Abstract
Foot complications are common among diabetic patients; foot ulcers are among the more serious consequences. These ulcers frequently become infected, with potentially disastrous progression to deeper spaces and tissues. If not treated promptly and appropriately, diabetic foot infections can become incurable or even lead to septic gangrene, which may require foot amputation. Diagnosing infection in a diabetic foot ulcer is based on clinical signs and symptoms of inflammation. Properly culturing an infected lesion can disclose the pathogens and provide their antibiotic susceptibilities. Specimens for culture should be obtained after wound debridement to avoid contamination and optimise identification of pathogens. Staphylococcus aureus is the most common isolate in these infections; the increasing incidence of methicillin‐resistant S. aureus over the past two decades has further complicated antibiotic treatment. While chronic infections are often polymicrobial, many acute infections in patients not previously treated with antibiotics are caused by a single pathogen, usually a gram‐positive coccus. We offer a stepwise approach to treating diabetic foot infections. Most patients must first be medically stabilised and any metabolic aberrations should be addressed. Antibiotic therapy is not required for uninfected wounds but should be carefully selected for all infected lesions. Initial therapy is usually empirical but may be modified according to the culture and sensitivity results and the patient's clinical response. Surgical intervention is usually required in cases of retained purulence or advancing infection despite optimal medical therapy. Possible additional indications for surgical procedures include incision and drainage of an abscess, debridement of necrotic material, removal of any foreign bodies, arterial revascularisation and, when needed, amputation. Most foot ulcers occur on the plantar surface of the foot, thus requiring a plantar incision for any drainage procedure.
Keywords: Amputation, Antibiotic therapy, Debridement, Diabetic foot infection, Surgical intervention
Introduction
In 2002, the Centers for Disease Control and Prevention reported that diabetes affects 18·2 million Americans or 6·3% of the population (1). Moreover, the World Health Organization predicts that the prevalence of diabetes will continue to grow and estimates that 24·5 million (8·9%) of the United States population will have diabetes by the year 2025 (2). Worldwide, the estimated number of people with diabetes is expected to rise dramatically over the coming decades, climbing to at least 228 million people in developing countries alone and some 300 million in all nations by 2025 (3).
Foot complications among people with diabetes are common. Ulcers, usually on the plantar surface or dorsal toes, are among the most serious and debilitating of these complications. An observational study reported that the cumulative incidence of developing a foot ulcer for patients with diabetes was 5·8% over 3 years (4). Another study showed that 15% of patients with diabetes will develop a foot ulcer during their lifetime (5). About half of all foot ulcers are clinically infected at the time the patient presents to a clinician, and ulcers are the most frequent predisposing factor to foot infections. These foot infections may begin superficially, but if untreated, they can spread to the contiguous subcutaneous tissues. Ultimately, the infectious process may involve muscle, tendon, bone, and joints. These deep infections are potentially disastrous and can rapidly progress to septic gangrene, which may eventually require a lower extremity amputation (6, 7, 8, 9).
At least 60% of non traumatic lower limb amputations occur among people with diabetes (1). In one study, 16% of all patients with foot ulcers (n = 514) and 36% of those who also had osteomyelitis (n = 79) had a lower extremity amputation during the follow‐up period (4). Other studies have shown that patients who have had one amputation have a 68% risk of having another in the next 5 years and have a 50% mortality rate in the 5 years following the initial amputation (10, 11). Thus, it is not surprising that lower extremity amputation is considered to be one of the most serious consequences of diabetes (12). This article reviews the diagnosis, bacteriology and treatment of diabetic foot infections and offers a stepwise approach to effective management that emphasises appropriate use of surgical interventions (Figure 1).
Figure 1.
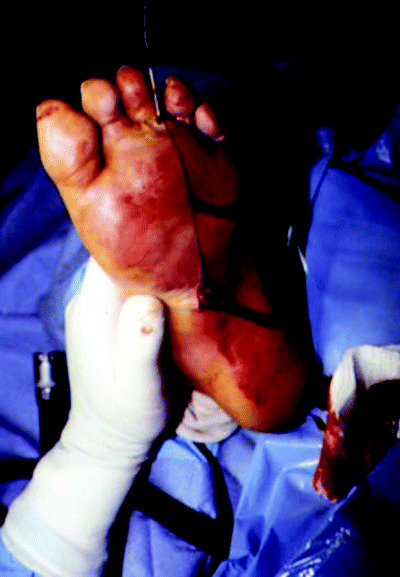
Diabetic foot infection with abscess in medial and central plantar compartments (photo courtesy of David G Armstrong, DPM).
Culture and sensitivity testing
Appropriate antibiotic therapy of a diabetic foot infection usually requires culturing the wound and performing sensitivity testing on isolated pathogens (12, 13). The accuracy of a wound culture depends on obtaining an appropriate specimen (12). This requires careful attention to sterile technique as well as selection of the optimal portion of the wound for sampling (13, 14). Before collecting a culture specimen, the wound should be debrided of all necrotic material and mechanically cleansed. Three methods commonly used for obtaining foot culture samples are swabbing, needle aspiration and wound biopsy. Deep tissue specimens generally are considered to provide the most reliable culture samples in diabetic foot ulcers (14, 15, 16, 17). One study compared culture specimens obtained by methods that minimise the likelihood of contamination (i.e. needle aspiration or biopsy) with those that cannot be obtained without contact with an ulcer or other openly draining lesions (i.e. superficial swabs) (18). Results showed that needle aspiration or biopsy techniques yielded more reliable information (fewer contaminants and more likely pathogens) compared with the swab techniques. Other studies demonstrated similar findings (19, 20, 21).
