomitant participation in other studies and previous participation in this study.
The study participants were allowed to take concomitant pain medication during the study, but the medication had to be constant at days 1–5 and at days 43–47 when pain was assessed.
Assessments
Persistent (chronic) and temporary pain (dressing change related) was assessed on days 1–5 and on days 43–47 (Figure 1). The persistent pain was rated in the morning and evening by the patients between dressing changes, using a diary. The persistent pain was rated on a pain relief 5‐point VRS (0 = no relief, 1 = slight relief, 2 = moderate relief, 3 = lots of relief, 4 = complete relief). Pain relief was regarded as a retrospective view of the treatment efficiency, and the high score was associated with high treatment efficacy 10, 11. In addition, pain intensity was measured on a validated 11‐point numeric box scale (NBS) from 0 to –10, with ‘0’ as no pain and ‘10’ as the worst imaginable pain. A low pain intensity score was regarded a preferred score 10, 11. Pain at dressing change was rated (NBS) by the patients at the visits on days 2 and 5 and on days 45 and 47, immediately after removal of the dressing and cleansing of the wound. Patients were asked to score their pain intensity on the 0–10 NBS.
The ulcer area was assessed at baseline, on days 15, 29 and on day 42 with wound tracings of the ulcer margins. Infection was assessed by coexistence of the classical signs of clinical infection: pain, erythema, oedema, heat and purulence. Delayed or stalled wound healing and one of the following three characteristics identified bacterial imbalance: increased level of exudate, granulation tissue, discoloration or odour; the combination led to study exclusion. Safety was monitored according to the European standards for testing medical devices on human subjects 12, 13. The causality of the adverse event was defined as related, possibly related and not related to the dressing. All adverse events in this study were reported spontaneously either by the patients or by the study personnel.
Materials
The two moist wound‐healing foam dressings used in this study absorb exudate and provide a physical barrier between the wounds and the environment. The ibuprofen‐foam (Biatain‐Ibu non adhesive foam dressing, Coloplast A/S) consists of a soft hydrophilic polyurethane foam containing ibuprofen (ibuprofen concentration: 0·5 mg/cm2) homogeneously dispersed throughout the foam. The foam is bound to a semipermeable polyurethane film. In the presence of exudate, there is a continuous release of a safe low‐dose ibuprofen into the wound bed (14). One dressing (15 × 15 cm) contains 112·5 mg of ibuprofen to be released between 1 and 7 days, compared to a maximum daily oral dose of 1200 mg and in special cases 3200 mg (15). A proof of concept study suggested an anti‐inflammatory and pain‐reducing effect of the dressing (16). The comparator foam consisted of the same materials and had a similar structure as the ibuprofen‐foam, but ibuprofen was not incorporated (Biatain non adhesive, Coloplast A/S). The backing film and the packaging of the two compared dressings were similar to ensure the double blinding.
Clinical endpoints
The ibuprofen‐foam was regarded as a therapeutically effective treatment if the pain relief was significantly higher and pain intensity ratings were significantly lower compared with the comparator product during days 1–5. The primary endpoint was persistent pain relief during the first 5 days. Secondary endpoints were reduction in persistent pain intensity on days 1–5, ulcer area reduction, difference between groups in adverse events and pain intensity on days 43–47. Other secondary endpoints were temporary pain at dressing change, occurrence of critical colonisation, and selected activities of daily living indicators such as well‐being, appetite, sleep and mood.
Data analysis and statistics
Sample size was determined by evaluating a previous study (16) on pain relief observed on a 5‐point scale during morning and evening where an overall mean pain relief of 3·8 for the ibuprofen‐foam and 3·3 for the comparator was observed. The common standard deviation was 0·79. With a 90% power, this yields a sample size of 54 per group (data on file). Empirically a drop‐out rate of 10% is observed in similar clinical studies, hence resulting in a sample size of 60 per group. Before the statistical analysis was performed, the data were entered twice and discrepancies resolved. All data analysis was performed on the intention‐to‐treat (ITT) population. The primary response variable was persistent pain relief on days 1–5. The secondary outcome variables were persistent pain intensity, ulcer size, pain at dressing change, activities of daily living and adverse events. The results were analysed according to the statistical analysis plan designed prior to study commencement (17). Alpha was set at 0·05. The persistent pain relief for days 1–5 was for each patient assessed during morning and evening on a 5‐point VRS (none, slight, moderate, lots, completely). After dichotomising data (relief, no relief), differences were analysed with an analysis of variance. Persistent pain intensity for each patient was assessed during morning and evening on an 11‐point NBS and analysed with an analysis of variance, and statistically significant (P < 0·05) parameters were reported together with adjusted treatment means. Pain at dressing change (temporary pain) was evaluated after each dressing change between day 1–5 and days 43–47. An analysis of variance was used to test differences between groups. For the follow‐up period, the visit on day 43 was used as the baseline for the dressing pain intensity. Adverse events were divided into the following categories: non device–related adverse events, non device–related serious adverse effects, device‐related adverse events and serious device‐related adverse effects. Any difference in observed adverse events between study groups was tested using a chi‐square test.
