Abstract
Most pressure ulcers occur over bony prominences such as heels and the sacrum. However, the National Pressure Ulcer Advisory Panel recognises that pressure ulcers can also occur on any tissue under pressure and thereby can develop beneath medical devices. This article reports on results from a secondary analysis of existing data collected by The Nebraska Medical Center on pressure ulcer quality improvement initiatives and outcomes. The purpose of this study was to quantify the extent of the problem and identify risk factors for medical device related (MDR) pressure ulcer development in hospitalised patients. A subset of data collected during eight quarterly pressure ulcer incidence and prevalence studies (N = 2178) was created and analysed. The overall rate of hospital‐acquired pressure ulcers was 5·4% (113 of 2079). The proportion of patients with hospital‐acquired ulcers related to medical devices was 34·5% (39 of 113). Findings indicate that if a patient had a medical device, they were 2·4 times more likely to develop a pressure ulcer of any kind. Numerous risk factors for pressure ulcer development were identified; however, none differentiated between those with MDR and traditional pressure ulcers.
Keywords: Incidence, Medical device pressure ulcer, Pressure ulcer
INTRODUCTION
Skin breakdown is an unfortunate reality of the already vulnerable hospitalised population. Skin breakdown in the form of pressure ulcers is defined as localised injury to the skin and/or underlying tissue usually over a bony prominence, as a result of pressure, or pressure in combination with shear (1). One source of pressure is the medical devices used to monitor and/or treat the patient's condition.
Medical devices are commonly used in hospital settings (Table 1). The device itself creates pressure. Humidity and heat develop between the device and the skin changing the microclimate of the skin. Often these devices must be secured tightly to assure a proper seal which, in turn, creates pressure in unusual areas rather than the bony prominences. The materials used to secure the device (e.g. tape, straps) may make it difficult to inspect the underlying skin beneath them. All of these factors increase the risk of pressure ulcers (2).
Table 1.
Medical devices in use in hospital settings
| Anti‐embolic stockings |
| Cervical collars |
| Endotracheal tubes/commercial endotracheal tube holders |
| Face masks for non‐invasive positive pressure ventilation |
| Faecal containment devices |
| Nasal cannulas |
| Pulse oximetry probes |
| Radial artery catheters |
| Sequential compression devices |
| Splints and braces |
| Urinary catheters |
REVIEW OF LITERATURE
Critically ill patients may be particularly vulnerable to medical device related (MDR) pressure ulcers for a number of reasons (3). Critically ill patients often have medical devices and are often unaware of the device and/or any pressure being applied by the device. Sedating medications, encephalopathy, neurologic disease/injury and severe neuropathy prevent awareness of pressure and movement in response to tissue ischaemia. Very ill patients are often weak and lack the strength to reposition themselves. The working hypothesis of this study was that decreased mobility, decreased sensory perception, decreased perfusion and higher usage of supportive medical devices in intensive care units (ICUs) placed these patients at higher risk for developing MDR pressure ulcers.
Research on pressure ulcers as a result of medical devices is scant. Davis et al. (4) reported that when cervical collars were in place for less than 5 days, pressure ulcers were present in 33% of patients. If the collar was present for over 5 days, pressure ulcers were present in 44% of the 99 patients, and 52% of these ulcers were full thickness.
Wille et al. (5) reported on a prospective study of 125 surgical ICU patients who had their pulse oximetry monitored by a clip‐on probe. The frequency rate of the pulse oximeter‐induced injury was 5% (6 of 125). Five patients who developed pulse oximeter‐induced injury received norepinephrine and dopamine and one received dopamine therapy only. This injury did not occur in any patient who did not receive vasopressor therapy.
Pressure ulcers from non‐invasive positive pressure ventilation (NIPPV) masks have been reported in the literature for many years 6, 7, and various dressings have been placed under masks in an attempt to prevent these ulcers 8, 9. NIPPV requires a tight seal of a mask over the bridge of the nose and chin to achieve the prescribed inspiratory and expiratory pressures.
