Abstract
Adult bone marrow‐derived stem cells may aid the healing of chronic lower extremity wounds by transplanting a population of progenitor cells locally into the wound. We present results from three cases in which bone marrow aspirate containing marrow‐derived cells was applied/injected locally into complex lower extremity chronic wounds of differing aetiologies. Our case series suggest that bone marrow aspirate, applied topically and injected into the wound periphery, may be a useful and potentially safe adjunct to wound simplification and ultimate closure.
Keywords: Diabetes mellitus, Foot, Stem cell, Ulcer, Wound healing
Introduction
Chronic lower extremity ulcers pose a serious burden on patients, their social support network and the health care system. Up to 25% of diabetic patients will suffer from foot ulceration during their lifetime (1). The cost of treating a diabetic foot ulceration has been estimated at $28 000 USD over 2 years (2). In Sweden, a review estimated that a single episode of foot ulceration costs $7850 USD rising to $52 920 USD in cases of amputation (3). Venous leg ulcers affect between 0.1% and 1% of the Western population 4, 5. The cost to heal a venous leg ulcer may be estimated at $6449 USD per year (6). Collectively, lower extremity ulcerations place a limb at risk for infection and amputation.
Macrovascular disease, hyperglycaemia and increased venous pressure are factors leading to delayed wound healing at the host level. Cells in the local environment are subjected to chronic inflammation, reactive oxygen species, proteolytic enzymes and recurrent ischaemia–reperfusion cycles, which contribute to wound chronicity (7). These stresses may inhibit fibroblast proliferation, resulting in a prematurely senescent phenotype populating the chronic wound (8). Senescent cells are unable to divide and are unresponsive to growth factors. Gurjala et al. induced exposed human dermal fibroblasts to oxidative stress with hydrogen peroxide and induced cellular senescence (9). Fibroblasts cultured from venous leg ulcers showed that a significant number of cells exhibited senescent‐associated biochemical markers (10). Work by Stanley and Osler suggests that a human venous leg ulcer with more than 15% senescent cells isolated in cultures signifies a ‘difficult to heal’ ulcer (11). Cellular senescence has been noted in pressure ulcers as well (12).
Adult bone marrow‐derived stem cells may aid the healing of chronic lower extremity wounds by transplanting a population of progenitor cells locally into the wound. Marrow aspirate can be obtained from the ileum, tibia or calcaneus, depending on the volume needed. There are two types of stem cells present in bone marrow stroma: haematopoietic stem cells and mesenchymal stem cells (MSC) (13). Stem cells are capable of self‐renewal and differentiating into mature cells. Plasticity refers to a cell’s ability to cross lineage barriers and phenotypically become a cell unique to other tissues (14). There are several proposed mechanisms of plasticity. The plasticity of bone marrow‐derived cells is well established, differentiating into islet cells (15), lung cells (16), cardiac and skeletal muscle (17), some central nervous system (CNS) cells (18) and the skin 19, 20, 21. Subsequent to dermal wounding, MSC in the bone marrow are mobilised and migrate to the injured tissue. While there are epidermal stem cells residing in the skin, marrow‐derived MSC also play a role in regeneration of dermal tissue. Badiavas et al. reported that wounding stimulated the engraftment of marrow cells into the skin near adnexal structures and subsequently differentiated into skin structures (19). Deng et al. transplanted fluorescent‐labelled bone marrow MSC into lethally irradiated mice and found that labelled cells gave rise to stem cells and committed cells in the skin (21). Another study found that 15–20% of the dermal fibroblasts originated from the bone marrow in a murine wound model (20).
Methods
We abstracted data from three cases in which bone marrow aspirate containing marrow‐derived cells was applied/injected locally into chronic wounds of differing aetiologies. Patient characteristics, past medical history, wound descriptions, duration of wound, time to complete granulation, time to skin grafting (two patients) and time to complete healing were obtained. Two patients were under an Integrated Scientific and Ethical Review Board (ISERB) approved (St Vincent Catholic Medical Centers, New York, NY) review of Charcot reconstruction using external fixation and wound healing, whose information was already available and de‐identified. The remaining patient was treated at a clinical affiliate of Rosalind Franklin University of Medicine and Science, where Institutional Review Board (IRB) approval was obtained.
