Abstract
To aid clinicians in selecting the appropriate approach for treating patients with diabetic foot infections, we investigated whether any baseline clinical findings predicted an unfavourable clinical outcome. Using data from a large, prospective treatment trial of diabetic foot infections (SIDESTEP), we assessed the association between clinical treatment failure and baseline history, physical and laboratory findings, by univariate and multivariate logistic regression analyses. Among 402 patients clinically evaluable 10 days after completing antibiotic therapy, baseline factors significantly (P < 0·05) associated by univariate analysis with treatment failure were ‘severe’ (versus ‘moderate’) University of Texas (UT) wound grade; elevated white blood cell count, C‐reactive protein or erythrocyte sedimentation rate; high wound severity score; inpatient treatment; low serum albumin; male sex; and skin temperature of affected foot >10°C above that of unaffected foot. By multivariate logistic regression only severe UT wound grade (odds ratio 2·1) and elevated white blood cell count [odds ratio 1·7 for a 1 standard deviation (2971 cells/mm3) increase] remained statistically significant. Clinical failure rates were 46% for patients with both risk factors compared with 10% for patients with no risk factors and 16–17% for patients with one risk factor. Increased white blood cell count and severe UT wound grade at baseline, but not other features, were significant independent and additive risk factors for clinical failure in patients treated for a diabetic foot infection.
Keywords: Clinical prediction, Diabetic foot infection, Treatment outcome, White blood cell count, Wound severity
Introduction
Infected foot wounds are a major cause of morbidity in persons with diabetes and the leading cause of lower extremity amputations in developed countries 1, 2, 3, 4. The potential severity of these infections and the complex treatments they require have resulted in foot wounds being the most frequent reason for diabetes‐related hospitalisation in the United States 5, 6. The clinician treating a patient with a diabetic foot infection must immediately address several issues 1, 7, 8, 9, 10, 11. Key among these are how broad‐spectrum the antibiotic regimen should be and by what route it should be administered, when to request urgent surgical or other specialty consultations and whether or not hospitalisation is required. These decisions will affect the cost of care, the likelihood of adverse events and presumably the clinical outcomes. Perhaps the most important factor affecting these decisions is the clinical severity of the infection. 1, 12, 13. Unfortunately, clinicians currently have little evidence‐based guidance for identifying which patients have a severe diabetic foot infection or which clinical findings are associated with a poor outcome (14).
Because providing high‐quality care should improve treatment outcomes, it would be useful to know if any patient‐ or wound‐related factors affect the results of treatment for diabetic foot complications (7). This study was designed to determine which, if any, of the many easily assessed baseline clinical findings predict the clinical outcome in patients being treated for a diabetic foot infection. Identifying such risk factors might help clinicians decide on the most appropriate type of care, the need for hospitalisation and the urgency with which various interventions should be undertaken.
Research design and methods
Data for this study were derived from a prospective, multicentre, double‐blind randomised controlled trial for diabetic foot infections (SIDESTEP) (15) that compared intravenous therapy with ertapenem versus piperacillin/tazobactam (with the option for follow‐on therapy in either arm with oral amoxicillin/clavulanate) for a moderate to severe diabetic foot infection. Infection was defined, in accord with the Infectious Diseases Society of America guidelines (1), as the presence of purulent wound drainage or ≥3 designated systemic or local inflammatory findings. The investigators excluded patients who did not require parenteral antibiotic therapy; had necrotising fasciitis or underlying osteomyelitis; or had critical limb ischaemia, extensive gangrene or any indwelling prosthetic material.
At baseline, enrolled patients were stratified based on the University of Texas (UT) diabetic wound classification 16, 17. This system, which includes four grades of depth (0–3) and four stages of comorbidity (A = non infected, B = infected C = ischaemic, D = infected and ischaemic), has been shown to predict outcome of a foot wound 17, 18. We enrolled patients with stage B or D, and categorised the severity of the wound as either ‘moderate’ (grade 0 or 1) or ‘severe’ (grade 2 or 3). The investigators were trained to asses the size, depth and presence and intensity of eight signs and symptoms of infection (related to findings of inflammation and wound discharge). This allowed us to use a custom‐developed scoring system to calculate a quantitative wound severity score (the validation of which is the subject of another study) at each visit.
In addition to the efficacy and safety outcomes, we obtained extensive descriptive and clinical characteristics, including patient demographics and medical history; prior and concomitant medications used; physical examination findings, emphasising the foot (including specified neurological and vascular assessments); standard serological and haematological tests and plain foot X rays (and any other imaging tests deemed necessary by the investigators). We also used a dermal thermometer to compare the skin temperature at the site of the infection with temperature at the same anatomic site on the uninfected foot. The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines (19) and was approved by each site’s institutional review board, and each patient provided written informed consent.