Even if the device initially fits properly, patients may develop oedema from fluid resuscitation and third spacing after the device is applied (10). Oedema can occur for a variety of reasons and in a variety of patients. Several situations exist in the acutely ill patient making them susceptible to oedema. Aggressive fluid resuscitation is used for hypovolemic, burned and septic patients and leads to systemic oedema. Localised oedema is also seen with lymphoedema or dependent oedema with heart failure. The oedematous skin stretches making the skin extremely fragile and prone to injury including pressure ulcers. Oedema may also have an effect on the susceptibility of deeper tissues for pressure ulcer formation. Blood vessels in oedematous deep tissue are compressed from the external pressure of the oedematous fluids, and oxygen transport from capillaries to cells is impaired in oedematous tissue.
Medical devices can also cause oedema by acting like a tourniquet if they do not fit properly. The oedematous tissue is subject to pressure‐induced ischaemia and the likelihood of pressure ulcers increases. Moisture also causes the skin to become more fragile. In addition, the presence of moisture enhances the deleterious effects of friction fivefold 2, 11, 12.
The purposes of this secondary analysis are to quantify the extent and nature of the problem, identify risk factors for MDR pressure ulcer development and explore potential preventive strategies in hospitalised patients. Based upon the lack of published information of pressure ulcers related to medical devices in hospitalised patients, the following research questions were addressed:
-
1
What proportion of pressure ulcers is related to medical devices?
-
2
What are the characteristics of MDR hospital‐acquired pressure ulcers (HAPUs)?
-
3
Are there unique risk factors for medical‐device related pressure ulcers in hospitalised patients?
-
4
Are hospitalised patients with medical devices at greater risk for developing pressure ulcers?
-
5
Do Braden scale total scores and/or subscale scores help differentiate between those with MDR pressure ulcer and those with traditional pressure ulcers?
-
6
Do ICU patients have a higher rate of MDR HAPUs than non ICU patients?
-
7
Is there an association between continuous quality improvement (CQI) initiatives and the number of MDR pressure ulcers over time?
METHODS
The data used in this secondary analysis were from a series of eight quarterly cross‐sectional point prevalence studies conducted at The Nebraska Medical Center to monitor prevalence and HAPU rates, risk factors and care processes. The study was approved by the Institutional Review Board of the University of Nebraska Medical Center.
Settings and subjects
There were 2500 subjects included in the original data collection effort. The inclusion criterion for the entire data collection set was all inpatients hospitalised on the specified survey dates. Psychiatric and obstetric patients with stays of less than 3 days were excluded from data collection. Other exclusions were patients not available because of surgery or medical tests and those who declined participation.
A subset of adult patients in ICU, medical surgical and step‐down units was created by excluding patients under the age of 17 years. This subset was used to determine prevalence rates in adult patients. Those with pressure ulcers on admission (n = 99) were then excluded, resulting in a subset containing a total of 2079 adult subjects who were pressure ulcer free on admission and residing in a critical care, step‐down or medical surgical unit on the days of the surveys. This subset was used to analyse HAPU rates, risk factors for HAPUs and care processes used to prevent pressure ulcers.
Study protocol
Data collection protocol.
Data collection tools for the large data set were developed to gather data on risk factors, preventive care processes and pressure ulcer outcomes. All data were collected under the direction of certified wound care nurses. Specific instructions for data collection were developed, including operational definitions for all variables. Routine reliability checks were also conducted on 5% of the sample with reliability at 95–100%.
Secondary analysis of data subset.
Prevalence and HAPU rates were determined. Prevalence is defined as ‘the number of patients with a pressure ulcer in a specific population at a specific time, usually evaluated on a one‐time cross‐sectional basis' (p. 491) (13). Hospital‐acquired rates are defined as ‘the number of patients who develop a pressure ulcer after admission to the health care organization’ (p. 491(13)). In the study procedure, hospital‐acquired ulcers were determined by identifying whether an ulcer was documented on admission assessment. If no ulcer was documented on admission, any new pressure ulcers were assumed to be hospital‐acquired. Newly acquired pressure ulcers were confirmed by the wound nurse present on each data collection team. In accordance with epidemiological methods, pressure ulcer rates were based on the number of patients with pressure ulcers, not the number of ulcers.