Superficial swab cultures are often used in the clinical setting, as some believe that they are cost effective, less invasive and adequately diagnostic (13). Two studies have reported that with proper technique, swabbing chronic wounds gave bacterial results similar to those obtained through the use of deep tissue culture techniques (13, 22). Other investigations have shown that the concurrence between quantitative bacteriology of swab and biopsy specimens, while not perfect, is adequate in most cases (23, 24). However, other data suggest that a swab of the infected ulcer can miss many anaerobic and some fastidious bacteria (19, 20, 21, 22). Furthermore, swab specimens are only minimally processed by many microbiology laboratories; the interpretation provided is often ‘mixed normal flora’. With tissue specimens, however, the laboratory usually will attempt to identify all potential pathogens. Whenever possible, the laboratory will culture material either from curettage of a debrided ulcer or a tissue biopsy to guide antibiotic therapy, especially in moderate‐to‐severe wounds (18, 25).
Bacteriology
Diabetic foot infections range in severity from minor superficial lesions to limb‐ or even life‐threatening deep tissue infections (16). Like all open wounds, diabetic foot ulcers are colonised with skin microorganisms (26,27); however, bacterial wound colonisation is not equivalent to infection. Infection is defined as microbial pathogens proliferating in a wound, causing tissue damage and eliciting a host inflammatory response (13). Many environmental and physiological factors simultaneously influence the life cycle of a wound. Some organisms, such as Staphylococcus aureus, are particularly virulent (26, 27, 28); others, such as coagulase‐negative staphylococci and diphtheroids, are relatively avirulent but can occasionally be true pathogens (26, 29, 42). The progression from colonisation of a wound to clinical infection cannot be predicted by the presence of a specific pathogen. Microbiological factors such as the quantity, type and interaction of pathogens present combined with host factors such as immune responses and tissue conditions act together to predispose to infection (27). Thus, infection in a diabetic foot ulcer is identified clinically, based on signs and symptoms (12, 17). The presence of purulent secretions, or at least two classic signs of inflammation, suggest infection. A patient with a wound that shows signs of infection should be referred to a specialist for clinical evaluation and potential treatment. Once a wound is deemed to be infected, a properly obtained culture specimen can define the causative pathogens. Initial antibiotic therapy is typically empirical and based on the clinical severity of the infection and any epidemiological or clinical clues. Definitive antibiotic treatment is based on the patient's clinical response to the empirical therapy, as well as the results of culture and sensitivity testing of the isolated pathogens (12, 17).
Many studies have reported that diabetic foot infections are usually caused by mixed flora (i.e. 3–5 species, including aerobic gram‐positive cocci and gram‐negative rods, as well as obligate anaerobes) (18, 20, 21, 30, 31). These studies were largely comprised of patients who had serious infections that failed to respond to previous antibiotic therapy. In contrast, in less severe infections, a single pathogen (usually a gram‐positive coccus) is more commonly noted (19, 26, 32, 33), most often S. aureus (12, 18, 19, 26, 28, 30, 31, 32, 34). Other frequently isolated aerobes include various Enterobacteriaceae, streptococci (especially groups A and B), enterococci, Proteus species, S. epidermidis, Pseudomonas aeruginosa (30, 32) and corynebacteria (29). Anaerobic species (e.g. Peptostreptococcus, Bacteroides and Clostridium species) are found less frequently (18, 30, 35, 36) but have been isolated from 13·5% (32) to 36% of infections in some studies (18). Infection caused by anaerobes is most frequent in wounds that are necrotic or ischaemic; anaerobic organisms should also be suspected if the wound has a putrid or fetid smell.
The increasing incidence of methicillin‐resistant S. aureus (MRSA) infection has further complicated the already difficult choice of selecting an antibiotic regimen for diabetic foot infections. In a 1999 report, MRSA organisms were isolated from 15% of diabetic foot ulcers in a British foot clinic (32). In 2001, the same group reported that the MRSA isolation rate from diabetic foot ulcers increased to 30% (P < 0·05), despite their infection control efforts (31). In the 1999 study, all patients who had MRSA isolated from their wounds had previously received prolonged antibiotic therapy (32). The time to healing of foot ulcers with MRSA (mean 35·4 [19–64] weeks) was longer than for patients whose ulcers were infected by methicillin‐susceptible S. aureus (mean 17·8 [8–24] weeks) (P = 0·03). Other reports have also found that isolating MRSA from a diabetic foot ulcer is associated with poor wound healing (37) and an increased risk of lower extremity amputation (37, 38).
Stepwise approach to management
Although numerous diabetic foot wound classification systems exist, few specifically focus on infection. Recently, interest in subclassifying wounds based on severity of infection has increased. The infection classification system summarised in the table is part of a larger wound classification system designed by the International Working Group on the Diabetic Foot for clinical research (17). It uses the acronym PEDIS, which stands for perfusion, extent/size, depth/tissue loss, infection and sensation. Each of these factors is assigned a number that corresponds with severity. The classification of the infection aspect is shown in Table 1.
Table 1.