Ulcer size was recorded at inclusion and after 15, 28 and 42 days of treatment. A t‐test was used to test differences in means reported for the ITT population. Wound healing was also tested using a linear healing parameter, as described by Gilman (18). This quantifies wound healing as the linear movement of the wound edge towards the centre of the wound.
Results
Baseline data
The study population consisted of 122 patients (62 in the ibuprofen‐foam group and 60 in the comparator group) with chronic, painful venous leg ulcers on the lower limb. Patients were recruited from 13 different centres: three in Great Britain, three in Lithuania, two in Denmark, two in Germany, two in the Czech Republic and one in Finland. The first patient was included on 19 September 2005, and last patient out was on 21 April 2006, with a clinical report approved by investigators on July 2006. Table 1 summarises the demographic profile of the study group. Table 2 summarises the medical history of the patients. In the ibuprofen‐foam group, 11% (7/62) of the patients dropped out of the study prematurely, and 5% (3/60) did not complete the study in in the comparator group. The dropouts were related to withdrawal of consent (4), protocol violation (3), pain in the study ulcer (1), serious non related adverse event (1) and suspected allergic reaction (1). In this trial, all patients were asked to keep their concomitant medication constant between days 1–5 and days 43–47, and no patient was excluded due to change in pain medication.
Table 1.
Demographics of patients in the two treatment groups
| Assessment | Ibuprofen‐foam | Comparator | |
|---|---|---|---|
| Age (years) | Mean | 66·0 | 70·0 |
| Standard deviation | 14·8 | 11·7 | |
| Gender | Female, n (%) | 43 (69) | 37 (62) |
| Height (cm) | Mean | 168 | 168 |
| Standard deviation | 1·3 | 1·2 | |
| Weight (kg) | Mean | 83·0 | 79·4 |
| Standard deviation | 2·6 | 2·1 | |
Table 2.
Important baseline values of patients in the two treatment groups
| Assessment | Ibuprofen‐foam | Comparator | P value | |
|---|---|---|---|---|
| Pain intensity at inclusion (numeric box scale) | Mean | 6·82 | 6·58 | NS |
| Standard deviation | 1·8 | 1·6 | ||
| Exudate level of ulcer | Moderate, n (%) | 41 (66) | 51 (85) | — |
| High, n (%) | 21 (34) | 9 (15) | ||
| Absolute ulcer area (cm2) | Mean | 11·0 | 7·3 | <0·05 |
| Standard deviation | 9·6 | 5·7 | ||
| Absolute ulcer circumference (cm2) | Mean | 15·5 | 12·5 | NS |
| Standard deviation | 9·1 | 6·9 | ||
—, not tested; NS, not significant.
Pain
Persistent pain relief on days 1–5
In the ibuprofen‐foam group, patients experienced significantly (P = 0.0003) more pain relief in the wound throughout the first 1–5 days of wear time compared with the comparator. In the first evening, 74% (46/62) of the patients in ibuprofen‐foam group had pain relief, compared with 58% (35/60) in the comparator group (Figure 2). Twenty‐eight percent (74 ‐ 58/58 × 100) more patients experienced pain relief the first evening in the ibuprofen‐foam group than in the comparator.
Figure 2.
