Abstract
PURPOSE:
African American men have historically had poorer prostate cancer biochemical and survival outcomes than Caucasians. However, emerging data suggest nononcologic factors drive much of this disparity. Prior evidence has suggested an association between a transient prostate specific antigen (PSA) bounce and improved biochemical control. However, racial differences in this relationship have remained relatively unexplored.
METHODS AND MATERIALS:
We identified 4477 men treated for low- or intermediate-risk prostate cancer within the U.S. Department of Veterans Affairs (VA) from 2000 to 2010 with brachytherapy alone or in combination with external beam radiotherapy without androgen deprivation. Longitudinal PSA data were used to define to biochemical failure and PSA bounce. Cox proportional hazard models were used explore racial differences in the relationship between the PSA bounce and time to biochemical failure.
RESULTS:
Thirty-one percent of our sample experienced a PSA bounce, with African Americans more likely to experience a bounce (42%) compared with Caucasians (29%); p < 0.001. Despite this, African Americans had a higher likelihood of biochemical failure (hazard ratio [HR] 1.4; p = 0.006). However, African American men experiencing a PSA bounce were less likely to experience a biochemical failure (HR = 0.64; p = 0.046), whereas this relationship was not statistically significant for Caucasians (HR = 0.78; p = 0.092). On multivariate analysis, African Americans receiving brachytherapy alone were most sensitive to the protective benefit of the PSA bounce (HR = 0.64).
CONCLUSIONS:
A PSA bounce was associated with improved biochemical control among patients receiving brachytherapy as part of their treatment for low- or intermediate-risk prostate cancer at the VA. African American men treated with brachytherapy had a particularly pronounced biochemical control benefit of a PSA bounce.
Keywords: PSA bounce, Prostate brachytherapy, Veterans affairs, Biochemical failure, Racial disparities
Introduction
African American men on aggregate have significantly worse prostate cancer outcomes, including overall survival (1), relative to Caucasian men, among all risk groups (2). These disparities have historically been assumed to be the result of the presence of a more aggressive cancer genotype among African American men. In more recent literature, the impact of genomic differences by race has been found to be relatively small and limited to patients with lower Gleason scores (3). The vast majority of racial disparity between black and nonblack patients appears to be driven by socioeconomic factors such as access to care (4), and racial differences in prostate cancer-specific mortality which is most pronounced among men with Gleason 6 disease (5). Furthermore, racial disparities persist in the utilization of brachytherapy which is an effective and commonly used treatment modality for men with low-risk disease (6).
Prostate brachytherapy offers several practical and dosimetric advantages compared with external beam radiation therapy (EBRT). Given the ability for more conformal dose distributions, and increasing evidence of the benefits of dose escalation using brachytherapy in combination with external beam radiotherapy (e.g., the ASCENDE-RT trial (7)), interest has also increased in its combination with additional treatment modalities, notably androgen deprivation and surgery. Despite this, the national trends in brachytherapy use for prostate cancer suggest a decline in utilization over time from 2004 to 2009 (8). Interestingly, however, the decline over this time found in this study was more marked for Caucasian men compared with African Americans.
Although a treatment failure is traditionally defined using prostate specific antigen (PSA) through the Phoenix criteria as the nadir + 2 ng/mL, a phenomenon was observed after brachytherapy in which a nonfailure interval PSA climbs by a value less than 2 ng/mL and then returns to the prerise PSA or below, which was coined by Wallner as PSA “bounce.” (9) Although the exact pathophysiologic significance of the bounce phenomenon is unclear, recent evidence suggests that it may be related to an increased T-cell response to the tumor (10). Several studies have analyzed the predictive and prognostic significance of the PSA bounce. Several patient series have found that the presence of a bounce was associated with improved biochemical progression-free survival (bPFS) (11–13), and in some analyses, with improved overall survival (14). However, there is reason to believe that these associations may not hold true for all patients. A recent analysis by Charret et al. (15) found that a PSA bounce was not associated with bPFS or overall survival among patients younger than 60 years who received low-dose-rate monotherapy. Furthermore, there are conflicting data regarding the prognostic value of a PSA bounce among patients receiving EBRT for prostate cancer. Whereas a recent analysis by Romesser et al. (16) found that the presence of a PSA bounce after treatment with dose-escalated EBRT increased the bPFS, prior studies considering this same question have found that a bounce is associated with decreased bPFS (17).