Bone marrow aspirate was harvested from the ipsilateral tibia using a BMA kit (Biomet/EBI, Parsippany, NJ). Under local or general anaesthesia, a stab incision deep to the periosteum was made at the anteromedial surface of the distal tibial metaphysis. The BMA trochar was passed through the incision and hand drilled through the cortex into the medulla of the bone. Between 10 and 20 cc of aspirate was collected. The aspirate was allowed to coagulate and placed on top of a well‐debrided wound, covered with petroleum‐impregnated gauze and left undisturbed for 48 hours. Additionally, in two patients, some aspirate was injected into the wound base and periwound area with a 20‐cc syringe and an 18‐gauge needle (Figure 1).
Figure 1.

The bone marrow aspirate trochar is inserted through the anterior‐medial face of the distal tibia into the medulla of the bone. A syringe can be connected to the exposed hub, and with negative pressure, the marrow harvested.
Results
Three patients with complex lower extremity chronic wounds, two undergoing Charcot reconstruction with external fixation and one undergoing aggressive wound debridement, were identified. The characteristics of the patients are listed in Table 1.
Table 1.
Summary table of patient characteristics and healing progression
| Age/gender | Etiology of wound | Duration of wound (months) | Time to 100% granulation (days) | Time to STSG (days) | Time to complete healing (days) | Adjunctive therapies used | |
|---|---|---|---|---|---|---|---|
| 1 | 47/M | HIV neuropathy | 3 | 13 | 29 | 60 | Wound V.A.C., STSG |
| 2 | 87/F | Venous leg ulcer | 4 | 19 | 31 | 50 | Wound V.A.C., STSG |
| 3 | 52/F | Diabetic neuropathy | 1 | n/a | n/a | 47 | Regranex® |
F, female; HIV, human immunodeficiency virus; M, male; STSG, split‐thickness skin graft.
n/a ‐ not applicable, did not undergo split thickness skin graft.
Patient 1 was a 47‐year‐old African‐American male with human immunodeficiency virus and peripheral neuropathy. He had a previous transmetatarsal amputation with a large full‐thickness ulcer involving the whole distal stump of 3‐month duration (Figure 2A). He suffered from Charcot arthropathy of the ankle that left his ankle in a fixed plantarflexed position. His deformity was corrected using an anterior closing‐wedge osteotomy of the distal tibial to mitigate distal pressure at the ulcer site and fixed with a static Ilizarov external fixator. Twenty millilitres of bone marrow aspirate was harvested from the distal tibial metaphysis and placed directly on the wound. The wound began to granulate after 48 hours. The wound V.A.C. (KCI, San Antonio, TX) was used to promote microdeformation of these new cells (22) and enhance granulation. In 13 days postapplication of marrow cells, he exhibited 100% granulation tissue (Figure 2B). At day 29, a split‐thickness skin graft (STSG) was applied over the granular tissue (Figure 2C). The STSG was completely incorporated, and the wound healed without drainage on day 60 (Figure 2D).
Figure 2.
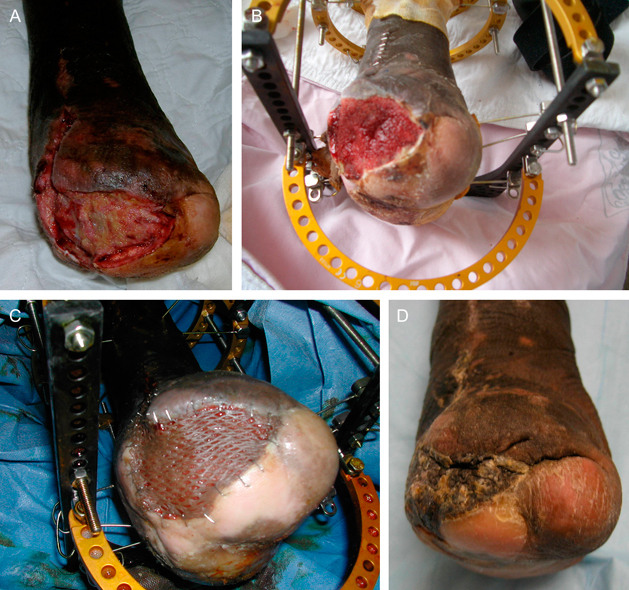
(A) A large distal transmetatarsal stump ulceration pre‐treatment with marrow‐derived stem cells (MDSC). (B) Thirteen‐day status post‐treatment with MDSC and V.A.C.®. (C) STSG applied at day 29. (D) Wound completely healed at day 60.