We evaluated the clinical response to treatment based on the changes in signs and symptoms of infection between the baseline visit and the follow‐up assessment (FUA), conducted ∼10 days after the end of all study‐designated antibiotic therapy. We designated the response as ‘favourable’ if all or most (i.e. more than half) of the pretherapy signs and symptoms of infection (and specifically fever, lymphangitis and purulent drainage) resolved and the patient did not need further antibiotic therapy, and ‘failure’ if there was persistence, progression or recrudescence of most (i.e. more than half) pretherapy signs and symptoms or the patient required additional systemic antibiotic therapy or surgical or other adjunctive treatments for their foot infection. A patient was clinically evaluable if there were data to assess the clinical response, no confounding factors and ≥48 hours of intravenous antibiotic therapy. Since favourable clinical response rates for evaluable patients at the FUA were statistically equivalent for patients who were treated with ertapenem (87%) and piperacillin/tazobactam (83%), we combined all patients for these analyses.
Statistical analysis
We performed a univariate analysis for each selected baseline parameter to determine if it was related to the clinical outcome. We used logistic regression to determine each parameter’s clinical and statistical significance by calculating odds ratios, 95% confidence intervals (CI) and P values (20). For continuous parameters, we standardised the odds ratios to express the risk associated with a 1 standard deviation increase. To examine as many risk factors as possible in the multivariate analysis, we included each baseline parameter with a possible relationship with clinical outcome (P ≤ 0·25) in the univariate analysis. Using a stepwise method of model selection, we entered and maintained parameters in the multivariate model that had a P ≤ 0·05. When modelling data, using a higher P value (e.g. 0·25) allows inclusion of factors in the univariate model that are statistically significant (P ≤ 0·05) as well as those that might become significant within the multivariate model. We assessed the relationship among the parameters included in the multivariate analysis using correlations for continuous parameters, Fisher’s exact test for categorical parameters, and one‐way analysis of variance tests for the relationship between continuous and categorical parameters (20).
Missing data and multivariate analysis
We assessed patterns of missing data for each variable and used five models to assess the predictors of outcome. Model 1 used no imputation of missing data and excluded patients with any missing data point. Model 2 also used no imputation and excluded patients lacking the parameter with the most missing data points (C‐reactive protein). Model 3 imputed data using the mean value of the parameter with the most missing data. Model 4 imputed the parameter with the most missing data using a predicted value based on a separate model‐building regression analysis. Model 5 used multiple imputations for missing data for five parameters, using a Markov Chain Monte Carlo method (21). We created a series of ten imputed data sets, conducted logistic regression analyses for each, then combined the estimates from each imputation and performed sequential model building. To determine the independent predictors of outcome and minimise the amount of missing data, we used the model‐building identified factors and conducted a logistic regression with no imputation.
Results
The SIDESTEP study randomised 586 patients, 402 of whom were clinically evaluable at the FUA. Among these, 342 (85%) had a favourable response and 60 had clinical failures. The mean duration of antibiotic therapy was similar for patients with a favourable clinical response and those who failed to respond (17·4 versus 17·6 days total therapy, and 10·7 versus 13·8 days just intravenous therapy). Table 1 summarises the continuous and dichotomous parameters selected for analysis as potential predictors of outcome.
Table 1.