One of the factors that has not been explicitly considered in the aforementioned analyses is examination of the potential variations of the association between PSA bounce and prostate cancer outcomes by race. In these studies, data on race are often not available; in those where racial composition of the sample is reported, African Americans are poorly represented. There are several reasons to consider the role of race on PSA kinetics in general, and the PSA bounce in particular. First, African American men are more likely to present with higher grade disease and have higher rates of biochemical recurrence after prostatectomy than Caucasian men (18, 19). Furthermore, there is increasing evidence that there are molecular differences in the prostate cancers themselves between African American and Caucasian men (20). The Veterans Administration Health System (VA) provides an ideal setting to examine this question. In addition to serving a racially diverse patient population, the VA treats a high volume of prostate cancer and has an electronic medical record system that allows longitudinal long-term followup of PSA outcomes.
Methods and materials
Patient sample
The sample is derived from patients diagnosed with prostate cancer within the United States Department of Veterans Affairs (VA) Health System, which is a single-payer, equal access health care system. Importantly for this study, the VA treats a significant proportion of minority patients from a diverse socioeconomic background, allowing for robust analysis of potential differences in PSA kinetics and outcomes by race.
Overall, we identified 109,579 patients who were classified as being diagnosed with prostate cancer between 2000 and 2010 from oncology raw database within the VA Informatics and Computing Infrastructure. Of these patients, 50,982 had clinical data and longitudinal PSA data that allow computation of our endpoints of interest. We excluded patients who had metastatic disease (5595) or no recorded treatment (6696). Given that the vast majority of patients were classified as either Caucasian or African American, and we are focused on differences between these two races, we excluded 1093 men who were classified as “other race.” Because the PSA outcomes of interest are profoundly impacted by the receipt of androgen deprivation, s, we restricted the sample to patients with a National Comprehensive Cancer Network classification of low- or intermediate-risk prostate cancer who received brachytherapy, with or without supplemental external beam irradiation leaving 4609 men. To remove the impact of antiandrogens on PSA outcomes, we abstracted the pharmacy records (Only prescriptions filled within the VA system were observed; any androgen deprivation received outside of the VA is not observed, even if other prostate cancer-directed therapy is offered through the VA.) of these men for antiandrogen or gonadotropin-releasing hormone agonist which excluded 132 men, rendering the final sample size of 4477 men. All brachytherapy procedures were low-dose-rate permanent implants. On average, patients had a mean of 8.35 PSA tests from 1 year before diagnosis to 5 years after diagnosis. There was no significant difference between the number of tests between African American and Caucasian patients (8.35 and 8.00, respectively).
Calculation of PSA metrics
Our primary PSA measure that we considered is the PSA bounce, which is defined as a posttreatment rise of PSA of 0.2 ng/mL or more which later falls to its prebounce level or less. We consider both the overall occurrence of a PSA bounce and the time to the bounce. Although various cut points have been explored in the context of the bounce, the biochemical failure predicted at a bounce cut point of 0.2 ng/mL is similar to those outcomes produced with higher cut points. (Robustness analyses considering higher PSA cut points to define a bounce [e.g., 0.4 ng/mL or 0.8 ng/mL] were performed. While increasing the PSA cutoff raised the probability that a bounce would predict a biochemical failure, there were changes in racial differences in biochemical failure based on PSA bounce status. These analyses are available from the authors on request.) And a bounce cut point of 0.2 ng/mL has been found to be reliable (21) and is most commonly used in prior literature. Biochemical failure is measured via the Phoenix definition which is defined a PSA exceeding the posttreatment nadir by 2 ng/mL or more. We consider the time to bounce as the time difference in months between the end of treatment and the time of the PSA bounce. Time to biochemical failure was defined as the length of time between the end of treatment and the first PSA value meeting the Phoenix definition.
Statistical analysis
Our descriptive analysis considers mean differences in clinical and demographic characteristics of our sample, stratified by whether the patient experienced a PSA bounce. We also calculated racial differences in the overall rate of PSA bounce, as well as in the mean time to and presence of a PSA bounce and biochemical failure for men who experienced these endpoints. We then explore Kaplan-Meier survival curves considering the association of the PSA bounce for both African American and Caucasian men in determining the overall time to biochemical failure. Finally, we consider Cox proportional hazard models to test the associations of race and PSA bounce on biochemical failure after adjusting for clinical and demographic characteristics, which included age, marital status, baseline PSA, T-stage, N-stage, National Comprehensive Cancer Network risk group, and year of diagnosis. We also explored the relationship between race and treatment (i.e., brachytherapy alone vs. brachytherapy with external beam radiotherapy), through separate Cox models considering two-way (including race and PSA bounce status) and three-way interactions (including treatment, race, and PSA bounce status). We used SAS 9.2 and Stata MP 15 to conduct our analyses. The proportional hazard assumptions were visually tested using the log(−log) plots and by testing for the nonzero slope of the scaled Schoenfeld residuals on time (22).