Patient 2 was an 87‐year‐old Caucasian female with venous insufficiency and a large ulcer 15 cm in diameter over her anterior ankle of 4‐month duration. The wound was extensively fibrotic and necrotic with exposed tibialis anterior tendon and deltoid ligament (Figure 3A). Her wound was debrided using the Versajet hydroscapel (Smith & Nephew, Memphis, TN), which included removal of a non viable tibialis anterior tendon. Twenty millilitres of bone marrow was aspirated from the distal anterior tibia, half of which was injected into the wound base and periwound area including the area of the deltoid ligament. The remaining aspirate was placed on top of the wound and covered with a non adherent dressing and was left undisturbed for 48 hours. At 48 hours, the wound V.A.C. was applied. Nineteen days later, 100% granulation tissue was noted (Figure 3B). On day 31, STSG was performed and the V.A.C. reapplied (Figure 3C). The wound completely healed at 50 days.
Figure 3.
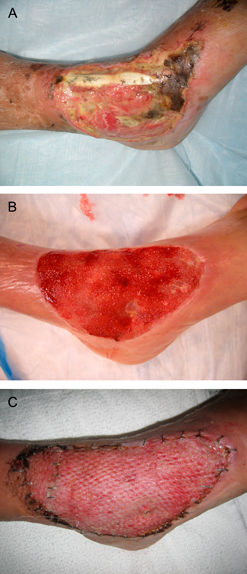
(A) A large anterior ankle ulcer with fibronecrotic base and exposed tibialis anterior tendon. (B) Nineteen days after debridement of tendon, application of MDSC and V.A.C.®. (C) STSG, 31 days after debridement (photo from day 39).
Patient 3 was a 52‐year‐old South Asian female with type 2 diabetes mellitus and peripheral neuropathy, who was to undergo Charcot foot reconstruction for a midfoot collapse. Three weeks prior to surgery, she presented with dorsal toe ulcers of digits 1 and 2 secondary to poorly fitted footwear (Figure 4A). At the time of surgical reconstruction, the wounds were thoroughly debrided of all non viable tissue, which resulted in exposed bone/joint of the second toe. Marrow aspirate was obtained from her distal tibial metaphysis and placed directly to the wound, covered with non adherent dressing and left in place for 48 hours. The first dressing change showed granulation tissue (Figure 4B). No adjunctive treatments were used, and the wound healed by secondary intention 47 days later with minimal scarring (Figure 4C).
Figure 4.
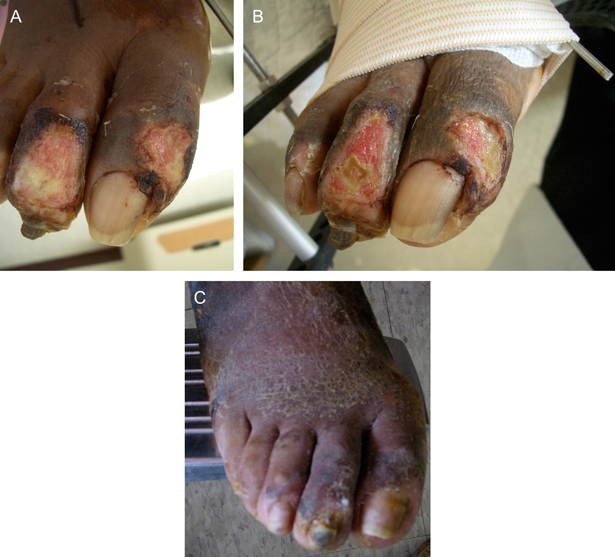
(A) Dorsal toe ulcers pre‐treatment with MDSC. (B) One week after treatment with MDSC, exposed bone and distal interphalangeal joint at the second digit. (C) Wounds completely healed at day 47 with minimal scarring (photo from day 65).