Descriptive statistics were used to identify the stage and location of pressure ulcers. Data collectors were also asked to determine whether each ulcer was present on admission and whether it was related to pressure from a medical device. Additional data analysis strategies were designed to identify risk factors for HAPU development in this subset of adult medical surgical and step‐down unit patients. Chi‐square analyses were used to determine statistically significant differences between those with and without pressure ulcers for risk factors represented as non‐parametric data (e.g. incontinence, immobility, perception). Odds ratios and 95% confidence intervals were determined for statistically significant univariate risk factors. Student's t‐tests were computed for risk factors represented by parametric data (e.g. age, albumin levels, Braden scores).
Compliance rates with prescribed preventive interventions and pressure ulcer rates were examined across the eight quarters of data collection to determine temporal patterns and correlations between interventions and outcomes. Several aspects of nursing care to inspect and protect skin beneath medical devices were measured.
RESULTS
Q1. What proportion of pressure ulcers is related to medical devices?
At the time of the data collection, 1·4% of patients had at least one MDR pressure ulcer. In this study, 1·3% of these patients had at least one hospital‐acquired MDR pressure ulcer. There were 83 patients in ICU with HAPUs. They had a total of 113 pressure ulcers, and 34·5% (39 of 113) of these ulcers were related to medical devices (Table 2).
Table 2.
Prevalence and HAPU rates: Overall and with/without MDR Pressure Ulcers
| Prevalence rate | |
| Overall | 9·7% (212 of 2178) |
| Excluding patients with only MDR ulcers | 8·3% (181 of 2178) |
| Patients with MDR ulcers | 1·4% |
| Hospital acquired rate | |
| Overall | 5·3% (113 of 2079) |
| Excluding patients with only MDR ulcers | 4·0% (83 of 2079) |
| Patients with MDR ulcers | 1·3% |
MDR, medical device related.
Q2. What are the characteristics of MDR HAPUs?
Most MDR HAPUs were stage I (35%); however; it is important to note that 24% were unstageable and 3% were stage III, a full‐thickness ulcer (Figure 1). The most common locations of MDR HAPU were the ears (35%), lower leg (11%) and heels (8%) (Figure 2). The three most common sites for non MDR HAPUs were sacrum‐coccyx, heels and buttocks; none was found on ears or lower leg.
Figure 1.
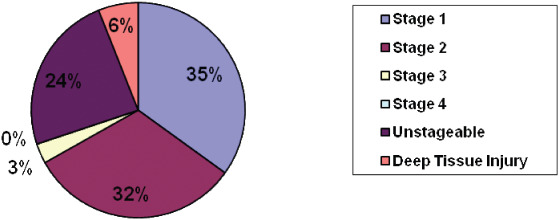
Distribution of stages of medical device related pressure ulcers. The most common stages of medical device related pressure ulcers were stage I and II. Unstageable and deep tissue injury pressure ulcers were present.
Figure 2.
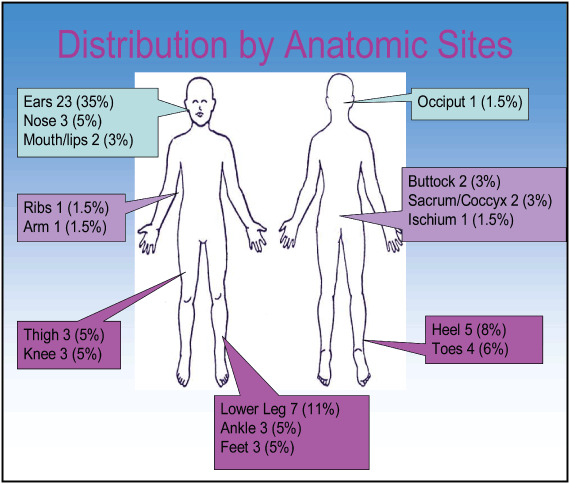
Locations of hospital acquired pressure ulcers on patients in critical care. The most common location for a medical device related pressure ulcer was the ear. However, they occurred on many body areas.
Q3. Are there unique risk factors for MDR pressure ulcers in hospitalised patients?
Age, gender, admitting diagnosis, body mass index, surgery during hospitalisation, oedema, diabetes mellitus and serum albumin level were analysed to determine their association with the development of MDR pressure ulcers. There were statistically significant differences for these common risk factors between those with and without pressure ulcers. However, the unique focus of the study was to detect differences in risk factor profiles between those with at least one MDR ulcer and those with traditional pressure ulcers. These two groups shared common risk factors for HAPUs. There were no unique risk factors that would allow early identification of patients at risk for MDR ulcers or differentiation between risks for MDR and traditional pressure ulcers.