Clinical outcome by baseline patient characteristics analysed for association with clinical outcome
| Continuous clinical factors (units) | Favourable response | Failure response | Total | |||
|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | N | Mean ± SD | |
| White blood cell count (cells/mm3) | 339 | 7933 ± 2576 | 60 | 9977 ± 4235 | 399 | 8240 ± 2971 |
| C‐reactive protein (mg/dl) | 204 | 3·7 ± 6·6 | 43 | 9·1 ± 10·1 | 247 | 4·6 ± 7·6 |
| Erythrocyte sedimentation rate (mm/hour) | 305 | 41·0 ± 29·9 | 55 | 54·4.0 ± 36·4 | 360 | 43·0 ± 31·3 |
| Total wound score* [3 (least)−49 (most) severe] | 322 | 15·5 ± 5·6 | 51 | 18·0 ± 5·4 | 373 | 15·8 ± 5·6 |
| Serum albumin (g/dl) | 324 | 3·8 ± 1·0 | 58 | 3·5 ± 0·5 | 382 | 3·7 ± 1·0 |
| Serum glucose (mg/dl) | 335 | 196·4 ± 104 | 59 | 220·8 ± 127 | 394 | 200·1 ± 108 |
| Haemoglobin (g/dl) | 339 | 13·1 ± 2·0 | 60 | 12·7 ± 1·7 | 399 | 13·0 ± 1·9 |
| Body mass index (kg/m2) | 307 | 33·4 ± 9·5 | 58 | 32·1 ± 8·1 | 365 | 33·2 ± 9·3 |
| Serum creatinine (mg/dl) | 337 | 1·3 ± 1·3 | 59 | 1·1 ± 0·6 | 396 | 1·2 ± 1·2 |
| Systolic blood pressure (mmHg) | 339 | 136·3 ± 19·7 | 60 | 134·5 ± 16·3 | 399 | 136·0 ± 19·2 |
| Diastolic blood pressure (mmHg) | 339 | 76·1 ± 11·9 | 60 | 75·8 ± 10·6 | 399 | 76·0 ± 11·7 |
| Haemoglobin A1C (%) | 291 | 8·7 ± 2·3 | 48 | 8·7 ± 2·3 | 339 | 8·7 ± 2·3 |
| Duration of diabetes diagnosis (years) | 330 | 12·5 ± 9·4 | 60 | 12·7 ± 11·1 | 390 | 12·5 ± 9·7 |
| Age (years) | 342 | 58·8 ± 13·6 | 60 | 58·6 ± 13·5 | 402 | 58·8 ± 13·5 |
| Weight (kg) | 309 | 99·0 ± 28·3 | 58 | 98·7 ± 26·8 | 367 | 98·9 ± 28·0 |
| Dichotomous clinical factors | Favourable response | Failure response | Total | |||
|---|---|---|---|---|---|---|
| N | n (%) | N | n (%) | N | n (%) | |
| Baseline severity by UT† score at baseline | 342 | 60 | 402 | |||
| Severe | 96 (28·1) | 29 (48·3) | 125 (31·1) | |||
| Moderate | 246 (71·9) | 31 (51·7) | 277 (68·9) | |||
| Outpatient | 342 | 60 | 402 | |||
| Initial treatment, inpatient | 114 (33·3) | 31 (51·7) | 145 (36·1) | |||
| Initial treatment, outpatient | 228 (66·7) | 29 (48·3) | 257 (63·9) | |||
| Gender | 342 | 60 | 402 | |||
| Male | 200 (58·5) | 44 (73·3) | 244 (60·7) | |||
| Female | 142 (41·5) | 16 (26·7) | 158 (39·3) | |||
| Dermal thermometry | 308 | 54 | 362 | |||
| ≥10°C | 32 (10·4) | 11 (20·4) | 319 (88·1) | |||
| <10°C | 276 (89·6) | 43 (79·6) | 43 (11·9) | |||
| Previous insulin use | 342 | 60 | 402 | |||
| Yes | 197 (57·6) | 41 (68·3) | 238 (59·2) | |||
| No | 145 (42·4) | 19 (31·7) | 164 (40·8) | |||
| Wound onset duration (months) | 338 | 60 | 398 | |||
| >6 | 40 (11·8) | 11 (18·3) | 51 (12·8) | |||
| ≤6 | 298 (88·2) | 49 (81·7) | 347 (87·2) | |||
| Treatment arm | 342 | 60 | 402 | |||
| Piperacillin/tazobactam | 162 (47·4) | 34 (56·7) | 196 (48·8) | |||
| Ertapenem | 180 (52·6) | 26 (43·3) | 206 (51·2) | |||
| Nylon monofilament test | 335 | 59 | 394 | |||
| ≥2 | 251 (74·9) | 48 (81·4) | 299 (75·9) | |||
| 0–1 | 84 (25·1) | 11 (18·6) | 95 (24·1) | |||
| Dorsalis pedis pulse | 338 | 60 | 398 | |||
| 0 to 1+ | 121 (35·8) | 19 (31·7) | 140 (35·2) | |||
| >1+ | 217 (64·2) | 41 (68·3) | 258 (64·8) | |||
| Foot X ray | 322 | 55 | 377 | |||
| Abnormal | 140 (43·5) | 25 (45·5) | 165 (43·8) | |||
| Normal | 182 (56·5) | 30 (54·5) | 212 (56·2) | |||
SD = standard deviation; n = sample size of the subgroup of the clinical factor; N= sample size of the total group of the clinical factor.
See text for details.
UT = University of Texas wound classification.