Although we harvested marrow aspirate from the ipsilateral tibia, there were no marrow site harvest complications in our patients.
Discussion
Bone marrow‐derived cells are multipotent cells capable of differentiation into multiple cell types including cartilage, bone, brain, fibroblasts and keratinocytes. They secrete large amounts of growth factors and cytokines (23). For medical purposes, marrow‐derived MSC can be harnessed by: (i) mobilisation and migration from the bone marrow, (ii) intravenous infusion, (iii) local injection or (iv) topical application. Although the previous body of evidence has shown that MSC migrate from the marrow niches to the sites of tissue injury, local application of a high concentration of cells is appealing. McFarlin et al. found that wound healing significantly improved after local injection of MSC in an animal wound model (23). Bauer et al. found that although extremity ischaemia is a powerful stimulant for marrow stem cell recruitment, fewer progenitor cells were able to migrate to the ischaemic wound (24). Perhaps marrow‐derived MSC use vascular conduits for mobilisation, and obstruction of these channels reduces engraftment. This may be of concern in patients with diabetes who are prone to macrovascular and microvascular diseases. Local application, topical or through injection, of MSC would place these progenitor cells at the site of injury, assisting homing and delivery.
The treatment of chronic wounds generally follows three phases, (i) debridement and infection mitigation, (ii) promotion of granulation tissue and (iii) wound closure. Because the wound environment is dynamic and frequently chronic wounds require many different treatments, it is unlikely that one type of treatment alone will bring a wound to complete closure. This is exemplified in the discussion involving the recent large randomised trial of negative pressure wound therapy (NPWT) via wound V.A.C. (25), where NPWT works best to promote granulation tissue and create a ‘healing’ environment inside a wound, but it does not specifically achieve wound closure in every case. It is, in essence, a wound simplification device – transforming a deep, complicated wound to a more manageable, simple wound. Therefore, measuring how the V.A.C. alone heals wounds (rather than how it prepares wounds for healing) might be less than ideal. We find the use of marrow aspirate analogous. It may promote quality granulation tissue and assists closure by an adjunctive method, such as skin grafting.
In our two patients who underwent adjunctive therapies, time to 100% granulation tissue was 13 and 19 days. The time to STSG application was 29 and 31 days. Patient 3 was allowed to heal secondarily because the location of her wounds on the dorsal toes precluded NPWT and STSG usage. These wounds did, interestingly, heal over exposed bone and joint with minimal scarring.
Badiavas and Falanga (26) recently published a prospective case–cohort using marrow‐derived cell in three patients with chronic wounds (one abdominal hernia dehiscence, one lower extremity arterial ulcer and one lower extremity mixed arterial/venous ulcer). They harvested the marrow from the ileum, applied a portion of the marrow aspirate directly to the wound and cultured the remaining cells for future application. Two patients required only one subsequent treatment, and the remaining patient required three subsequent treatments before complete healing was obtained. These investigators augmented healing with Apligraf (Organogenesis, Canton, MA), a bioengineered skin substitute in one patient. They achieved similar results with the three wounds healing at 29, 16 and 2 weeks and remaining closed at a follow‐up of 1–2 years.
Our case series suggests that bone marrow aspirate, applied topically and injected into the wound periphery, may be a useful and safe adjunct to wound simplification and ultimate closure. More work is needed to understand the complex communication of cytokines that stimulate MSC to differentiate to mature cells of various tissues. Application of certain cytokines may allow scientists to direct the differentiation of cells when expanding cultures in vitro and use in vivo.