Results
Our sample encompasses 4477 men who received brachytherapy as monotherapy or in combination with EBRT as primary treatment for their prostate cancer. Of these men, 1404 experienced a PSA bounce and 3037 experienced no bounce. Their demographic and clinical characteristics are reported in Table 1. The average age of the sample was 64.4 years, although men who experienced a bounce were significantly younger (age, 62 years) than men who did not experience a bounce (age, 65 years). African American men are significantly more likely to experience a PSA bounce (N = 382/904; 42%) than Caucasian men (N = 1022/3573; 29%, p < 0.001). As such, African Americans make up 27% of the men who experienced a PSA bounce compared with only 17% among those who did not (p < 0.001). In addition, men who experienced a bounce were more likely to receive brachytherapy as monotherapy, have lower Gleason’s score and initial PSA, and low-risk disease. Those experiencing a bounce were marginally more likely to experience a biochemical failure than those who did not (6.4% vs. 7.8%; p = 0.0793).
Table 1.
Baseline clinical and demographic patient characteristics
| Variable | N | No bounce (N = 3073) | Bounce (N = 1404) | p-Value |
|---|---|---|---|---|
| Biochemical failure | 339 | 245 (7.8%) | 94 (6.4%) | 0.0741 |
| Bounce | 1404 | 0 (0.0%) | 1404 (100.0%) | <0.001 |
| Race | <0.001 | |||
| African American | 904 | 522 (17.0%) | 382 (27.2%) | |
| Caucasian | 3573 | 2551 (83.0%) | 1022 (72.8%) | |
| Treatment | 0.0379 | |||
| Brachytherapy | 4004 | 2731 (88.9%) | 1273 (90.7%) | |
| EBRT + brachytherapy | 473 | 342 (11.1%) | 131 (9.3%) | |
| T-stage | 0.1178 | |||
| T2a or lower | 4117 | 2815 (91.6%) | 1302 (92.7%) | |
| T2b-T2c | 342 | 242 (7.9%) | 100 (7.1%) | |
| Unknown | 18 | 16 (0.5%) | 2 (0.1%) | |
| N-stage | 0.1352 | |||
| N0 | 4342 | 2974 (96.8%) | 1368 (97.4%) | |
| N unknown | 135 | 99 (3.3%) | 36 (2.6%) | |
| Baseline PSA | 0.0299 | |||
| <10 | 3882 | 2643 (86.0%) | 1239 (88.3%) | |
| 10–20 | 583 | 424 (13.8%) | 159 (11.3%) | |
| Unknown | 12 | 6 (0.2%) | 6 (0.4%) | |
| Gleason’s score | 0.001 | |||
| <7 | 3024 | 2022 (65.8%) | 1002 (71.4%) | |
| 7 | 1355 | 979 (31.2%) | 376 (26.8%) | |
| Unknown | 98 | 72 (2.3%) | 26 (1.9%) | |
| Marital status | 0.1279 | |||
| Married | 2296 | 1607 (52.3%) | 689 (49.1%) | |
| Not married | 2177 | 1463 (47.6%) | 714 (50.9%) | |
| Unknown | 4 | 3 (0.1%) | 1 (0.1%) | |
| Risk group | <0.001 | |||
| Low | 2552 | 1688 (54.9%) | 865 (61.6%) | |
| Intermediate | 1924 | 1385 (45.1%) | 539 (38.4%) | |
| Age at diagnosis | 64.4 (6.6) | 65.3 (6.7) | 62.4 (6.3) | <0.001 |
EBRT = external beam radiation therapy.
The sample includes patients who received brachytherapy either alone or in combination for low- or intermediate-risk prostate cancer within the Department of Veterans Affairs.
In exploring the PSA kinetics among men who experienced both a bounce and biochemical failure by race (Fig. 1), there are several trends that emerge. First, individuals who experience a PSA bounce do so considerably earlier than biochemical failure, at approximately 18 months vs. 80–90 months, on average, respectively. In terms of racial differences, there was little difference in the average time to a PSA bounce between African American and Caucasian men, with those experiencing the bounce approximately 18 months after treatment regardless of race. Similarly, Caucasian and African American men had similar times to biochemical failure (on average 75.5 months after treatment) compared with African American men (75.5 vs. 81.4 months; p = 0.11). Overall, our findings of kinetics for PSA bounce and biochemical failure, specifically that a bounce occurs substantially earlier than a biochemical failure, is largely consistent with values previously reported in the literature (23–25).