Q4. Are hospitalised patients with medical devices at greater risk for developing pressure ulcers?
Those patients with medical devices were significantly more likely to develop a pressure ulcer (χ 2 = 6·98, P = 0·008). If a patient had a medical device in place, they were 2·4 times more likely to develop a pressure ulcer of any kind (P = 0·10, 95% CI = 1·2–4·8). Pressure ulcers developed on usual and unusual body areas in patients with medical devices. Pressure ulcers were found on the lips from endotracheal tubes (Figure 3), on the hand from splints (Figure 4), on the arm from arterial line tubing (Figure 5) and on the occiput from neck collars (Figure 6). Sometimes the medical device seems to prevent skin assessment. In Figure 7, a patient developed a pressure ulcer on the heel, but it was not discovered because of an anti‐embolism stocking.
Figure 3.
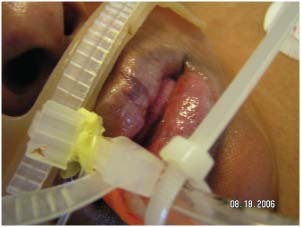
Pressure ulcer on the lip from an endotracheal tube.
Figure 4.
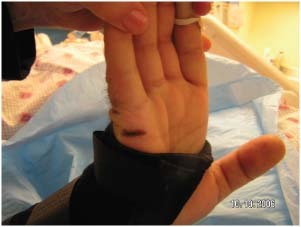
Pressure ulcer from wrist splint.
Figure 5.
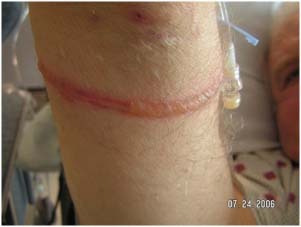
Pressure ulcer from arterial line tubing.
Figure 6.
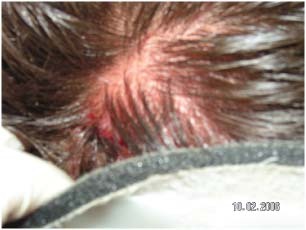
Occipital pressure ulcer from a cervical collar.
Figure 7.
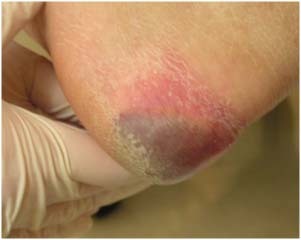
Deep tissue injury pressure ulcer on the heel. The patient was wearing antiembolism stockings.
Q5. Do Braden scale total scores and/or subscale scores help differentiate between those with MDR pressure ulcers and those with traditional ulcers?
This facility's pressure ulcer prevention programme is based on the areas of risk as shown in the subscales of the Braden scale (e.g. immobility, activity) and the total score. Admission, current and lowest Braden scale score in 10 days, as well as all six subscales showed statistically lower values for those patients with pressure ulcers. However, significant differences in these risk profiles were not present between groups of patients with MDR compared with traditional pressure ulcers. Patient risk profiles for MDR and traditional pressure ulcers are more alike than different.
Q6. Do ICU patients have a higher rate of MDR HAPUs than non ICU patients?
There was no significant difference in MDR pressure ulcers between patients in critical care units and medical/surgical or step‐down units. (Figure 8).
Figure 8.
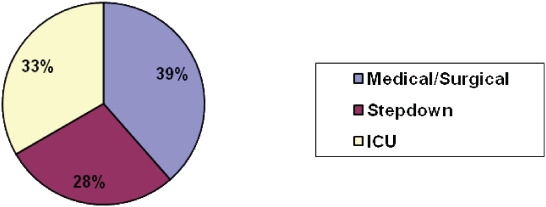
Locations in the hospital where medical device related ulcers were found. Pressure ulcers from medical devices occurred in almost equal frequency in intensive care unit, step‐down and general medical surgical nursing units.
Q7. Is there an association between CQI initiatives and the number of pressure ulcers over time?