References
- 1. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. [DOI] [PubMed] [Google Scholar]
- 2. Ramsey SD, Newton K, Blough D, McCullough DK, Sandhu N, Reiber GE, Wagner EH. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999;22:382–7. [DOI] [PubMed] [Google Scholar]
- 3. Apelqvist J. Wound healing in diabetes. Outcome and costs. Clin Podiatr Med Surg 1998;15:21–39. [PubMed] [Google Scholar]
- 4. Phillips TJ. Chronic cutaneous ulcers: etiology and epidemiology. J Invest Dermatol 1994;102:38S–41S. [DOI] [PubMed] [Google Scholar]
- 5. Callam MJ, Ruckley CV, Harper DR, Dale JJ. Chronic ulceration of the leg: extent of the problem and provision of care. Br Med J (Clin Res Ed) 1985;290:1855–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morrell CJ, Walters SJ, Dixon S, Collins KA, Brereton LM, Peters J, Brooker CG. Cost effectiveness of community leg ulcer clinics: randomised controlled trial. BMJ 1998;316:1487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mustoe TA, O’Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg 2006;117:35S–41S. [DOI] [PubMed] [Google Scholar]
- 8. Harding KG, Moore K, Phillips TJ. Wound chronicity and fibroblast senescence – implications for treatment. Int Wound J 2005;2:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gurjala AN, Liu WR, Mogford JE, Procaccini PS, Mustoe TA. Age‐dependent response of primary human dermal fibroblasts to oxidative stress: cell survival, pro‐survival kinases, and entrance into cellular senescence. Wound Repair Regen 2005;13:565–75. [DOI] [PubMed] [Google Scholar]
- 10. Mendez MV, Stanley A, Park HY, Shon K, Phillips T, Menzoian JO. Fibroblasts cultured from venous ulcers display cellular characteristics of senescence. J Vasc Surg 1998;28:876–83. [DOI] [PubMed] [Google Scholar]
- 11. Stanley A, Osler T. Senescence and the healing rates of venous ulcers. J Vasc Surg 2001;33:1206–11. [DOI] [PubMed] [Google Scholar]
- 12. Vande Berg JS, Rudolph R, Hollan C, Haywood‐Reid PL. Fibroblast senescence in pressure ulcers. Wound Repair Regen 1998;6:38–49. [DOI] [PubMed] [Google Scholar]
- 13. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997;276:71–4. [DOI] [PubMed] [Google Scholar]
- 14. Herzog EL, Chai L, Krause DS. Plasticity of marrow‐derived stem cells. Blood 2003;102:3483–93. [DOI] [PubMed] [Google Scholar]
- 15. Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose‐competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest 2003;111:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Theise ND, Henegariu O, Grove J, Jagirdar J, Kao PN, Crawford JM, Badre S, Saxena R, Krause DS. Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrow. Exp Hematol 2002;30:1333–8. [DOI] [PubMed] [Google Scholar]
- 17. Van Laake L, Hassink R, Doevendans P, Mummery C. Heart repair and stem cells. J Physiol 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 2000;290:1779–82. [DOI] [PubMed] [Google Scholar]
- 19. Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol 2003;196:245–50. [DOI] [PubMed] [Google Scholar]
- 20. Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow‐derived cells to skin: collagen deposition and wound repair. Stem Cells 2004;22:812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deng W, Han Q, Liao L, Li C, Ge W, Zhao Z, You S, Deng H, Murad F, Zhao RC. Engrafted bone marrow‐derived flk‐(1+) mesenchymal stem cells regenerate skin tissue. Tissue Eng 2005;11:110–9. [DOI] [PubMed] [Google Scholar]
- 22. Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum‐assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114:1086–96; discussion 1097–1088. [DOI] [PubMed] [Google Scholar]
- 23. McFarlin K, Gao X, Liu YB, Dulchavsky DS, Kwon D, Arbab AS, Bansal M, Li Y, Chopp M, Dulchavsky SA, Guatam SC. Bone marrow‐derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen 2006;14:471–8. [DOI] [PubMed] [Google Scholar]
- 24. Bauer SM, Goldstein LJ, Bauer RJ, Chen H, Putt M, Velazquez OC. The bone marrow‐derived endothelial progenitor cell response is impaired in delayed wound healing from ischemia. J Vasc Surg 2006;43:134–41. [DOI] [PubMed] [Google Scholar]
- 25. Armstrong DG, Lavery LA. Negative pressure wound after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–10. [DOI] [PubMed] [Google Scholar]
- 26. Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow‐derived cells. Arch Dermatol 2003;139:510–6. [DOI] [PubMed] [Google Scholar]