Fig. 1.

PSA kinetics of men who experienced a PSA bounce and biochemical failure. PSA bounce defined as a PSA increase of 0.2 ng/mL or more which comes down to its prebounce level or less. Probability density is depicted on the y-axis.
Turning to time to biochemical failure as an outcome, Fig. 2 presents four cumulative hazard plots exploring both the role of PSA bounce and race in biochemical failure. Plot A considers the cumulative hazard of biochemical failure for both African American and Caucasian patients. Overall, African American men tend to experience biochemical failure somewhat earlier than Caucasian men (Panel A; HR 1.40; p = 0.006), consistent with prior literature. Also consistent with prior literature, patients who experience a PSA bounce are less likely to experience a biochemical failure than those who did not (Panel B; HR = 0.74; p = 0.018). In stratifying this relationship by race, African American men appear to have a protective benefit from a PSA bounce (Panel D; HR = 0.64; p = 0.046) compared with the statistically nonsignificant benefit among Caucasian men (Panel C; HR = 0.78; p = 0.092).
Fig. 2.
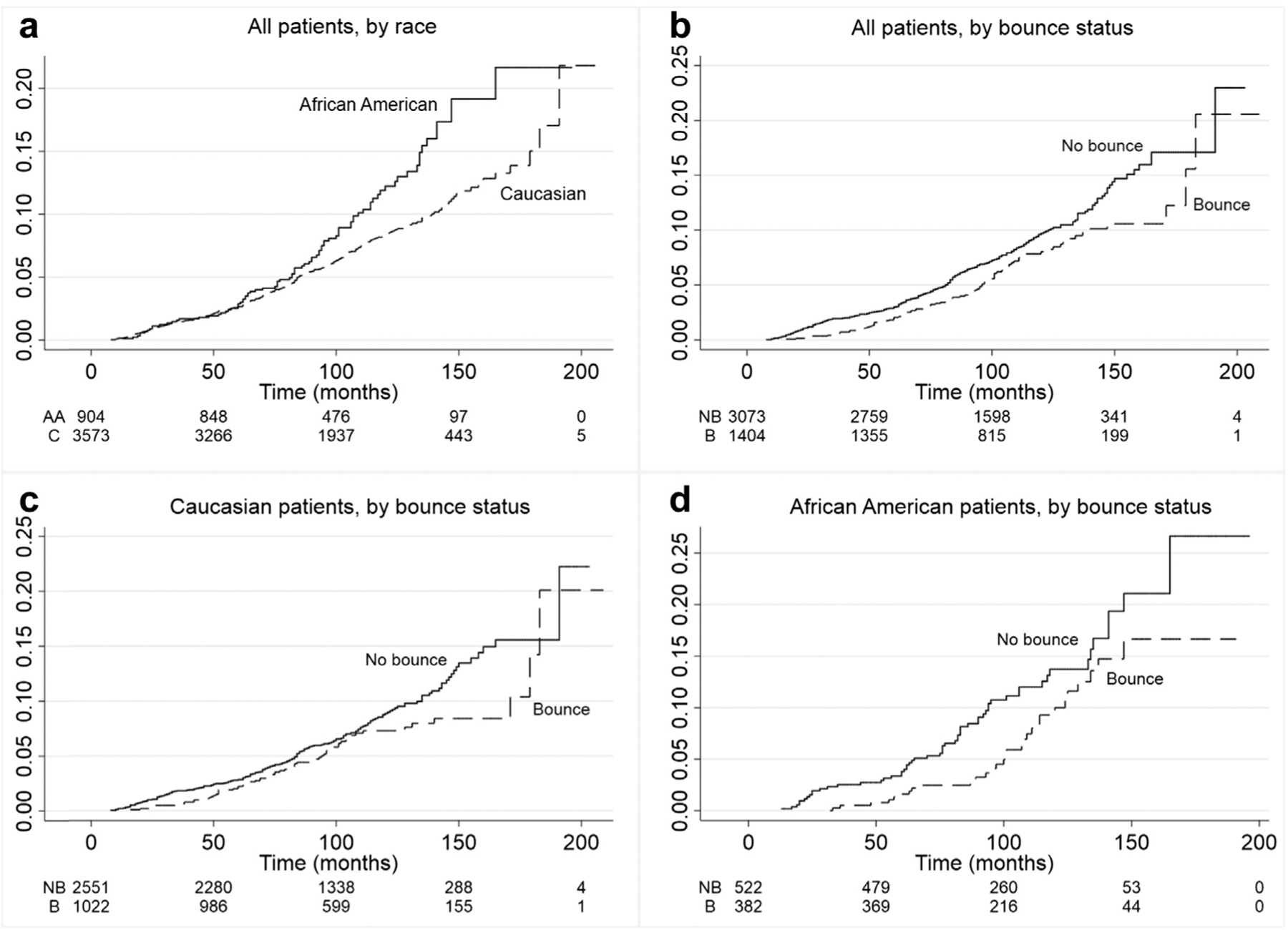
Cumulative hazard plots of biochemical failure over time by race and PSA bounce status. Biochemical failure (y-axis) is defined by the Phoenix criteria of PSA nadir + 2 ng/mL. Time refers to the number of months between end of treatment and time of biochemical failure. Plots (a) and (b) depict biochemical outcomes for all patients stratified by their race and PSA bounce status, respectively. Plot (c) depicts biochemical outcomes among Caucasian patients stratified by PSA bounce status, whereas Plot (d) depicts this relationship among African Americans. AA, African American; C, Caucasian; NB, no PSA bounce; B, PSA bounce.