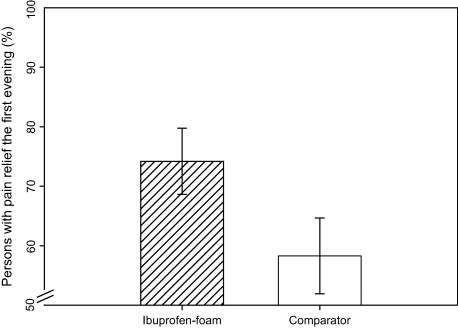
Percentage of patients with pain relief the first evening including standard deviations. The first evening there were 28% more persons with pain relief in the ibuprofen – foam (74%) group, than the comparator group (58%).
Persistent pain intensity on days 1–5
Wound pain was significantly reduced with the ibuprofen–foam during days 1–5 (P < 0·0003). The wound pain intensity levels were reduced from 6·8 at inclusion to 4·1, a reduction of 40% with the ibuprofen‐foam, and from 6·6 at inclusion to 4·6, a reduction of 30% with the comparator. Women reported less pain intensity than men, and pain intensity decreased with increasing age. Furthermore, pain intensity increased with increasing baseline pain intensity and increasing wound size (Table 3).
Table 3.
Patients’ pain intensity depended on the following statistically significant variables from the analysis of variance (d.f. = 914) on morning and evening pain intensity values from day 1 to 5
| Effect | P value | Interpretation |
|---|---|---|
| Treatment (ibuprofen‐foam versus comparator alone) | 0·0003 | Ibuprofen‐foam reduces painintensity |
| Time (between assessments) | <0·0001 | Pain intensity for all patients decreases over time |
| Gender (women) | 0·01 | Women have less pain |
| Age | <0·0001 | Older people have less pain |
| Baseline pain intensity | <0·0001 | High baseline pain level, associated with high levels of pain at any time or with any treatment or other included variables |
| Baseline ulcer size | <0·0001 | Larger ulcers, associated with higher pain levels |
Pain at dressing change
Dressing change–related pain intensity increased in the former ibuprofen‐foam group when the non adhesive comparator was introduced between days 43 and 47. A significant difference (P < 0·05) in pain intensity was detected at dressing removal (Figure 3). The pain intensity increased significantly for the previous ibuprofen‐foam group from baseline NBS value of 0·3 to 0·9 (0·9/0·3 × 100 = 300%) in contrast to constant levels in the comparator group with an average NBS value of 2·0 at baseline. At days 1–5, it was not possible to detect a difference in dressing change pain.
Figure 3.
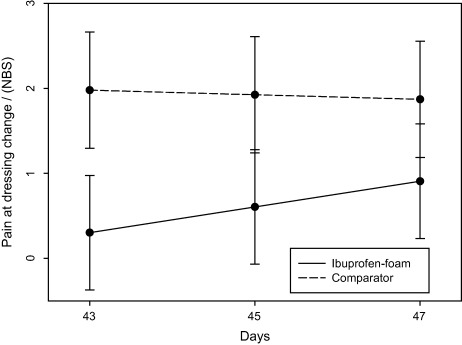
Pain at dressing change on days 43–47 increased in the former ibuprofen–foam group (P = 0·05). NBS, numeric box scale.
Ulcer area
The ulcers decreased on average from 11·2 to 7·9 cm2 in the ibuprofen‐foam group and from 7·2 to 3·8 cm2 in the comparator group (Figure 4). The decrease in ulcer area between the two groups was comparable, with no statistical difference. The wound area decrease between inclusion to day 43 divided by the average wound circumference was not significantly different between the groups using the Gilman (18) method. Both study group wound perimeters moved towards the centre of the wounds with comparable speed. Ten ulcers in the comparator group and nine ulcers in the ibuprofen‐foam group healed during the trial, and there was no statistical difference in healing time.
Figure 4.
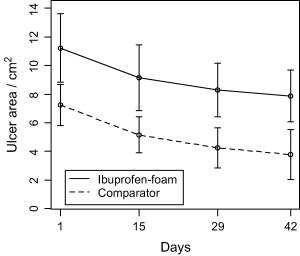
Absolute change in ulcer area (cm2) on the intention‐to‐treat population. There was no statistical significant difference on ulcer area reduction (P = 0·26) between the treatment groups.