Classification of diabetic foot infections
| GRADE 1 | No symptoms or signs of infection |
| GRADE 2 | Infection involving the skin and the subcutaneous tissue only (without involvement of deeper tissues and without systemic signs as described below). At least two of the following signs are present: |
| •Local swelling or induration | |
| •Erythema >0·5–2 cm around the ulcer | |
| •Local tenderness or pain | |
| •Local warmth | |
| •Purulent discharge (thick, opaque to white secretion). Other causes of an inflammatory response of the skin should be excluded (e.g. trauma, gout, acute Charcot neuro‐osteoarthropathy, fracture, thrombosis and venous stasis) | |
| GRADE 3 | Erythema >2 cm plus one of the signs described above (swelling, tenderness, warmth, discharge) OR |
| Infection involving structures deeper than skin and subcutaneous tissues such as abscess, osteomyelitis, septic arthritis and fasciitis | |
| No systemic inflammatory response signs as described below | |
| GRADE 4 | Any foot infection with the following signs of a systemic inflammatory response syndrome (SIRS). This response is manifested by two or more of the following conditions: |
| •Temperature >38°C or <36°C | |
| •Heart rate >90 beats/minute | |
| •Respiratory rate >20 breaths/minute | |
| •PaCO2 <32 mmHg | |
| •White blood cell count >12·000 or <4·000/mm3 | |
| •10% immature (band) forms |
Adapted from (17) with permission from IDF Consultative Section on the Diabetic Foot/IWGDF. Copyright © 2003, IDF Consultative Section on the Diabetic Foot/IWGDF. All rights reserved.
Many diabetic foot infections are superficial, that is, they do not extend below the subcutaneous fascia. However, some foot infections are complicated by deep soft tissue involvement. Clues to deep infection may include an unexplained delay in the healing process, the presence of a purulent discharge, a fullness in the plantar space, unexpected pain or tenderness in a previously insensate foot, or a deep sinus tract (39). Of even greater concern are imminently limb‐ or life‐threatening deep tissue infections. These may be characterised by superficial bullae, petechiae or ecchymoses, soft tissue crepitus, rapid spread of infection and tissue gas on X‐rays (39, 40). All but the most superficial and mild infections should be clinically and radiographically evaluated for the presence of deeper involvement (16, 39).
Deep tissue infections rarely respond to antimicrobial therapy alone and generally require surgical procedures (41). In patients with evidence of systemic toxicity (e.g. fever, leucocytosis and severe metabolic aberrations), urgent intervention may be needed. If there is any possibility of retained purulence (e.g. an abscess, especially under pressure), compartment syndrome or advancing infection despite appropriate antimicrobial therapy, surgical exploration should be considered. Possible surgical interventions include incision and drainage, wound debridement, bone resection, tissue revascularisation and amputation.
Incision
Most deep foot infections require incision and drainage. The most common site of foot ulceration in diabetic patients is the plantar surface (42). Some of the earliest work detailing the surgical anatomy of the plantar fascial spaces of the foot was that of Grodinsky, published in 1929 (43). Among several smaller fascial spaces, he identified three major plantar spaces. These included the medial, central (superficial and deep) and lateral spaces. Although the logical approach to draining infections in these spaces would be a plantar incision, a scar on the plantar surface of the foot might be a source of discomfort; thus, Grodinsky recommended a medial approach. However, experience has shown that with careful tissue dissection and handling, a plantar incision can drain infection without a sensitive scar (44).
A plantar incision typically begins posterior to the medial malleolus and extends laterally and distally towards the midline, ending between the heads of the first and second metatarsals (44). Any portion of this incision can be used for surgical debridement or drainage, depending on the area of infectious involvement. For example, as shown in Figure 2, an incision for an infection in the central plantar space might end distally between the third and fourth metatarsals. Most often, only a portion of this incisional approach is required for adequate exposure.
Figure 2.
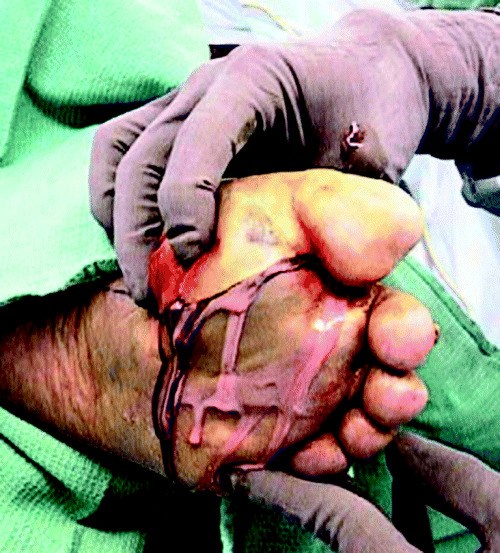
Skin incision for plantar approach to foot infections (photo courtesy of David G Armstrong, DPM).
The incision can then be carried through the plantar aponeurosis to pass through the medial and central spaces. From there it can go through the interval between the abductor hallucis and flexor digitorum brevis, approaching the deep central space where the plantar arteries and nerves are located (44). Detaching the abductor hallucis and flexor digitorum brevis from the calcaneus by means of anterior retraction allows visualisation of the quadratus plantae muscles. The deep aspect of the central compartment is entered after separating the flexor hallucis longus tendon from the quadratus, and the dissection is then carried distally to visualise the plantar nerves. Figure 3 illustrates the plantar spaces of the foot.