The multivariate analysis, considering the aforementioned relationships after controlling for demographic and baseline clinical variables (listed in Table 1), is presented in Table 2. In addition, year of diagnosis variables were included in all models. Overall, the significant relationships in Figs. 2a and 2b of race and PSA bounce in predicting biochemical failure outcomes remain robust on multivariate analysis, with the overall hazard ratios changing little from the unadjusted analysis in Fig. 2. Remaining variables that were predictive of a higher risk of biochemical failure included intermediate-risk grouping (HR = 1.57; p < 0.01) compared with low risk and baseline PSA of 10–20 (HR = 1.42; p < 0.05). We also considered several models considering interactions of race and PSA bounce (Table 3, Panel A), as well as three-way interactions of treatment, race, and PSA bounce (Table 3, Panel B). The hazard ratios generated from these analyses show that all men who experienced a PSA bounce had a trend toward a lower risk of biochemical failure, with African Americans with a PSA bounce trending toward a more protective benefit than Caucasians experiencing a PSA bounce, although the presence of a bounce was associated with a lower rate of biochemical failure regardless of race. The strongest protective effect of a PSA bounce tended to be among African American men treated with brachytherapy as monotherapy although none of the hazard ratios were significant at the 5% level.
Table 2.
Multivariate predictors of biochemical failure after brachytherapy (N = 4609)
| HR (SE) | |
|---|---|
| Race (ref: Caucasian) | |
| African American | 1.43 (0.13)*** |
| PSA bounce (ref: No bounce) | |
| PSA bounce | 0.76 (0.12)** |
| Treatment (ref: brachytherapy + EBRT) | |
| Brachytherapy | 0.99 (0.17) |
| T-stage (ref: T2a or lower) | |
| T2b-T2c | 0.77 (0.22) |
| N-stage (ref: N0) | |
| N unknown | 0.74 (0.39) |
| Baseline PSA (ref: <10 ng/mL) | |
| 10–20 | 1.42 (0.16)** |
| Unknown | 1.93 (0.73) |
| Marital status (ref: married) | |
| Not married | 1.11 (0.11) |
| Unknown | 7.00 (1.01)* |
| Risk group (ref: low risk) | |
| Intermediate risk | 1.57 (0.13)*** |
| Age at diagnosis | 1.00 (0.01) |
EBRT = external beam radiation therapy; HR = hazard ratios reported; SE = standard errors.
Results represent output of Cox regression model.
p < 0.01;
p < 0.05;
p < 0.10.
Model included diagnosis year variables.
Table 3.
Multivariable predictions of biochemical failure by treatment, race, and PSA bounce status
| 95% Confidence interval | |||
|---|---|---|---|
| Hazard ratio | Lower | Upper | |
| Panel A: Two-way interaction with race and PSA bounce | |||
| Men receiving either brachytherapy alone or in combination with EBRT | |||
| African American and PSA bounce vs. no bounce | 0.69 | 0.44 | 1.07 |
| Caucasian and PSA bounce vs. no bounce | 0.79 | 0.59 | 1.05 |
| Panel B: Three-way interactions with treatment, race, and PSA bounce | |||
| Men receiving brachytherapy alone | |||
| African American and PSA bounce vs. no bounce | 0.64 | 0.40 | 1.05 |
| Caucasian and PSA bounce vs. no bounce | 0.77 | 0.57 | 1.05 |
| Men receiving brachytherapy in combination with EBRT | |||
| African American and PSA bounce vs. no bounce | 0.98 | 0.33 | 2.89 |
| Caucasian and PSA bounce vs. no bounce | 0.93 | 0.41 | 2.10 |
EBRT = external beam radiation therapy.