Adverse events
In total, 31 adverse events on 19 persons were reported (Table 4). In the ibuprofen‐foam group, 12 patients experienced 21 adverse events, and in the comparator group, seven patients experienced ten adverse events. One patient was hospitalised (serious adverse event) because of a non device–related pulmonary oedema and was excluded from the study. In the ibuprofen‐foam group, 19% (12/62) adverse events were reported compared with 12% in the comparator group (7/60). The relation to the device is reported in Table 4. Most of these adverse events had no relation to either the ulcer or the dressing. There were no serious device‐related adverse events reported in the study. The device‐related adverse events were categorised into four groups: study ulcer pain, infection in study ulcer, local skin reactions and other. Skin reactions were, in the ibuprofen‐foam group, observed with four patients; one had urticaria and later eczema, one had eczema alone, and two had blisters. In the comparator group, four patients had skin reactions: two had eczema and two had blisters. In the ibuprofen‐foam group, allergic dermatitis was suspected in one patient. A patch test was applied on the patient’s normal skin. The patch test showed a weak, potentially allergic reaction to the complete product, but not to ibuprofen‐standardised patch test (petrolatum base), or to the comparator product. The negative patch tests to the components of the dressing controverts a contact irritant dermatitis, although there is a slight chance that the patient is allergic to a metabolite of ibuprofen.
Table 4.
Adverse events separated into mild, moderate and severe and relation to device for the ibuprofen‐foam and the comparator group
| Type of adverse event | Ibuprofen‐foam* | Comparator† | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Unrelated | Possible related | Related | Total | Unrelated | Possible related | Related | Total | ||
| Total N (%) | 3 (14) | 5 (24) | 13 (62) | 21 (100) | 1 (10) | 3 (30) | 6 (60) | 10 (100) | NS‡ |
Infection in study ulcer, three patients; eczema, two patients; blisters, two patients; urticaria, one patient; study ulcer pain, one patient; other, 12 patients (13 incidences unrelated to the dressing).
†Infection in study ulcer, two patients; bullae, one patient; eczema, two patients; blisters, one patient; other, four patients (six incidences unrelated to the dressing).
‡NS, not significant.
Discussion
This study has confirmed the benefit of foam dressing with low‐dose ibuprofen for the treatment of persistent chronic wound pain. In proof‐of‐concept studies, Jørgensen et al. (16), Sibbald et al. (19) and Flanagan et al. (5) have demonstrated that the ibuprofen–foam combines effective moist wound healing and ibuprofen release. This combined action reduced persistent pain, reduced pain at dressing change and increased quality of life, without being able to detect ibuprofen in the blood plasma (16). This randomised, controlled, double‐blind study was designed for a 42‐day treatment period plus an additional follow‐up period of 5 days, where all patients were treated with the comparator foam dressing alone without ibuprofen. Based on results from preclinical and human pilot studies 5, 16, 19, 20, a treatment period of 42 days should be sufficient time for evaluating adverse events and following the change in wound healing (e.g. ulcer area). Biatain Non adhesive foam dressing was considered the appropriate comparator product, as the presence of ibuprofen was the only difference between the two dressings. The addition of the follow‐up period days 43–47 was to test whether patients from the ibuprofen‐foam group would experience increase in pain during the last 5 days due to the withdrawal of the local low‐dose release of ibuprofen.
Patient population
The patients in previous studies that compared the analgesic effect of topical ibuprofen on intact skin with systemic use of ibuprofen were rarely above 50 years of age 21, 22, 23. In the ibuprofen‐foam group of this study, the patients were on average 66 years of age, and 70 years of age in the comparator group (Table 1). The ideal patient in a pain trial may be often younger and male because pain‐relieving medication is more likely to result in improved pain scores (24). For this reason, there are few previous reports of topical pain trials in an older, often female‐predominant polymedicated population. Therefore, this investigation, together with the few clinical trials on interventions on the elderly population, showing statistical differences has high societal value.
Pain
Patients’ perception of pain is known to change over time (11). Therefore, to get comparable results, pain interventions must be evaluated over relatively short periods (10).