Figure 3.
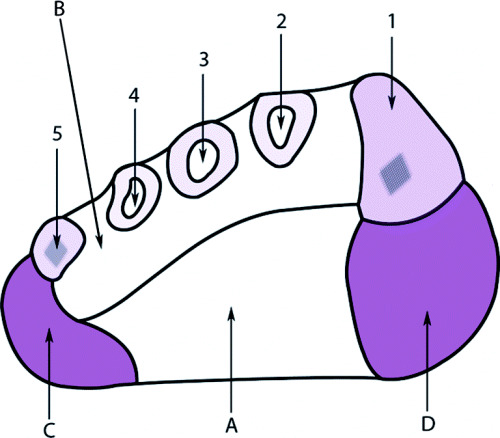
Schematic diagram of a cross‐section of the foot. Numbers 1–5 indicate metatarsals; A, central plantar space; B, deep interosseous space; C, lateral plantar space; D, medial plantar space (44).
Debridement
Devitalised tissue in a wound can delay healing, predispose to infection and interfere with adequate assessment (39, 45, 46, 47, 48). Removal of devitalised tissue and callus is generally accomplished by debridement. The most commonly used technique for diabetic foot ulcers is sharp or surgical debridement using a scalpel, tissue forceps or similar instrument (49, 50, 51). Surgical debridement of non viable tissue exposes the healthy tissue; this helps to begin wound healing, reduces the risk of infection by removing microbial contaminants, decreases wound malodor (27) and has been associated with shortening healing times (52). Patients should be warned in advance to expect bleeding, and that with the full extent of the wound exposed, the lesion will be larger than before the procedure.
Because surgical debridement should be sufficiently extensive to remove all infected and necrotic tissue, the procedure may require more than one session (14). For some patients, weekly debridement may be needed as part of routine local wound care for diabetic ulcers (25). Sharp debridement is the most controlled and efficient method. However, alternative debridement techniques may be preferred in some circumstances, such as in patients for whom bleeding is a concern, when necessary anaesthesia is unavailable or the clinic's facilities are inadequate to perform aggressive surgical debridement. In these instances, consider non surgical debridement methods, including wet‐to‐dry dressings, various types of topical enzymes or moisture‐retentive dressings (39, 48, 55, 56, 57).
Investigation
Following debridement, the ulcer should be gently but thoroughly examined with a sterile metal probe. The examiner should determine the wound's depth and seek the presence of any foreign bodies, abscesses, sinus tracts or exposed bone (39, 58). In cases where the ulcer extends to the bone, osteomyelitis or joint infection are often present (39).
Wound lavage
Wound infection has been reported to occur after 15% of surgical cases classified as ‘contaminated’ and 40% of those classified as ‘dirty’ (59). The number of bacteria present in the wound margins at the end of surgery appears to be the most important factor in determining the likelihood of a wound infection (58, 61). Thus, proper cleansing and preparation of a wound should lower its bacterial counts (58). Energetic rinsing of the wound can eliminate necrotic tissue and blood clots that may interfere with natural defense mechanisms. Thus, wound irrigation, either alone or in combination with antibiotic prophylaxis, is widely used to prevent postoperative wound infection.
Animal studies have produced variable results using saline irrigation alone on infected wounds. In one study, saline irrigation significantly decreased aerobic and anaerobic bacterial counts and subsequent infection, compared with untreated controls (P < 0·001) (58). In another study, saline irrigation alone reduced bacterial wound contamination at a rate similar to that of irrigation with povidone iodine solution, cefazolin solution or no irrigation (60). In addition, scrubbing the wound with an antiseptic combined with povidone iodine or cefazolin irrigation solutions before irrigation resulted in a statistically significant decrease in bacterial counts compared with controls. Recent animal studies also show that tap water may be as effective as normal saline, which has normally been used for irrigation (61, 62). In a study of 46 patients with hand lacerations admitted to an emergency department, there was no difference in the number of infected wounds at the 48‐hour follow‐up visit between those irrigated with tap water and those irrigated with normal saline at the time of repair (63). Thus, wound irrigation with saline or water as a complement to prophylactic systemic antibiotics appears to be safe in reducing wound infection rates before closing contaminated wounds, such as diabetic foot ulcers.
Antibiotic treatment
Opinions vary on the most appropriate use of antibiotics for diabetic foot infections. This is especially so because of the concern about increasing antibiotic resistance in infection treatment and control (64). For clinically uninfected wounds, antibiotic therapy usually is not necessary (17). However, when the clinical presentation indicates infection, antibiotic therapy is virtually always needed. This should be directed at the most commonly identified pathogens and should be started promptly (12, 26). The initial regimen is typically empirical but may be targeted more specifically after culture and sensitivity results are available. For severe infection, parenteral broad‐spectrum antibiotics that have been proven clinically effective for diabetic foot infections are recommended; these include imipenem/cilastatin, newer fluoroquinolones (e.g. levofloxacin and ciprofloxacin), third‐ or fourth‐generation cephalosporins (e.g. ceftazidime and cefuroxime) and beta‐lactam/beta‐lactamase inhibitors (e.g. ampicillin/sulbactam, piperacillin/tazobactam) (33). In addition, agents with activity against MRSA, such as vancomycin or linezolid, should be considered for patients at risk of infection with MRSA, in light of its association with worse clinical outcomes (32, 37, 38, 67). Patients with less serious infections (i.e. mild‐to‐moderate and not life‐threatening) who have not been treated with antibiotics can generally be treated on an outpatient basis with narrower spectrum oral antibiotics (19). There is insufficient evidence available to recommend topical antibiotics for superficial ulcers (25).