Hazard ratios generated from multivariate Cox regression model controlling for age, marital status, baseline PSA, risk group, and diagnosis year. Model with two-way interactions include an interaction term for race and PSA bounce status. Model with three-way interactions include an interaction term for treatment, race and PSA bounce status. Panel A contains a two-way interaction between race and PSA bounce. Panel B contains a three-way interaction between treatment modality, race, and PSA bounce.
Discussion and conclusions
The presence of a PSA bounce has been characterized in the literature as a positive prognostic factor and may be considered as a biological marker of future biochemical control. This analysis considers the association of PSA bounce on biochemical failure in a large, racially diverse sample of men treated at the VA. Our initial findings were largely consistent in both magnitude and direction with previous literature regarding an African American predilection for higher risk of biochemical failure after brachytherapy, with a hazard ratio of approximately 1.4 for failure than Caucasian men (26). In addition, men who experienced a PSA bounce also were found to have a lower risk of biochemical recurrence, consistent with previous reports of this association (16,21,27–29) (including a meta-analysis (11)). However, the magnitude of the association of a PSA bounce we found was somewhat lower than that reported by the meta-analysis, whereas we found a hazard ratio of 0.7–0.8 for PSA bounce, whereas Bernstein et al reported a hazard ratio of approximately 0.4 for the role of a PSA bounce in predicting against biochemical failure.
In the stratification of the bounce by race, African American men had a protective association of the PSA bounce (HR = 0.61; p < 0.05), whereas this association was not significant among Caucasian men (HR = 0.76; p = 0.064). On multivariate analysis of a two-way interaction (Panel A of Table 3), there is no significant difference at the 5% level, whereas in the three-way interaction model (Panel B of Table 3), African American men who received brachytherapy had the most favorable biochemical profile after a PSA bounce (HR for failure = 0.64), although this difference did not meet statistical significance at the 5% level. To the authors’ knowledge, this analysis is the first to explore the differential role of race in the relationship of PSA bounce with biochemical failure; with the exception of one study focusing on African-Caribbean patients (30), many of the aforementioned series considering the relationship of PSA bounce either have very small representations of patients of African descent (often no more than 10–15% of the sample), which can make it difficult to detect a relationship, or omit race in the analysis altogether.
The reasons that African American men who experience a PSA bounce are more likely to experience a prolonged biochemical recurrence-free survival than their Caucasian counterparts may be driven in part by baseline genomic aspects of their tumors (3), although there is evidence that African American men may be more likely to opt for treatment at earlier stages and their tumors therefore may represent a more radiosensitive phenotype. There is also evidence that androgen receptor expression may be higher in African American patients with prostate cancer as compared with their Caucasian counterparts (31, 32), as well as having a decreased expression level of double-strand break repair, rendering the tumors more radiosensitive (32).
Aside from the potential biological aspects of the tumors by race, there are other clinical and nonclinical factors that may potentially drive this disparity. First, there may be differences in preferences, although the VA is an integrated, equal-access system and there may be differences in treatment preferences by race. For instance, a previous analysis of 125,083 veterans found that African American men with low-risk prostate cancer were more likely to choose active treatment (which may include brachytherapy) over conservative management (33), which may render the distribution of tumors among African American men in this cohort to be more biologically favorable. Furthermore, several studies analyzing racial disparities in VA patient populations have found little to no association of race in cancer survival outcomes (including lung (34) and prostate (35) cancer), potentially driven by the nature of the VA as an open-access, integrated health system.
Although these findings do support further examination of racial differences in response to prostate cancer radiotherapy, there are several limitations to be acknowledged. First, although most veterans seek care completely within the VA system, we did not observe treatment (e.g., definitive therapy or androgen deprivation) or followup (including PSA) among patients who sought care outside of the VA system. We cannot account for the possibility that some patients may have received partial treatment or hormonal therapy outside the VA system and later received brachytherapy inside the VA system, although it is likely that this makes up a very small portion of our patient population. We also did not observe dosimetric characteristics, including measures such as D90, of the brachytherapy implant, which are known to be associated with outcomes. Furthermore, patients from this sample come from only 15 VA centers which had an active brachytherapy program at some point during this period. Finally, there are some facilities and practitioners that have higher volumes than others. As such, we cannot account for potential selection bias because of geographic site or operator dependence.