Persistent pain relief on days 1–5
The primary endpoint of this study was pain relief. This study demonstrated that patients treated with the ibuprofen‐foam had more pain relief than the comparator group (P < 0·05). This significant result is in line with a pilot cross‐over study that demonstrated a reduction in pain intensity when changing from a comparator to the ibuprofen‐foam (16). More patients (28%) in the ibuprofen‐foam group had pain relief in the first evening (Figure 2), indicating that the observed pain relief has a quick onset of action. In a meta‐analysis of topical non steroidal anti‐inflammatory drug gels by Mason et al. (25), comparable results were shown, where the active treatment had an effect on 48% of patients, compared with 26% from placebo. In this case, we observed pain relief in 74% of patients in the active group compared with 58% in the comparator group. Ibuprofen is mainly reported to have an effect on pain that is of a nociceptive origin (22). Therefore, it is not expected that the ibuprofen‐foam would be effective in all patients. There is evidence in the literature (26) that moist wound healing provides pain relief. This study shows that by adding ibuprofen to a moist wound healing foam, an even stronger pain relieving effect is experienced by patients.
Persistent pain intensity on days 1–5
In this study, the data demonstrated that the pain intensity levels were reduced with the ibuprofen‐foam, 40% from baseline compared with a 30% reduction for the comparator. Farrar et al. (27) found in a meta‐analysis of pain trials, where the 11‐point NBS scale was used, that a 30% reduction in pain intensity was clinically relevant. This means that both the ibuprofen‐foam and the comparator product had clinically relevant pain reductions, with the ibuprofen‐foam having a slight advantage. The pain intensity levels were also influenced by gender, age, initial pain level, and wound size.
Pain at dressing change
Pain at dressing change or removal has been identified as a key patient concern 28, 29. The ibuprofen‐foam seemed to be associated with decreased pain at dressing change. On days 43–47, the former ibuprofen‐foam group experienced an increment in their dressing‐change pain. This increase was interpreted as a response to the removal of the analgesic effect. In other studies by Jørgensen et al. (16) and Sibbald et al. (19) similar results were observed, where the ibuprofen‐foam reduced pain at dressing change. Pain at dressing change was not significantly different between the two comparative groups on days 1–5. Pain perception can be influenced by the effect of care (30); thus, measuring pain when crossing all patients over to the comparator foam is interpreted as a new baseline for the moist wound‐healing parameters of the foam dressing alone. On day 43, the patients were accustomed to the best standard care. Therefore, differences observed during days 43–47 could be interpreted as free of the effect of care. Indirectly, it was, therefore, shown that the patients in the former ibuprofen‐foam group versus the comparator had experienced less pain at dressing change.
Ulcer area
The ibuprofen‐foam dressing was regarded as successful if the healing properties were similar to or better than those of the comparator foam. In this study, the patients in the ibuprofen‐foam study had larger ulcers at baseline compared with the comparator group (P < 0·05). Area‐based comparisons of the ulcer, therefore, gave biased results (18). In such case, a comparison of the linear healing parameter is more appropriate as it would not be affected by the difference in baseline wound area. Using this method (as well as mean area), no difference in wound healing between the ibuprofen‐foam and the comparator group was found.
Ibuprofen has an anti‐inflammatory effect, and some literature discusses the effect of ibuprofen on wound healing 31, 32, 33, 34. Theoretically, ibuprofen could inhibit the normal inflammatory wound‐healing stage by reducing the amounts of prostaglandin. Most of the reported research has been performed on acute wounds, or with extremely high doses of ibuprofen in preclinical studies on animals, with variable outcomes (35). Often, patients with a chronic wound suffers from chronic medical disorders and the wounds may be stuck in the inflammatory phase (36). Therefore, low‐dose ibuprofen use in chronic wounds is less likely to significantly influence healing of chronic wounds (16). Both methods used in this study to quantify wound healing showed that there was no difference in the chronic wound–healing rate between comparative groups, and the healing rate was comparable with earlier randomised controlled trials on the comparator foam (37).
Healing venous leg ulcers requires compression bandaging (6). In this study, patients were required to receive compression bandaging before inclusion and throughout the study period. However, patients often discontinue compression treatment because of the associated pain. In a study by Briggs and Closs (6) on 96 venous leg ulcer patients, 44% of patients could not adhere to their compression therapy because of persistent wound pain. Consequently, for chronic ulcer patients, pain is a serious barrier to wound healing. An ibuprofen–foam may be a good solution for increasing patient’s compression bandage adherence. In addition, for the 30% of patients who are unlikely to heal within 5 years (38), pain control should be considered as important to patients as healing.