Association between clinical failure of treatment and baseline parameters
Univariate analysis
From the univariate analysis, 13 baseline parameters reached a P value of ≤0·25 (see Table 2). There was no effect of sex or ethnicity on outcome. Of the dichotomous factors, a severe baseline wound was associated with the highest increase in odds of failure (odds ratio = 2·40, P = 0·002, comparing moderate with severe wounds). Clinical failure was noted in 23% of the patients with a severe wound at baseline compared with 11% with a moderate wound. Among the continuous variables, an elevated white blood cell count had the highest association with clinical failure [odds ratio = 1·80, P < 0·001, for an increase of 1 standard deviation (2971 cells/mm3) in white blood cell count]. The overall mean baseline (±standard deviation) white blood cell count was 8240 (±2971) cells/mm3; it was 9977 (±4235) cells/mm3 for the patients who failed treatment compared with 7933 (±2576) cells/mm3 for those with a favourable clinical response.
Table 2.
Level of association of clinical factors at baseline with clinical treatment failure from the univariate analyses (follow‐up assessment clinically evaluable population)
| Clinical factor | Odds ratio (OR) | 95% confidence interval (CI) | P value |
|---|---|---|---|
| White blood cell count | 1·80 | 1·39–2·32 | <0·001 |
| C‐reactive protein | 1·74 | 1·31–2·31 | <0·001 |
| Baseline severity by UT* score (severe versus moderate) | 2·40 | 1·37–4·19 | 0·002 |
| Erythrocyte sedimentation rate | 1·48 | 1·13–1·93 | 0·004 |
| Total wound score | 1·51 | 1·14–1·98 | 0·004 |
| Outpatient (initial treatment inpatient versus outpatient) | 2·14 | 1·23–3·72 | 0·007 |
| Serum albumin | 0·52 | 0·33–0·83 | 0·007 |
| Gender (male versus female) | 1·95 | 1·06–3·60 | 0·032 |
| Dermal thermometry (≥10°C versus <10°C) | 2·21 | 1·04–4·70 | 0·040 |
| Serum glucose | 1·23 | 0·95–1·59 | 0·112 |
| Previous insulin use (yes versus no) | 1·59 | 0·89–2·85 | 0·121 |
| Wound onset duration (>6 months versus ≤6 months) | 1·67 | 0·80–3·48 | 0·169 |
| Treatment arm (piperacillin/tazobactam versus ertapenem) | 1·45 | 0·84–2·53 | 0·185 |
| Haemoglobin | 0·85 | 0·64–1·12 | 0·243 |
| Nylon monofilament test (≥2 versus 0–1) | 1·46 | 0·73–2·94 | 0·290 |
| Body mass index | 0·86 | 0·64–1·17 | 0·335 |
| Serum creatinine | 0·79 | 0·43–1·45 | 0·454 |
| Systolic blood pressure | 0·91 | 0·69–1·20 | 0·507 |
| Dorsalis pedis pulse (0 to 1+ versus >1+) | 0·85 | 0·47–1·54 | 0·597 |
| Foot X ray (abnormal versus normal) | 1·08 | 0·61–1·93 | 0·785 |
| Diastolic blood pressure | 0·97 | 0·74–1·278 | 0·833 |
| Haemoglobin A1C | 1·03 | 0·756–1·39 | 0·875 |
| Duration of diabetes diagnosis | 1·02 | 0·78–1·34 | 0·876 |
| Age | 0·98 | 0·75–1·29 | 0·902 |
| Weight | 0·99 | 0·75–1·31 | 0·937 |
UT = University of Texas wound classification. Single logistic regression was used to estimate the OR, 95% CI and P values. OR for the continuous variables represent the increased odds of clinical failure based on a 1 standard deviation increase in the risk factor.
Multivariate analysis
We found no relationship between missing data and erythrocyte sedimentation rate or dermal thermometry, but for C‐reactive protein, there were more missing data points among patients with a severe baseline wound (54%) than among those with a moderate wound (32%). Women (45%) had more missing values than men (34%), and outpatients had more (43%) missing values than inpatients (31%).
In running five models (see Research design and Methods) to build the final multivariate model, baseline wound severity and white blood cell count were consistently the most predictive of clinical outcome. Dermal thermometry was a significant predictor in Model 5 (P = 0·04), which used multiple imputation. Total wound score nearly achieved significance in Models 2 and 4 (P = 0·06), both of which imputed C‐reactive protein. Differences in skin temperatures by dermal thermometry and the results of the total wound score were not statistically significant predictors of clinical outcome when tested independently in models containing baseline severity and white blood cell count.