In conclusion, our findings demonstrate that African Americans treated with brachytherapy for their prostate cancer are more likely than Caucasians to experience a PSA bounce. Our findings support the growing body of evidence that African American patients may have a higher likelihood of harboring tumors that are biologically more sensitive to radiation, including brachytherapy, but overall poorer outcomes in aggregate compared with their Caucasian counterparts. Further research is needed to explore the molecular, genomic, and nonclinical correlates that drive these disparities.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Hunter Holmes McGuire VA Medical Center. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Services in support of the research project were generated by the VCU Massey Cancer Center Biostatistics Shared Resource, supported, in part, with funding from NIH-NCI Cancer Center Support, United States Grant P30 CA016059.
Footnotes
Conflict of interest: The authors have nothing to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- [2].Sundi D, Ross AE, Humphreys EB, et al. African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: Should active surveillance still be an option for them? J Clin Oncol 2013;31:2991–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mahal BA, Alshalalfa M, Spratt DE, et al. Prostate cancer genomic-risk differences between African-American and white men across Gleason scores. Eur Urol 2019;75:1038–1040. [DOI] [PubMed] [Google Scholar]
- [4].Krimphove MJ, Cole AP, Fletcher SA, et al. Evaluation of the contribution of demographics, access to health care, treatment, and tumor characteristics to racial differences in survival of advanced prostate cancer. Prostate Cancer Prostatic Dis 2019;22:125–136. [DOI] [PubMed] [Google Scholar]
- [5].Mahal BA, Berman RA, Taplin M-E, et al. Prostate cancer-specific mortality across Gleason scores in black vs nonblack MenProstate cancer-specific mortality across Gleason scores in black and nonblack MenLetters. JAMA 2018;320:2479–2481. [DOI] [PubMed] [Google Scholar]
- [6].Burt LM, Shrieve DC, Tward JD. Factors influencing prostate cancer patterns of care: An analysis of treatment variation using the SEER database. Adv Radiat Oncol 2018;3:170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rodda S, Tyldesley S, Morris WJ, et al. ASCENDE-rt: An analysis of treatment-related morbidity for a randomized trial comparing a low-dose-rate brachytherapy boost with a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2017;98:286–295. [DOI] [PubMed] [Google Scholar]
- [8].Mahmood U, Pugh T, Frank S, et al. Declining use of brachytherapy for the treatment of prostate cancer. Brachytherapy 2014;13:157–162. [DOI] [PubMed] [Google Scholar]
- [9].Wallner KE, Blasko J, MJ D. Prostate brachytherapy made complicated. Seattle, WA: Smart Medicine Publishing; 1997. [Google Scholar]
- [10].Yamamoto Y, Offord CP, Kimura G, et al. Tumour and immune cell dynamics explain the PSA bounce after prostate cancer brachytherapy. Br J Cancer 2016;115:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bernstein MB, Ohri N, Hodge JW, et al. Prostate-specific antigen bounce predicts for a favorable prognosis following brachytherapy: A meta-analysis. J Contemp Brachytherapy 2013;5:210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mitchell DM, Swindell R, Elliott T, et al. Analysis of prostate-specific antigen bounce after I125 permanent seed implant for localised prostate cancer. Radiother Oncol 2008;88:102–107. [DOI] [PubMed] [Google Scholar]
- [13].Patel C, Elshaikh MA, Angermeier K, et al. PSA bounce predicts early success in patients with permanent iodine-125 prostate implant. Urology 2004;63:110–113. [DOI] [PubMed] [Google Scholar]
- [14].Hinnen KA, Monninkhof EM, Battermann JJ, et al. Prostate specific antigen bounce is related to overall survival in prostate brachytherapy. Int J Radiat Oncol Biol Phys 2012;82:883–888. [DOI] [PubMed] [Google Scholar]
- [15].Charret J, Baumann AS, Eschwege P, et al. Prostate-specific antigen bounce in patients treated before 60 years old by iodine 125 brachytherapy for prostate cancer is frequent and not a prognostic factor. Brachytherapy 2018;17:888–894. [DOI] [PubMed] [Google Scholar]