Wound closure
Surgery involving heavily contaminated wounds, such as amputation due to gangrenous diabetic foot ulcers, carries a risk of wound breakdown. This may require revision of the amputation to a higher level (66). Primary closure means the skin is closed at the end of surgery, allowing healing by primary intention. In secondary closure, the wound is left open at the end of the surgery, and heals by granulation and contraction. A study by Fisher and associates (67) reported fewer wound complications attributable to the surgical technique (P = 0·05) in patients undergoing a delayed primary closure (i.e. closure a few days after the first amputation and infection drainage) rather than a guillotine amputation followed by more proximal revision with primary closure at the time of the revisional procedure. Ultimately, the choice for primary, delayed primary or healing by secondary intention is contingent upon (a) the degree of drainage at the time of decision making, (b) the amount of tissue available to close the wound and (c) any residual infection distal to the site of intended wound closure. Unless an amputation is performed considerably proximal to the area of infection, wounds rarely are primarily closed at the time of incision and debridement of infection.
Local signs and symptoms of infection, or any evidence of systemic toxicity, often dictate the decision on timing of delayed primary closure. The most reliable single indicator of success for delayed primary closure in the well‐perfused extremity is the absence of substantial wound drainage (68). In many complex wounds, delayed primary closure is used in tandem with healing by secondary intention. Topical negative pressure therapy, full‐ and split‐thickness grafts, and local and distant flaps also are useful adjuncts for closing deep complex wounds following debridement.
Revascularisation
Arterial perfusion is necessary for healing and antibiotic delivery; therefore, ischaemia should be suspected in patients with diabetic foot ulcers that fail to heal after appropriate stepwise management (14). Other indications suggesting lower‐extremity ischaemia include symptoms of leg claudication, absence of palpable foot pulses, hair loss, poor capillary refill, skin atrophy and nail cornification (14, 69). A patient with a non healing ulcer in whom ischaemia is suspected should be referred to a vascular surgeon to determine whether the patient is a suitable candidate for a revascularisation procedure. These include various types of percutaneous transluminal angioplasty or a surgical bypass graft (14). Limb revascularisation success rates in patients with diabetes have been shown to be comparable to those in patients without diabetes. These procedures have helped to heal ulcerations and eliminate pain, often permitting a return of function, improved well‐being and a decreased need for amputation at all levels (70).
Amputation
Every effort should be made to avoid an amputation, especially at a high limb level. However, in some cases, amputation is needed to save the rest of a patient's limb or even his life. Indications for lower‐extremity amputation in patients with diabetes may include extensive gangrene, peripheral arterial occlusion, a non healing ulcer, severe soft tissue infection and extensive osteomyelitis (5). Risk factors for a patient with diabetes to require an amputation include lower‐extremity ischaemia, peripheral neuropathy, elevated glycated haemoglobin levels, a history of foot ulcers and retinopathy. Although the decision to amputate is difficult for both patient and provider, it is sometimes preferred when a patient has undergone unsuccessful treatment over a long period (14). Unless the patient has a life‐threatening infection that requires emergency amputation, taking time for counselling may help the patient in the decision‐making process.
After a decision has been made to amputate, the goal should be to perform the most distal amputation that will heal (71). Larsson et al. (72) conducted a prospective study in 189 patients with diabetes who had achieved healing of either a minor (i.e. below the ankle) or major (i.e. above the ankle) amputation. Investigators found that major amputations were associated with greater mortality (P < 0·001), shorter recovery time (as indicated by return to previous walking capacity [P < 0·001] and previous living condition [P < 0·001]) and a decreased requirement of new major amputations (P < 0·01), but no difference in the overall rate of re‐amputation.
Conclusion
Foot ulcers and their consequent infections are a common and serious cause of morbidity in patients with diabetes. Properly identifying and counselling persons at risk of ulceration or infection can prevent the dire consequences of diabetic foot ulcers, such as lower‐extremity amputation. Similarly, aggressive and appropriate assessment and treatment of ulcers and infections can improve patient outcomes. These measures include proper surgical debridement, drainage and wound lavage. Obtaining appropriate specimens from infected wounds for culture and sensitivity testing can help to direct antibiotic therapy. For patients whose wounds do not heal after adequate treatment with this stepwise approach, the best long‐term outcome may be achieved through revascularisation or, in some cases, judicious amputation.
References
- 1. Centers for Disease Control and Prevention, US Department of Health and Human Services . National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2003. Atlanta, GA: US Centers for Disease Control and Epidemiology; 2003. [Google Scholar]
- 2. King H, Aubert RD, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates and projections. Diabetes Care 1998; 21: 1414–31. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization . Global burden of diabetes: WHO projects a 170% growth in the number of people with diabetes in developing countries by 2025. World Health Organization 1998 (retrieved January 26, 2004, from http://www/who.int/inf‐pr‐1998/en/pr98‐63.html).