There were a number of CQI initiatives, including skin protection under oxygen tubing, development of a new pressure ulcer protocol and changes to pulse oximeter use policy. Figure 9 depicts the number of MDR pressure ulcers related to the implementation of continuous quality initiatives. The number of MDR HAPUs decreased as these initiatives were implemented.
Figure 9.
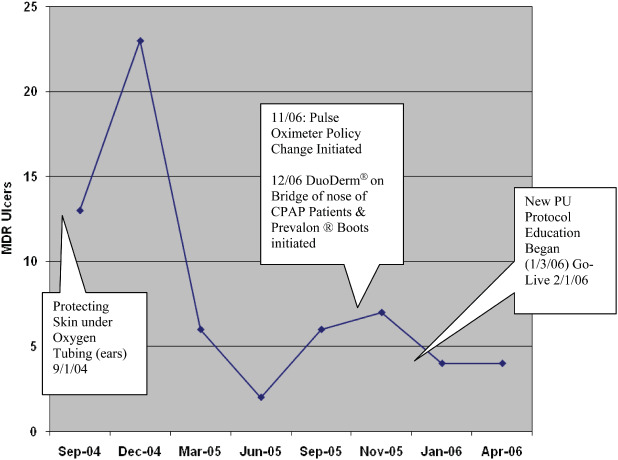
Continuous quality improvement efforts were associated with fewer medical device related hospital‐acquired pressure ulcers over time.
DISCUSSION
Overall, MDR pressure ulcers accounted for one third of all pressure ulcers in this sample. Stage I and II pressure ulcers were the most common; however, unstageable pressure ulcers and deep tissue injury were found. The significant numbers and seriousness of pressure ulcer stages indicate that if MDR pressure ulcers were prevented, the overall incidence and prevalence rates would decline.
In this study, the specific medical device the patient was wearing was not recorded. However, conclusions can be made based on the location, shape and size of the pressure ulcer. For instance, a linear‐shaped pressure ulcer on the top of the ear can be attributed to oxygen tubing and a small round ulcer on the ear lobe itself can be correlated to a clip‐on oxygen saturation monitoring probe.
It was expected that the largest concentration of MDR pressure ulcers would occur in the ICU because of the fragile patient population and the greater number of medical devices used to treat and monitor these patients. However, this study highlighted that MDR pressure ulcers occur on progressive care/step‐down and medical surgical units at nearly the same frequency. Reasons for the presence of MDR pressure ulcers on general patient care units could be that patients are lower acuity, thought to be able to report pressure changes from devices or that the pressure‐induced changes in the skin did not manifest until the patient left critical care.
It was expected that risk factors using the Braden risk tool could clearly identify those at risk for MDR pressure ulcers, so that appropriate preventive strategies could be used. Analysis of the data indicated that the risk profiles were comparable for those with MDR and traditional pressure ulcers. The key or critical risk factor for those with developing MDR pressure ulcer was placement of the medical device itself.
IMPLICATIONS FOR PRACTICE
More frequent and thorough skin and neurovascular assessments must be performed on patients with medical devices. These assessments should include loosening and removing the devices on each shift (if the patient's medical condition allows) for a thorough skin inspection (14). Patients of particular concern are those with a significant amount of oedema already, those at risk for developing oedema and immobilised patients with sensory deficits who are unable to feel the increasing pressure or alert the nurse that the device is painful and tight. Pressure ulcers located on the lips, cheeks, ear lobes, bridge of the nose, occiput, fingertip and webbing of the thumb have no other explanation for their development other than a medical device.
Communication and collaboration with other health care providers are essential to prevent MDR pressure ulcers. Key health care providers include occupational therapy (OT), physical therapy, respiratory therapy (RT) and orthopedic surgeons. OT is instrumental with the creation of splints and braces for patients. For example, a nurse can collaborate with OT in developing a splint for a patient that may include padding over bony prominences or restructuring the brace to avoid an already injured area. Partnership with RT is essential in the ICU for patients on the ventilator. The endotracheal tube should be moved from side to side of the mouth every shift to assess lips, skin on the cheeks, teeth and tongue. Face masks and oxygen tubing should also be removed to inspect the skin. At this time, no protective device is available to pad the face from NIPPV devices; perhaps one can be created. Many medical devices in use today have not been redesigned for years; it may be a good time to work with the manufacturers to improve the designs of the devices.