The final multivariate model demonstrated that only two features were statistically significant independent predictors of clinical failure: 1) UT wound grade classification of ‘severe’ compared with ‘moderate’ (odds ratio 2·1, 95% CI 1/33, 2·2, P < 0·001) and 2) elevated white blood cell count [odds ratio 1·7 with an increase in 1 standard deviation (2971 cells/mm3) above the mean, 95% CI 1·2–3·8, P = 0·01). The white blood cell count was significantly and directly related at all values to clinical failure rates at the FUA assessment (Figure 1). At baseline, patients with neither independent risk factor had a failure rate of 22/222, or 9·9% (95% CI 6·3–14·6); this increased to 23/144, or 16% (95% CI 19·4–23·0) for patients with one of these risk factors and to 15/33, or 45·5% (28·1, 63·6) for patients with both risk factors.
Figure 1.
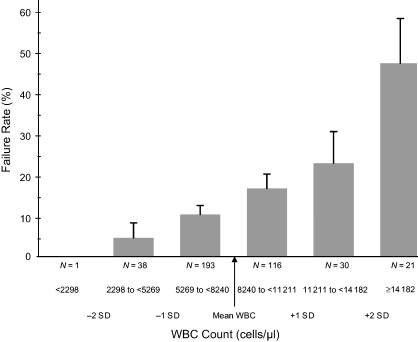
Clinical treatment failure rate by white blood cell count. SD = standard deviation; N = sample size of the group; WBC = white blood cell.
Conclusions
Many of the risk factors for a diabetic patient developing a foot infection are known 22, 23, but there is little information on which, if any, clinical findings predict the outcome of infection. Among the grading systems developed for classifying diabetic foot wounds 11, 16, 24, 25, 26, 27, only those from the International Working Group on the Diabetic Foot 11, 28, 29 and the Infectious Diseases Society of America (1) specifically grade the severity of the infection component. Neither of these, however, has yet been validated, and it is unknown if they predict the likelihood of successful treatment. Furthermore, other factors that might potentially help predict the clinical outcome of a diabetic foot infection have not been prospectively analysed.
Knowing which clinical factors predict an unfavourable outcome could help the clinician know when to be especially vigilant and to consider more aggressive diagnostic and therapeutic interventions. The costliest decision in treating a diabetic foot infection is when to hospitalise the patient 30, 31. While several medical and psychosocial factors play into this decision, the most important is the severity of the infection (1). A second key decision point is selecting the appropriate antibiotic therapy. Most patients are initially treated empirically while awaiting culture and sensitivity results. Patients with mild to moderate diabetic foot infections can often be treated with relatively narrow‐spectrum oral antibiotic agents, but for severe infections it is usually safest to start with broad‐spectrum and parenteral agents 3, 32.
Our prospective study identified numerous factors by univariate analyses that were associated with a worse clinical outcome with moderate to severe diabetic foot infections. These included demographic, clinical and laboratory parameters, each of which would be easily assessable at the patient’s initial presentation. Among these, we used the nine parameters that achieved statistical significance (P < 0·05) and the five parameters with a P value <0·25 level for the multivariate analysis. Our multivariate regression model found that only two baseline parameters, a severe (rather than moderate) wound as determined by the UT grade of depth and a higher white blood cell count, were statistically significantly associated with an unfavourable clinical outcome.
These two parameters have been found to be associated with worse clinical outcomes in previously published retrospective studies, each of which (unlike ours) included some patients with bone infection. In evaluating the UT classification, Armstrong et al. found that the risk for high‐level limb amputation was 11‐fold higher for wounds that penetrated to bone than for those that did not involve deep structures (17). Oyibo et al. (18) found that both the UT grade and stage, as well as aspects of the older Wagner classification grade (26), were significantly correlated with the risk of amputation. For UT stage, the risk of amputation significantly increased with the presence of infection, both alone and in combination with ischaemia, but not with ischaemia alone. Increasing stage, regardless of grade, was associated with an increased risk of amputation and prolonged ulcer healing time, and the UT system was a better predictor of outcome than the Wagner system (18). Similarly, Eneroth et al. found, using logistic regression analysis, that a diabetic foot infection deep enough to expose bone was associated with limb amputation (33). Calhoun et al. also noted that more severe or extensive foot infections (by Wagner score) were associated with a higher treatment failure rate than milder infections (24).