- 4. Ramsey SD, Newton K, Blough D, McCollough DK, Sandhu N, Reiber G et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999;22: 382–7. [DOI] [PubMed] [Google Scholar]
- 5. Reiber GE, Boyko EJ, Smith DG. Lower extremity foot ulcers and amputations in diabetics. In: Diabetes in America, 2nd edn. Rockville, MD: National Institute of Diabetes and Digestive and Kidney Disease, National Institutes of Health, 1995: 409–28. [Google Scholar]
- 6. Armstrong DG, Lavery LA, Harkless LB, Van Houtum WH. Amputation and reamputation of the diabetic foot. J Am Podiatr Med Assoc 1997;87: 255–9. [DOI] [PubMed] [Google Scholar]
- 7. Durham JR, McCoy DM, Sawchuk AP, Meyer JP, Schwarcz TH, Eldrup‐Jorgensen J et al. Open transmetatarsal amputation in the treatment of severe foot infections. Am J Surg 1989;158: 127–30. [DOI] [PubMed] [Google Scholar]
- 8. McIntyre KE. Control of infections in the diabetic foot. the role of microbiology, immunopathy, antibiotics and guillotine amputation. J Vasc Surg 1987;5: 787–90. [DOI] [PubMed] [Google Scholar]
- 9. Tan JS, Friedman NM, Hazelton‐Miller C, Flanagan JP, File TM. Can aggressive treatment of diabetic foot infections reduce the need for above‐ankle amputations? Clin Infect Dis 1996;23: 286–91. [DOI] [PubMed] [Google Scholar]
- 10. Goldner MG. The fate of the second leg in the diabetic amputee. Diabetes 1960;9: 100–3. [DOI] [PubMed] [Google Scholar]
- 11. Whitehouse FW, Jurgensen C, Block MA. The later life of the diabetic amputee: another look at the fate of the second leg. Diabetes 1968;17: 520–1. [DOI] [PubMed] [Google Scholar]
- 12. Lipsky BA. A current approach to diabetic foot infections. Curr Infect Dis Rep 1999;1: 253–60. [DOI] [PubMed] [Google Scholar]
- 13. Neil JA, Munro CL. A comparison of two culturing methods for chronic wounds. Ostomy Wound Manage 1997;43: 20–30. [PubMed] [Google Scholar]
- 14. Calhoun JH, Overgaard KA, Stevens CM, Dowling JPF, Mader JT. Diabetic foot ulcers and infections: current concepts. Adv Skin Wound Care 2002; 15: 31–45. [DOI] [PubMed] [Google Scholar]
- 15. Pellizzer G, Strazzabosco M, Presi S, Furlan F, Lora L, Benedetti P et al. Deep tissue biopsy vs. superficial swab culture monitoring in the microbiological assessment of limb‐threatening diabetic foot infection. Diabet Med 2001;18: 822–7. [DOI] [PubMed] [Google Scholar]
- 16. Lipsky BA. Diabetic foot infections: pathophysiology, diagnosis, and treatment. Int J Dermatol 1991;30: 560–2. [PubMed] [Google Scholar]
- 17. International Working Group on the Diabetic Foot . International consensus on the diabetic foot [CD‐ROM]. Amsterdam, The Netherlands: International Diabetes Federation, 2003. [Google Scholar]
- 18. Wheat LJ, Allen SD, Henry M, Kernek CB, Siders JA, Kuebler T et al. Diabetic foot infections: bacteriologic analysis. Arch Intern Med 1986;246: 1935–40. [PubMed] [Google Scholar]
- 19. Lipsky BA, Pecoraro RE, Larson SA, Hanley ME, Ahroni JH. Outpatient management of uncomplicated lower‐extremity infections in diabetic patients. Arch Intern Med 1990;150: 790–7. [PubMed] [Google Scholar]
- 20. Sapico FL, Canawati HN, Witte JL, Montgomerie JZ, Wagner FW Jr, Bessman AN. Quantitative aerobic and anaerobic bacteriology of infected diabetic feet. J Clin Microbiol 1980;12: 413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sapico FL, Witte JL, Canawati HN, Montgomerie JZ, Bessman AN. The infected foot of the diabetic patient: quantitative microbiology and analysis of clinical features. Rev Infect Dis 1984;6 (Suppl. 1): S171–6. [DOI] [PubMed] [Google Scholar]
- 22. Perry CR, Pearson RL, Miller GA. Accuracy of cultures of material from swabbing of the superficial aspect of the wound and needle biopsy in the preoperative assessment of osteomyelitis. J Bone Joint Surg 1991;73‐A: 745–9. [PubMed] [Google Scholar]
- 23. Bill TJ, Ratliff CR, Donovan AM, Knox LK, Morgan RF, Rodeheaver GT. Quantitative swab culture versus tissue biopsy: a comparison in chronic wounds. Ostomy Wound Manage 2001;47: 34–7. [PubMed] [Google Scholar]
- 24. Stotts NA. Determination of bacterial bioburden in wounds. Adv Wound Care 1995;8: 46–52. [PubMed] [Google Scholar]
- 25. Apelqvist J, Bakker K, Van Houten WH, Nabuurs‐Franssen MH, Schaper NC, on behalf of the International Working Group on the Diabetic Foot . International consensus and practical guidelines on the management and the prevention of the diabetic foot. Diabet Metabol Res Rev 2000;16 (Suppl. 1):S84–92. [DOI] [PubMed] [Google Scholar]