Any tube can create pressure no matter where it is. Finding the tube and checking the skin around it is essential to quality nursing care. While positioning a patient, the key is to know where each tube or monitoring device is and to be certain that the patient is not lying on it. Each and every tube and/or medical device is the nurse's responsibility to make sure it does not harm the patient.
CONCLUSION
The findings from this study confirm that MDR pressure ulcers are a significant problem in the health care industry. Future research on this subject should test the previously stated nursing implications to ascertain their impact on the number of MDR pressure ulcers. Future research could also illuminate how long any device can be in place before it needs to be moved or removed to examine the skin. Specific devices and their unique risk could also be explored.
Medical devices can cause pressure ulcers. This study reported that one third of all pressure ulcers in patients were from medical devices. Interventions that could protect the patient from pressure related injury were discussed.
ACKNOWLEDGEMENTS
The authors continue to appreciate the daily dedication of the nursing staff of The Nebraska Medical Center to prevent pressure ulcers. We also acknowledge the work of the Skin and Wound Advisory Team for their data collection used in this study.
REFERENCES
- 1. National Pressure Ulcer Advisory Panel (NPUAP) and European Pressure Ulcer Advisory Panel. International pressure ulcer prevention and treatment guidelines 2010 [WWW document]. URL http://www.npuap.org [accessed 1 April 2010].
- 2. Reger S, Ranganathan V, Sahgal V. Support surface interface pressure, microclimate and the prevalence of pressure ulcers: an analysis of the literature. Ostomy Wound Manage 2007;53:50–8. [PubMed] [Google Scholar]
- 3. Wolverton CL, Hobbs LA, Beeson T, Benjamin M, Campbell K, Forbes C, Huff N, Kieninger M, Luebbehusen M, Myers M, White S. Nosocomial pressure ulcer rates in critical care [Electronic version]. J Nurs Care Qual 2004;20:56–62. [DOI] [PubMed] [Google Scholar]
- 4. Davis JW, Parks SN, Detlefs CL, Williams GG, Williams JL, Smith RW. Clearing the cervical spine in obtunded patients: the use of dynamic fluoroscopy. J Trauma 1995;39:435–8. [DOI] [PubMed] [Google Scholar]
- 5. Wille J, Braams R, Van Harren W, Van Der Werken C. Pulse oximeter‐induced digital injury: frequency rate and possible causative factors. Crit Care Med 2000;28:3555–7. [DOI] [PubMed] [Google Scholar]
- 6. Meecham‐Jones DJ, Braid GM, Wedzicha JA. Nasal masks for domiciliary positive pressure ventilation: patient usage and complications. Thorax 1994;49:811–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sleilati FH, Stephan HA, Nasr MW, Riachy MA. An unusual pressure sore of the nasal bridge. Br J Oral Max Surg 2008; 46:411–2. [DOI] [PubMed] [Google Scholar]
- 8. Wood K, Flaten A, Backe W. Inspissated secretions: a life‐threatening complication of prolonged noninvasive ventilation. Resp Care 2000;45:491–3. [PubMed] [Google Scholar]
- 9. Weng MH. The effect of protective treatment in reducing pressure ulcers for non‐invasive ventilation patients. Int Crit Care Nurs 2008;24:295–99. [DOI] [PubMed] [Google Scholar]
- 10. Callaghan S, Trapp M. Evaluating two dressings for the prevention of nasal bridge pressure sores. Prof Nurs 1998;13:361. [PubMed] [Google Scholar]
- 11. Grey JE, Enoch S, Harding, KG. Pressure ulcers. Br Med J 2006;332:472–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kokate J, Leland K, Held A. Temperature modulated pressure ulcers: a porcine model. Arch Phys Med Rehab 1995;76:666–73. [DOI] [PubMed] [Google Scholar]
- 13. Whittington K, Briones R. National prevalence and incidence study: a 6‐year sequential acute care data. Adv Skin Wound Care 2004;17:490–4. [DOI] [PubMed] [Google Scholar]
- 14. Webber‐Jones J, Thomas C, Bordeaux R. The management and prevention of rigid cervical collar complications. Ortho Nurs 2002;21:19–25. [DOI] [PubMed] [Google Scholar]