- [16].Romesser PB, Pei X, Shi W, et al. Prostate-specific antigen (PSA) bounce after dose-escalated external beam radiation therapy is an independent predictor of PSA recurrence, metastasis, and survival in prostate adenocarcinoma patients. Int J Radiat Oncol Biol Phys 2018;100:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Horwitz EM, Levy LB, Thames HD, et al. Biochemical and clinical significance of the posttreatment prostate-specific antigen bounce for prostate cancer patients treated with external beam radiation therapy alone. Cancer 2006;107:1496–1502. [DOI] [PubMed] [Google Scholar]
- [18].Mahal BA, Aizer AA, Ziehr DR, et al. Racial disparities in prostate cancer-specific mortality in men with low-risk prostate cancer. Clin Genitourin Cancer 2014;12:e189–e195. [DOI] [PubMed] [Google Scholar]
- [19].Pettaway CA, Troncoso P, Ramirez EI, et al. Prostate specific antigen and pathological features of prostate cacner in black and white patients: A comparitive study based on radical prostatectomy specimens. J Urol 1998;160:437–442. [PubMed] [Google Scholar]
- [20].Bhardwaj A, Srivastava SK, Khan MA, et al. Racial disparities in prostate cancer: A molecular perspective. Front Biosci (Landmark Ed) 2017;22:772–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Caloglu M, Ciezki JP, Reddy CA, et al. PSA bounce and biochemical failure after brachytherapy for prostate cancer: A study of 820 patients with a minimum of 3 Years of follow-up. Int J Radiat Oncol Biol Phys 2011;80:735–741. [DOI] [PubMed] [Google Scholar]
- [22].Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–526. [Google Scholar]
- [23].Kanzaki H, Kataoka M, Nishikawa A, et al. Kinetics differences between PSA bounce and biochemical failure in patients treated with 125I prostate brachytherapy. Jpn J Clin Oncol 2015;45:688–694. [DOI] [PubMed] [Google Scholar]
- [24].Mazeron R, Bajard A, Montbarbon X, et al. Permanent 125I-seed prostate brachytherapy: Early prostate specific antigen value as a predictor of PSA bounce occurrence. Radiat Oncol 2012;7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Thompson A, Keyes M, Pickles T, et al. Evaluating the Phoenix definition of biochemical failure after 125I prostate brachytherapy: Can PSA kinetics distinguish PSA failures from PSA bounces? Int J Radiat Oncol Biol Phys 2010;78:415–421. [DOI] [PubMed] [Google Scholar]
- [26].Yamoah K, Stone N, Stock R. Impact of race on biochemical disease recurrence after prostate brachytherapy. Cancer 2011;117:5589–5600. [DOI] [PubMed] [Google Scholar]
- [27].Åström L, Sandin F, Holmberg L. Good prognosis following a PSA bounce after high dose rate brachytherapy and external radiotherapy in prostate cancer. Radiother Oncol 2018;129:561–566. [DOI] [PubMed] [Google Scholar]
- [28].Hauck CR, Ye H, Chen PY, et al. Increasing fractional doses increases the probability of benign PSA bounce in patients undergoing definitive HDR brachytherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2017;98:108–114. [DOI] [PubMed] [Google Scholar]
- [29].Engeler DS, Schwab C, Thöni AF, et al. PSA bounce after 125I-brachytherapy for prostate cancer as a favorable prognosticator. Strahlenther Onkol 2015;191:787–791. [DOI] [PubMed] [Google Scholar]
- [30].Leduc N, Atallah V, Creoff M, et al. Prostate-specific antigen bounce after curative brachytherapy for early-stage prostate cancer: A study of 274 African-Caribbean patients. Brachytherapy 2015;14:826–833. [DOI] [PubMed] [Google Scholar]
- [31].Gaston KE, Kim D, Singh S, et al. Racial differences in androgen receptor protein expression in men with clinically localized prostate cancer. J Urol 2003;170:990–993. [DOI] [PubMed] [Google Scholar]
- [32].Spratt DE, Dess RT, Hartman HE, et al. Androgen receptor activity and radiotherapeutic sensitivity in african-American men with prostate cancer: A large scale gene expression analysis and meta-analysis of RTOG trials. Int J Radiat Oncol Biol Phys 2018;102:S3. [Google Scholar]
- [33].Loeb S, Byrne N, Makarov DV, et al. Use of conservative management for low-risk prostate cancer in the veterans Affairs integrated health care system from 2005–2015conservative management of low-risk prostate cancer in the VA system, 2005–2015. JAMA 2018;319:2231–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ganti AK, Subbiah SP, Kessinger A, et al. Association between race and survival of patients with non-small-cell lung cancer in the United States veterans Affairs population. Clin Lung Cancer 2014;15:152–158. [DOI] [PubMed] [Google Scholar]
- [35].Daskivich TJ, Kwan L, Dash A, et al. Racial parity in tumor burden, treatment choice and survival outcomes in men with prostate cancer in the VA healthcare system. Prostate Cancer Prostatic Dis 2015; 18:104. [DOI] [PubMed] [Google Scholar]