Many diabetic patients with a severe infection do not have leukocytosis (34), but our study demonstrated that an elevated white blood cell count should raise special concerns. We found only two other (retrospective) studies with similar findings. The investigation by Eneroth et al. (33) of deep infections found that a white blood cell count of >12 000 cells/mm3 was an independent predictor of limb amputation. Similarly, Akanji et al. (35) reported that leukocytosis was associated with worse clinical outcomes with diabetic foot infection. In a retrospective cohort study of diabetic foot infections by Pittet et al., however, the neutrophil count was not among the independent factors that predicted treatment failure (36).
Of note is that the results of our large prospective study of diabetic patients with moderate to severe foot infections showed that many factors that might help predict clinical outcome, such as male gender, increased serum inflammatory markers (sedimentation rate and C‐reactive protein), long duration of diabetes, poor glycaemic control, elevated serum creatinine, and diminished pedal pulses, did not provide additional information beyond that provided by UT grade of depth and white blood cell count. Previous smaller, retrospective studies, which did not exclude patients with osteomyelitis, have revealed additional factors that correlated with outcome. Pittet et al. reported that independent factors associated with treatment failure were the presence of fever, increased serum creatinine, prior hospitalisation for a diabetic foot lesion and a gangrenous wound (36). Other patient characteristics, however, including various demographic variables, duration of diabetes, neutrophil count or the anatomical site of the lesion, failed to predict outcome (36). Benotmane et al. found that the factors associated with a worse outcome in a group of patients with an infected diabetic foot wound included male gender, delay in proper management, poor‐quality medical treatment, ‘negative surgical attitudes’ and an inadequate initial amputation level (37). In a study by Eneroth et al., long duration of diabetes and inadequate foot perfusion were also independent risk factors predicting limb amputation (33). Limb ischaemia was the major factor associated with a poor outcome in patients with a severe diabetic foot infection in a report by Diamantopoulos et al. (38). In a prospective antibiotic trial of diabetic foot infections reported by Grayson et al., treatment failure was associated with antibiotic‐resistant pathogens and nosocomial acquisition of infection (39). Criado et al., in a retrospective stepwise discriminate analysis of vascular surgery patients, noted that patients most likely to require a major amputation during the initial hospitalisation were those with an absent dorsalis pedis pulse or with a polymicrobial infection (40). In addition, non insulin‐dependent diabetic men and those undergoing delayed amputation had worse outcomes (40).
Some of the outcomes from these studies differ from our findings. This may be partly explained by the selection bias that is often introduced by retrospective studies; they are more likely to include sicker patients with several abnormal baseline parameters than in our prospective antimicrobial trial. In addition, unlike these retrospective studies, we excluded patients with osteomyelitis (that was not resected), with critical limb ischaemia requiring surgical intervention or with any immediately life‐threatening comorbidity. Although limb ischaemia may be an important predictor of outcomes for diabetic foot infections, our study included too few of these patients to allow any meaningful analysis of this factor. The relatively high cure rate in both arms of our trial also limited our ability to investigate treatment failures. While the mean white blood cell counts for the patients who failed therapy were just within the normal range, an adverse outcome was directly related to the white blood cell count, and a count above ∼10 000 appears to predict a poor outcome. Certainly, our findings should be validated by another prospective trial.
In summary, we found that at baseline, a severe wound by the UT grade and an elevated white blood cell count were the only two statistically significant independent predictors of an unfavourable clinical outcome in diabetic patients with a moderate to severe foot infection. The importance of these clinical parameters is supported by the fact that their risk was more than additive when combined, that the white blood cell count was linearly related to outcome, and the similar findings in some previously published retrospective case series. Of interest is that other factors previously found to be associated with a poor outcome in retrospective studies were not in our prospective study. Knowing which baseline clinical findings predict a worse clinical outcome may help clinicians formulate an effective management strategy for a patient with a diabetic foot infection.
Potential conflict of interest
Benjamin A. Lipsky, MD, Peter Sheehan, MD, David G. Armstrong, DPM, PhD, and Alan D. Tice, MD severed as consultants to, been members of the speaker’s bureau for and have received research grants from Merck & Co., Inc. Adam B. Polis, MA and Murray A. Abramson, MD, MPH are employees of and stockholders in Merck & Co., Inc.
Acknowledgements
We thank Wendy Horn (manuscript preparation), David Morgenstern and Sandy Rawlins (study administration) and Diane Tipping (statistical analysis and programming). Merck & Co., Inc., West Point, PA, sponsored the SIDESTEP study and provided financial and material support for this analysis. All the authors had full access to the primary data and their analyses and independence in reporting of results and the content of the manuscript.
Presented in part at the Infectious Diseases Society of America Annual Meeting in San Francisco, CA, on 7 October 2005.