- 26. El‐Tahawy AT. Bacteriology of diabetic foot infections. Saudi Med J 2000;21: 344–7. [PubMed] [Google Scholar]
- 27. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001; 14: 244–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lipsky BA, Pecoraro RE, Wheat LJ. The diabetic foot: soft tissue and bone infection. Infect Dis Clin North Am 1990;4: 409–32. [PubMed] [Google Scholar]
- 29. Bessman AN, Geiger PJ, Canawati H. Prevalence of Corynebacteria in diabetic foot infections. Diabetes Care 1992;15: 1531–3. [DOI] [PubMed] [Google Scholar]
- 30. Armstrong DG, Liswood PJ, Todd WF. Prevalence of mixed infections in the diabetic pedal wound: a retrospective review of 112 infections. J Am Podiatr Med Assoc 1995;85: 533–7. [DOI] [PubMed] [Google Scholar]
- 31. Dang CN, Prasad YDM, Boulton AJM, Jude EB. Methicillin‐resistant Staphylococcus aureus in the diabetic foot clinic: a worsening problem. Diabet Med 2003;20: 159–61. [DOI] [PubMed] [Google Scholar]
- 32. Tentolouris N, Jude EB, Smirnof I, Knowles EA, Boulton AJM. Methicillin‐resistant Staphylococcus aureus: an increasing problem in a diabetic foot clinic. Diabet Med 1999;16: 767–71. [DOI] [PubMed] [Google Scholar]
- 33. Lipsky BA, Baker PD, Landon GC, Fernau R. Antibiotic therapy for diabetic foot infections: comparison of two parenteral‐to‐oral regimens. Clin Infect Dis 1997;24: 643–8. [DOI] [PubMed] [Google Scholar]
- 34. Lipsky BA, Berendt AR. Principles and practice of antibiotic therapy of diabetic foot infections. Diabetes Metab Res Rev 2000;16 (Suppl. 1):S42–6. [DOI] [PubMed] [Google Scholar]
- 35. Johnson S, Lebahn F, Peterson LR, Gerding DN. Use of an anaerobic collection and transport swab device to recover anaerobic bacteria from infected foot ulcers in diabetes. Clin Infect Dis 1995;20 (Suppl. 2):S289–90. [DOI] [PubMed] [Google Scholar]
- 36. Gerding DN. Foot infections in diabetic patients: the role of anaerobes. Clin Infect Dis 1995;29 (Suppl. 2): S283–8. [DOI] [PubMed] [Google Scholar]
- 37. Wagner A, Reike H, Angelkort B. Highly resistant pathogens, especially methicillin‐resistant Staph aureus, in diabetic foot infections. Dtsch Med Wochenschr 2001;126: 1353–6. [DOI] [PubMed] [Google Scholar]
- 38. Fejfarova, V , Jirkovaska A, Skibova J, Petkov V. Pathogen resistance and other risk factors in the frequency of lower limb amputations with the diabetic foot syndrome. Vnitr Lek 2002;48: 302–6. [PubMed] [Google Scholar]
- 39. Boulton AJ, Meneses P, Ennis WJ. Diabetic foot ulcers: a framework for prevention and care. Wound Repair Regen 1999;7: 7–16. [DOI] [PubMed] [Google Scholar]
- 40. Eneroth M, Larsson J, Apelqvist J. Deep foot infections in patients with diabetes and foot ulcer: an entity with different characteristics, treatments, and prognosis. J Diabetes Complications 2000;13: 254–63. [DOI] [PubMed] [Google Scholar]
- 41. Caputo GM, Cavanaugh PR, Ulbrecht JS, Gibbons GW, Karchmer AW. Assessment and management of foot disease in patients with diabetes. N Engl J Med 1994;331: 854–60. [DOI] [PubMed] [Google Scholar]
- 42. Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. Diabetes Care 1998;21: 855–9. [DOI] [PubMed] [Google Scholar]
- 43. Loeffler RD, Ballard A. Plantar fascial spaces of the foot and a proposed surgical approach. Foot Ankle 1980;1: 11–14. [DOI] [PubMed] [Google Scholar]
- 44. Grodinsky M. A study of the fascial spaces of the foot and their bearing on infections. Surg Gynecol Obstet 1929;49: 739–51. [Google Scholar]
- 45. Jones V. Debridement of diabetic foot lesions. The Diabetic Foot 1998;1: 88–94. [Google Scholar]
- 46. Rauwerda JA. Foot debridement: anatomic knowledge is mandatory. Diabet Metabol Res Rev 2000;16 (Suppl. 1):S23–6. [DOI] [PubMed] [Google Scholar]
- 47. Sibbald RG, Williamson D, Orstead HL, Campbell K, Keast D, Krasner D et al. Preparing the wound bed – debridement, bacterial balance, and moisture balance. Ostomy Wound Manage 2000; 46: 14–35. [PubMed] [Google Scholar]
- 48. Singhal A, Reis ED, Kerstien MD. Options for nonsurgical debridement of necrotic wounds. Adv Skin Wound Care 2001;14: 96–103. [DOI] [PubMed] [Google Scholar]
- 49. Murray HJ, Young MJ, Hollis S, Boulton AJ. The association between callus formation, high pressures and neuropathy in diabetic foot ulceration. Diabetic Med 1996;13(11):979–82. [DOI] [PubMed] [Google Scholar]