Adverse events
The usual adverse events reported for ibuprofen are related to relatively high dose with oral intake (15). None of the most frequently reported oral ibuprofen adverse events (15) were observed in this study. The 15 × 15 cm ibuprofen‐foam chosen in this investigation contains 112·5 mg of ibuprofen to be released into the wound over 7 days, depending on the exudate level (14). This is much lower than the recommended daily dose of 1200 mg, and in special cases 3200 mg (39). It has been shown in another study that the ibuprofen could not be detected systemically when the ibuprofen‐foam was used (16).
The data from this study and other clinical studies on the ibuprofen‐foam including more than 373 patients 5, 15, 19, 20, 39 have not detected any cases of allergic or contact dermatitis as adverse reactions to the ibuprofen‐foam. A meta‐analysis on topical use of ibuprofen including studies from 1500 patients has demonstrated no difference in adverse reactions (including allergies) between placebo and topical use of ibuprofen gels (25). Based on the results from this study and the literature, the likelihood of adverse events with the ibuprofen‐foam would be considered low. The likelihood of adverse events following long‐term use of the ibuprofen‐foam is unknown.
Conclusion
This is a report of a multinational and multicentre, randomised, double‐blind, clinical investigation on ibuprofen‐foam. The study compared two moist wound‐healing dressings and included 122 patients, with 62 randomised to the ibuprofen‐foam and 60 to the comparator. The primary outcome, the persistent wound pain relief during the first 1–5 days, was significantly higher in the ibuprofen‐foam group. There was a quick onset of action, with a significant pain relief in the first evening. Women reported less pain intensity than men, and pain intensity decreased with increasing age. In addition, pain intensity increased with increasing initial pain intensity and increasing wound size. Wound healing was similar in the ibuprofen‐foam group to that of the group that used comparator foam alone. No difference in adverse events between comparator and local sustained release of low‐dose ibuprofen was observed in this study. There was no difference between dressing absorption capacity between the two compared dressings. This study has demonstrated that the ibuprofen‐foam dressing is beneficial for persistent pain relief and reducing persistent and temporary wound pain intensity without compromising healing or safety.
Conflicts of interest
R. R. has acted as a paid researcher for Coloplast and received funding for that research. V. V. received funding for the salary of the research nurse involved in this work. P. V. and K. E. received renumeration for the extra time involved in study participation according to international guidelines. The authors are all members of the International Pain Advisory Board for Coloplast A/S and have been reimbursed for their services.
Acknowledgements
This study was supported by Coloplast A/S, Holtedam 1, 3050 Humlebæk, Denmark, and its affiliates. Our appreciations to Clifo A/S for handling patient monitoring, double data entry and cleaning and statistical data analysis. Thanks to Rikke Holt Agerslev, Søren Nymand Lophaven and Ole K. Nielsen for their contributions to writing the manuscript.
References
- 1. Cooper SM, Hofman D, Burge SM. Leg ulcers and pain: a review. Int J Low Extrem Wounds 2003;2:189–97. [DOI] [PubMed] [Google Scholar]
- 2. Hofman D, Ryan TJ, Arnold F, Cherry GW, Lindholm C, Bjellerup M, Glynn C. Pain in venous leg ulcers. J Wound Care 1997;6:222–4. [DOI] [PubMed] [Google Scholar]
- 3. Phillips TJ, Stanton B, Provan A, Lew R. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol 1994;31:49–53. [DOI] [PubMed] [Google Scholar]
- 4. Briggs M, Nelson EA. Topical agents or dressings for pain in venous leg ulcers. Cochrane Database Syst Rev 2005. (1): 23. [DOI] [PubMed] [Google Scholar]