References
- 1. Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, LeFrock JL, Lew DP, Mader JT, Norden C, Tan JS. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2004;39:885–910. [DOI] [PubMed] [Google Scholar]
- 2. Singh N, Armstrong, DG , Lipsky, BA . Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. [DOI] [PubMed] [Google Scholar]
- 3. Reiber G, Boyko, EJ , Smith, DG . Diabetes in America, 2nd edition. Chapter 18. Lower extremity foot ulcer and amputations in diabetes [WWW document] URL http://diabetes.niddk.nih.gov/dm/pubs/america/contents.htm [accessed 27 June 2005].
- 4. Lipsky B, Pecoraro, RE , Wheat, LJ . The diabetic foot. Soft tissue and bone infection. Infect Dis Clin North Am 1990;4:409–32. [PubMed] [Google Scholar]
- 5. Cavanagh P, Lipsky BA, Bradbury A. Treating diabetic foot ulcers. Lancet 2005;366:1725–35. [DOI] [PubMed] [Google Scholar]
- 6. U.S. Centers for Disease Control Prevention . Number (in thousands) of hospital discharges with peripheral arterial disease (PAD), ulcer/inflammation/infection (ULCER), or neuropathy as first‐listed diagnosis and diabetes as any‐listed diagnosis, United States, 1980–2002 [cited 16 June 2005]. URL http://www.cdc.gov/diabetes/statistics/hosplea/fig1.htm. Accessed 11 November 2006
- 7. Jeffcoate W, Harding, KG . Diabetic foot ulcers. Lancet 2003;361:1545–51. [DOI] [PubMed] [Google Scholar]
- 8. Lipsky B. Evidence‐based antibiotic therapy of diabetic foot infections. FEMS Immunol Med Microbiol 1999;26:267–76. [DOI] [PubMed] [Google Scholar]
- 9. Consensus development conference on diabetic foot wound care . Diabetes Care 1999;22:1354–60. [DOI] [PubMed] [Google Scholar]
- 10. American Diabetes Association . Peripheral arterial disease in people with diabetes. Diabetes Care 2003;26:3333–41. [DOI] [PubMed] [Google Scholar]
- 11. Peters EJG, Lavery LA. Effectiveness of the diabetic foot risk classification system of the International Working Group on the Diabetic Foot. Diabetes Care 2001;24:1442–47. [DOI] [PubMed] [Google Scholar]
- 12. International Working Group on the Diabetic Foot . International Consensus on the Diabetic Foot. May 2003; International Diabetes Federation (CD‐ROM available for purchase at www.idf.org/bookshop).
- 13. Pinzur MS, Slovenkai MP, Trepman E, Shields NN. Guidelines for diabetic foot care: recommendations endorsed by the Diabetes Committee of the American Orthopaedic Foot and Ankle Society. Foot Ankle Int 2005;26:113–9. [DOI] [PubMed] [Google Scholar]
- 14. Macfarlane R, Jeffcoate, WJ . Factors contributing to the presentation of diabetic foot ulcers. Diabet Med 1997;14:867–70. [DOI] [PubMed] [Google Scholar]
- 15. Lipsky BA, Armstrong DG, Citron DM, Tice AD, Morgenstern DE, Abramson MA. The SIDESTEP study of diabetic foot infection: a randomized, controlled, double‐blinded, multicenter trial of ertapenem versus piperacillin/tazobactam. Lancet 2005;366:1695–1703. [DOI] [PubMed] [Google Scholar]
- 16. Lavery L, Armstrong, DG , Harkless, LB . Classification of diabetic foot wounds. J Foot Ankle Surg 1996;35:528–31. [DOI] [PubMed] [Google Scholar]
- 17. Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998;21:855–59. [DOI] [PubMed] [Google Scholar]
- 18. Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Harkless LB, Boulton AJM. A comparison of two diabetic foot ulcer classification systems: the Wagner and the UT wound classification systems. Diabetes Care 2001;24:84–8. [DOI] [PubMed] [Google Scholar]
- 19. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) . E6 Good Clinical Practice: Consolidated Guidance. 1996 [cited 2005 May 9] URL http://www.fda.gov/cder/guidance/959fnl.pdf. Accessed 11 November 2006
- 20. Kirkwood BR. Essentials of medical statistics. Oxford: Blackwell, 1988. [Google Scholar]