- 50. Pitei DL, Foster A, Edmonds M. The effect of regular callus removal on foot pressures. J Foot Ankle Surg 1999;38(4):251–5. [DOI] [PubMed] [Google Scholar]
- 51. Young MJ, Cavanagh PR, Thomas G, Johnson MM, Murray H, Boulton AJ. The effect of callus removal on dynamic plantar foot pressures in diabetic patients. Diabet Med 1992;9(1):55–7. [DOI] [PubMed] [Google Scholar]
- 52. Steed DL, Donohoe D, Webster MW, Lindsley L and the Diabetic Ulcer Study Group . Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. J Am Coll Surg 1996; 183: 61–4. [PubMed] [Google Scholar]
- 53. Smith J, Thow J. Is debridement effective for diabetic foot ulcers? A systematic review 1. Diabetic Foot 2001;4: 10–4. [Google Scholar]
- 54. Smith J, Thow J. Is debridement effective for diabetic foot ulcers? A systematic review 2. Diabetic Foot 2001;4: 77–80. [Google Scholar]
- 55. Smith J, Thow J. Update of systematic review on debridement. The Diabetic Foot 2003;6: 12–6. [Google Scholar]
- 56. Grayson ML, Gibbons GW, Balough K, Levin E, Karchmer AW. Probing to bone in infected pedal ulcers: a clinical sign of underlying osteomyelitis in diabetic patients. JAMA 1995;273: 721–3. [PubMed] [Google Scholar]
- 57. Cruse PJE, Foord R. The epidemiology of wound infection: a 10 year prospective study of 62,939 wounds. Surg Clin North Am 1980;60: 27–40. [DOI] [PubMed] [Google Scholar]
- 58. Badia JM, Torres JM, Tur C, Sitges‐Serra A. Saline wound irrigation reduces the postoperative infection rate in guinea pigs. J Surg Res 1996;63: 457–9. [DOI] [PubMed] [Google Scholar]
- 59. Raahave D. Bacterial density in laparotomy wounds during gastro‐intestinal operations. Scand J Gastroenterol 1976;37: 135–42. [PubMed] [Google Scholar]
- 60. Howell JM, Stair TO, Howell AW, Mundt DJ, Falcone A, Peters SR. The effect of scrubbing and irrigation with normal saline, povidone iodine, and cefazolin on wound bacterial counts in a guinea pig model. Am J Emerg Med 1993;11: 134–8. [DOI] [PubMed] [Google Scholar]
- 61. Moscati R, Mayrose J, Fincher L, Jehle D. Comparison of normal saline with tap water for wound irrigation. Am J Emerg Med 1998;16: 370–81. [DOI] [PubMed] [Google Scholar]
- 62. Moscati R, Reardon R, Lerner E, Mayrose J. Wound irrigation with tap water. Acad Emerg Med 1998;5: 1076–80. [DOI] [PubMed] [Google Scholar]
- 63. Bansal BC, Wiebe RA, Perkins SD, Abramo TJ. Tap water for irrigation of lacerations. Am J Emerg Med 2002;29: 469–72. [DOI] [PubMed] [Google Scholar]
- 64. Hart CA. Antibiotic resistance: an increasing problem? Br Med J 1998;316: 1255–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lipsky BA, Itani K, Norden C, and the Linezolid Diabetic Foot Infections Study Group . Treating foot infections in diabetic patients: a randomized, multicenter, open‐label trial of linezolid versus ampicillin‐sulbactam/amoxicillin‐clavulanate. Clin Infect Dis 2004;38: 17–24. [DOI] [PubMed] [Google Scholar]
- 66. Senkowsky J, Money MK, Kerstein MD. Lower extremity amputation: open versus closed. Angiology 1990;41: 222–7. [DOI] [PubMed] [Google Scholar]
- 67. Fisher DF, Clagett GP, Fry RE, Humble TH, Fry WJ. One‐stage versus two‐stage amputation for wet gangrene of the lower extremity: a randomized study. J Vasc Surg 1998;8: 428–33. [PubMed] [Google Scholar]
- 68. Armstrong DG, Attinger CE, Boulton AJM, Frykberg RG, Kirsner RS, Lavery LA et al. Guidelines regarding topical negative pressure (VAC) therapy in the diabetic foot: results of the Tucson Expert Consensus Conference on Negative Pressure Wound Therapy. Ostomy Wound Manage 2004;50(4 Suppl 1B):3S–27S. [PubMed] [Google Scholar]
- 69. Gibbons GW. Vascular evaluation and long‐term, results of distal bypass surgery in patients with diabetes. Clin Podiatr Med Surg 1995;12: 129–39. [PubMed] [Google Scholar]
- 70. Gibbons GW, Burgess AM, Guadagnoli E, Pomposelli FB Jr, Freeman DV, Campbell DR et al. Return to well‐being and function after infrainguinal revascularization. J Vasc Surg 1995; 21: 35–45. [DOI] [PubMed] [Google Scholar]
- 71. American Diabetes Association . Consensus development conference on diabetic foot wound care: 7–8 April 1999, Boston Massachusetts. Diabetes Care 1999;22: 1354–60. [DOI] [PubMed] [Google Scholar]
- 72. Larsson J, Agardh C‐C, Apelqvist J, Stenstrom A. Long‐term prognosis after healed amputation in patients with diabetes. Clin Orthop 1998;350: 149–58. [PubMed] [Google Scholar]