- 5. Flanagan M, Vogensen H, Haase L. Case series investigating the experience of pain in patients with chronic venous leg ulcers treated with a foam dressing releasing ibuprofen. World Wide Wounds 2006. [WWW document]. URL http://www.worldwidewounds.com/2006/april/Flanagan/Ibuprofen‐Foam‐Dressing.html [accessed on 2 March 2007]
- 6. Briggs M, Closs SJ. Patients’ perceptions of the impact of treatments and products on their experience of leg ulcer pain. J Wound Care 2006;15:333–7. [DOI] [PubMed] [Google Scholar]
- 7. Bosley BN, Wiener DK, Rudy TE, Granieri E. Is chronic nonmalignant pain associated with decreased appetite in older adults? Preliminary evidence. J Am Geriatr Soc 2004;52:247–51. [DOI] [PubMed] [Google Scholar]
- 8. Hyde C, Ward B, Horsfall J, Winder G. Older women’s experience of living with chronic leg ulceration. Int J Nurs Pract 1999;5:189–98. [DOI] [PubMed] [Google Scholar]
- 9. Noonan L, Burge SM. Venous leg ulcers: is pain a problem? Phlebology 1998;13:14–19. [Google Scholar]
- 10. Bernstein SL, Bijur PE, Gallagher EJ. Relationship between intensity and relief in patients with acute severe pain. Am J Emerg Med 2006;24:162–6. [DOI] [PubMed] [Google Scholar]
- 11. Angst MS, Brose WG, Dyck JB. The relationship between the visual analog pain intensity and pain relief scale changes during analgesic drug studies in chronic pain patients. Anesthesiology 1999;91:34–41. [DOI] [PubMed] [Google Scholar]
- 12. Anonymous. ISO 14155–1: Clinical investigation of medical devices for human subjects — Part 1: General requirements. 2003. [Google Scholar]
- 13. Anonymous. ISO 14155–2: Clinical investigation of medical devices for human subjects — Part 2: Clinical investigation plans. 2003. [Google Scholar]
- 14. Steffansen B, Herping SPK. Novel wound models for characterizing the effects of exudates levels on the controlled release of ibuprofen from foam dressings. In: Gottrup F, Bale S, Franks P, Hofman D, Francu M, Price P, Romanelli M, Rybak Z, editors. European Wound Management Association Conference; 2006 May 1720; Prague. Frederiksberg: Congress Consulting. [Google Scholar]
- 15. Thomson MICROMEDEX . DrugPoints® Ibuprofen. MICROMEDEX(R) Healthcare Series. 2006, 128. Thomson Micromedex, 6200 South Syracuse Way, Suite 300, Greenwood Village, Colorado. [Google Scholar]
- 16. Jørgensen B, Friis GJ, Gottrup F. Pain and quality of life for patients with venous leg ulcers: proof of concept of the efficacy of Biatain‐Ibu, a new pain reducing wound dressing. Wound Repair Regen 2006;14:233–9. [DOI] [PubMed] [Google Scholar]
- 17. Habicht A, Lophaven SN, Aagaard EB, Agerslev RH. Statistical analysis plan, DK071WO. Humlebaek, Denmark: Coloplast A/S, 2006. [Google Scholar]
- 18. Gilman T. Wound outcomes: the utility of surface measures. Int J Low Extrem Wounds 2004;3:125–32. [DOI] [PubMed] [Google Scholar]
- 19. Sibbald RG, Coutts P, Fierheller M. Improved persistent wound pain with a novel sustained release ibuprofen foam dressing. In: Symposium for Advanced Wound Care; Poster 185; May 2006; San Antonio (TX), USA. [Google Scholar]
- 20. Gad P, Shewale S, Drewes A, Arendt‐Nielsen L. Effect of a local ibuprofen dressing on healing of experimentally induced laser wounds. In: Gottrup F, Bale S, Franks P, Hofman D, Francu M, Price P, Romanelli M, Rybak Z, editors. European Wound Management Association Conference; 2006 May 1720; Prague. Frederiksberg: Congress Consulting. [Google Scholar]
- 21. Whitlam JB, Brown KF, Crooks MJ, Room GF. Transsynovial distribution of ibuprofen in arthritic patients. Clin Pharmacol Ther 1981;29:487–92. [DOI] [PubMed] [Google Scholar]
- 22. Machen J, Whitefield M. Efficacy of a proprietary ibuprofen gel in soft tissue injuries: a randomised, double‐blind, placebo‐controlled study. Int J Clin Pract 2002;56:102–6. [PubMed] [Google Scholar]