- 21. Allison PD. Missing data. Sage University Papers Series on Quantitative Applications in the Social Sciences, series no. 07‐136. Thousand Oaks: Sage, 2002. [Google Scholar]
- 22. Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006;29:1288–93. [DOI] [PubMed] [Google Scholar]
- 23. Peters EJ, Lavery LA, Armstrong DG. Diabetic lower extremity infection: influence of physical, psychological, and social factors. J Diabetes Complications 2005;19:107–12. [DOI] [PubMed] [Google Scholar]
- 24. Calhoun JH, Cantrell J, Cobos J, Lacy J, Valdez RR, Hokanson J, Mader J. Treatment of diabetic foot infections: Wagner classification, therapy, and outcome. Foot Ankle 1988;9:101–6. [DOI] [PubMed] [Google Scholar]
- 25. Armstrong DG, Peters EJ. Classification of wounds of the diabetic foot. Curr Diab Rep 2001;1:233–8. [DOI] [PubMed] [Google Scholar]
- 26. Wagner F. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle 1981;2:62–122. [DOI] [PubMed] [Google Scholar]
- 27. Jeffcoate WJ, Macfarlane RM, Fletcher EM. The description and classification of diabetic foot lesions. Diabet Med 1993:10:676–9. [DOI] [PubMed] [Google Scholar]
- 28. Schaper NC, Apelqvist J, Bakker K. The international consensus and practical guidelines on the management and prevention of the diabetic foot. Curr Diab Rep 2003;3:475–9. [DOI] [PubMed] [Google Scholar]
- 29. Lipsky BA. A report from the international consensus on diagnosing and treating the infected diabetic foot. Diabetes Metab Res Rev 2004;20 Suppl. 1:S68–77. [DOI] [PubMed] [Google Scholar]
- 30. Girod I, Valensi P, Laforet C, Moreau‐Defarges T, Guillon P, Baron F. An economic evaluation of the cost of diabetic foot ulcers: results of a retrospective study on 239 patients. Diabetes Metab 2003;29:269–77. [DOI] [PubMed] [Google Scholar]
- 31. Stockl K, Vanderplas A, Tafesse E, Chang E. Costs of lower‐extremity ulcers among patients with diabetes. Diabetes Care 2004;27:2129–34. [DOI] [PubMed] [Google Scholar]
- 32. Caputo G, Cavanagh P, Ulbrecht J, Gibbons G, Karchmer A. Assessment and management of foot disease in patients with diabetes. N Engl J Med 1994;331:854–60. [DOI] [PubMed] [Google Scholar]
- 33. Eneroth M, Apelqvist J, Stenstrom A. Clinical characteristics and outcome in 223 diabetic patients with deep foot infections. Foot Ankle Int 1997;18:716–22. [DOI] [PubMed] [Google Scholar]
- 34. Armstrong DG, Lavery LA, Sariaya M, Ashry H. Leukocytosis is a poor predictor of acute osteomyelitis of the foot in diabetes mellitus. J Foot Ankle Surg 1996;35:280–3. [DOI] [PubMed] [Google Scholar]
- 35. Akanji AO, Famuyiwa OO, Adetuyibi A. Factors influencing the outcome of treatment of foot lesions in Nigerian patients with diabetes mellitus. Q J Med 1989;73:1005–14. [PubMed] [Google Scholar]
- 36. Pittet D, Wyssa B, Herter‐Clavel C, Kursteiner K, Vaucher J, Lew PD. Outcome of diabetic foot infections treated conservatively: a retrospective cohort study with long‐term follow‐up. Arch Intern Med 1999;159:851–6. [DOI] [PubMed] [Google Scholar]
- 37. Benotmane A, Mohammedi F, Ayad F, Kadi K, Azzouz A. Diabetic foot lesions: etiologic and prognostic factors. Diabetes Metab 2000;26:113–7. [PubMed] [Google Scholar]
- 38. Diamantopoulos EJ, Haritos D, Yfandi G, Grigoriadou M, Margariti G, Paniara O, Raptis SA. Management and outcome of severe diabetic foot infections. Exp Clin Endocrinol Diabetes 1998;106:346–52. [DOI] [PubMed] [Google Scholar]
- 39. Grayson ML, Gibbons GW, Habershaw GM, et al. Use of ampicillin/sulbactam versus imipenem/cilastatin in the treatment of limb‐threatening foot infections in diabetic patients. Clin Infect Dis 1994;18:683–93. [DOI] [PubMed] [Google Scholar]
- 40. Criado E, De Stefano AA, Keagy BA, Upchurch GR Jr, Johnson G Jr. The course of severe foot infection in patients with diabetes. Surg Gynecol Obstet 1992;175:135–40. [PubMed] [Google Scholar]