- 23. Simon D, Backonya M, Mersky H, Solomon S, Saper J, Lippe P, Haddox D, Aronoff G, Brose W, Gallagher RM, Krames E, Marcus N, Mauskop A, North R, Payne R, Portenoy R, Rachlin E, Ready B, Rowlingson JC, Rucker K, Stanton‐Hichs M, Warfield C, Wilson P. The necessity for early evaluation and treatment of the chronic pain patient. Glenview, IL: American Academy of Pain Medicine, 1997. [Google Scholar]
- 24. Eriksen J, Sjøgren P. Epidemiologiske forhold vedrørende langvarige/kroniske non‐cancersmertetilstande i Danmark. Ugeskrift for læger 2006;168:1947–50. [PubMed] [Google Scholar]
- 25. Mason L, Moore RA, Edwards JE, Derry S, McQuay HJ. Topical NSAIDs for chronic musculoskeletal pain: systematic review and meta‐analysis. BMC Musculoskelet Disord 2004;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wiechula R. The use of moist wound‐healing dressings in the management of split‐thickness skin graft donor sites: a systematic review. Int J Nurs Pract 2003;9:S9–17. [DOI] [PubMed] [Google Scholar]
- 27. Farrar JT, Young JP, LaMoreau L, Wert JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11‐point numerical pain rating scale. Pain 2001;94:149–58. [DOI] [PubMed] [Google Scholar]
- 28. Moffat CJ, Franks PJ, Hollinworth H. Understanding wound pain and trauma: an international experience. In: Briggs M, Hollinworth H, Meaume S, Pediani R, Romanelli M, Agreda JS, Torra i Bou JET, Vanscheidt W, editors. EWMA position document: pain at wound dressing changes. London: Medical Education Partnership Ltd., 2003:2–7. [Google Scholar]
- 29. Briggs M, Ferris FD, Harding K, Hofman D, Hollinworth H, Krasner D, Lindholm C, Moffat CJ, Price P, Romanelli M, Sibbald RG, Stacey M, Téot L. Minimising pain at wound dressing‐related procedures: a consensus document. WUWHS consensus statement. London: Medical Education Partnership Ltd, 2004. [PubMed] [Google Scholar]
- 30. Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on {micro}‐opioid receptors. J Neurosci 2005;25:7754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Muller‐Decker K, Hirschner W, Marks F, Furstenberger G. The effects of cyclooxygenase isozyme inhibition on incisional wound healing in mouse skin. J Invest Dermatol 2002;119:1189–95. [DOI] [PubMed] [Google Scholar]
- 32. Proper SA, Fenske NA, Burnett SM, Luria LW. Compromised wound repair caused by perioperative use of ibuprofen. J Am Acad Dermatol 1988;18:1173–9. [DOI] [PubMed] [Google Scholar]
- 33. Muscara MN, McKnight W, Asfaha S, Wallace JL. Wound collagen deposition in rats: effects of an NO‐NSAID and a selective COX‐2 inhibitor. Br J Pharmacol 2000;129:681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dvivedi S, Tiwari SM, Sharma A. Effect of ibuprofen and diclofenac sodium on experimental would healing. Indian J Expt Biol 1997;35:1243–5. [PubMed] [Google Scholar]
- 35. Radi ZA, Kahn NK. Effects of cyclooxygenase inhibition on bone, tendon, and ligament healing. Inflamm Res 2005;54:358–66. [DOI] [PubMed] [Google Scholar]
- 36. Abd‐El‐Aleem SA, Ferguson MW, Appleton I, Bhowmick A, McCollum CN, Ireland GW. Expression of cyclooxygenase isoforms in normal human skin and chronic venous ulcers. J Pathol 2001;195:616–23. [DOI] [PubMed] [Google Scholar]
- 37. Andersen KE, Franken CPM, Gad P, Larsen AM, Larsen JR, Van Neer PAFA, Vuerstaek J, Wuite J, Neumann HAM. A randomized, controlled study to compare effectiveness of two foam dressings in the management of lower leg ulcers. Ostomy/Wound Manage 2002;48:31–41. [Google Scholar]
- 38. Nelzen O, Bergquist D, Lindhagen A. Venous and non venous leg ulcers: clinical history and appearance in a population study. Br J Surg 1994;81:182–7. [DOI] [PubMed] [Google Scholar]
- 39. Ricci E, Romanelli M. An open, comparative, randomised international real life study on the clinical performance and cost‐effectiveness of Biatain‐Ibu versus local best practice in the treatment of painful exuding wounds. Napoli, Italy: Associazione Italiana Ulcere Cutanee (AIUC), 2006. [Google Scholar]


