Abstract
Randomised clinical trials (RCTs) to evaluate diabetic foot wound therapies have systematically eliminated large acute wounds from evaluation, focusing only on smaller chronic wounds. The purpose of this study was to evaluate the proportion and rate of wound healing in acute and chronic wounds after partial foot amputation in individuals with diabetes treated with negative pressure wound therapy (NPWT) delivered by the vacuum‐assisted closure (VAC®) device or with standard wound therapy (SWT). This study constitutes a secondary analysis of patients enrolled in a 16‐week RCT of NPWT: 162 open foot amputation wounds (mean wound size = 20·7 cm2) were included. Acute wounds were defined as the wounds less than 30 days after amputation, whereas chronic wounds as the wounds greater than 30 days. Inclusion criteria consisted of individuals older than 18 years, presence of a diabetic foot amputation wound up to the transmetatarsal level and adequate perfusion. Wound size and healing were confirmed by independent, blinded wound evaluators. Analyses were done on an intent‐to‐treat basis. There was a significantly higher proportion of acute wounds (SWT = 59; NPWT = 63) than chronic wounds (SWT = 26; NPWT = 14), evaluated in this clinical trial (P = 0·001). There was no significant difference in the proportion of acute and chronic wounds achieving complete wound closure in either treatment group. Despite this finding, the Kaplan–Meier curves demonstrated statistically significantly faster healing in the NPWT group in both acute (P = 0·030) and chronic wounds (P = 0·033). Among the patients treated with NPWT via the VAC, there was not a significant difference in healing as a function of chronicity. In both the acute and the chronic wound groups, results for patients treated with NPWT were superior to those for the patients treated with SWT. These results appear to indicate that wound duration should not deter the clinician from using this modality to treat complex wounds.
Keywords: Amputation, Diabetes, Ulcer, VAC, Wound
Introduction
Diabetic foot wounds are frequently the cause of morbidity, including infection, gangrene and amputation 1, 2. Preventing these wounds is of critical importance, and in recent years, renewed attention has been focus on wound healing (3). Negative pressure wound therapy (NPWT), delivered through the VAC System™ (Vacuum‐Assisted Closure; KCI USA, San Antonio, TX) (4), is one of the most promising modalities to heal complex wounds. A recent randomised clinical trial (RCT) evaluated clinical outcomes of NPWT and standard wound therapy (SWT) in the treatment of acute and chronic wounds after partial foot amputation in individuals with diabetes. Results suggested that VAC therapy heals a higher proportion of wounds and accomplishes the healing faster than high‐quality standard moist wound healing (5). While these data appear promising, they do not include an analysis of wounds based on age.
When considering healing based on wound ‘age’, we might expect acute wounds to heal faster when treated with either NPWT or SWT. However, we are unaware of any studies that have evaluated this hypothesis in a large population. Previous RCTs on diabetic foot wound have systematically eliminated large acute wounds from evaluation, focusing only on smaller chronic wounds 6, 7, 8, 9, 10. This study compares the wounds studied in the aforementioned RCT in an attempt to determine if chronicity determines clinical outcomes.
Dichotomising the study population into acute and chronic wounds was not part of the a priori analysis. While the average duration of wounds was similar in both NPWT and SWT treatment arms, we planned a post hoc analysis to evaluate whether there was a difference in the proportion of ‘acute’ versus ‘chronic’ wounds treated in each arm of the study and whether the wound chronicity resulted in a difference in response.
Methods
This analysis was conducted as part of a 16‐week (112 day) multicentre, randomised study comparing NPWT as delivered using the VAC System™ and SWT (including alginates, hydrocolloids, foams or hydrogels) in the treatment of diabetic foot amputation wounds. Eighteen study sites participated in the clinical trial. The study was approved by the presiding institutional review board at each site. One hundred sixty‐two subjects were recruited and randomised, with 85 study patients randomised to the SWT arm and 77 patients randomised to the NPWT treatment arm. Wounds were stratified according to wound duration. For wounds that were less than or equal to 30 days after surgery, the wound classification was considered as acute. Conversely, wounds that were greater than 30 days in age were considered as chronic. Further explanation of these methods is described in a previous study (5).
The VAC System™ is a non invasive wound closure system that uses controlled, localised negative pressure to help promote healing in chronic and acute wounds. It uses a latex‐free and sterile polyurethane or polyvinyl alcohol foam dressing that is fitted at the bedside to the appropriate size for each patient’s wound and then covered with an adhesive drape to create an airtight seal. Overlying the foam within the drape, a TRAC Pad® (Kinetic Concepts Inc., San Antonio, TX, USA) accurately senses, monitors, and maintains the target pressure at the wound site to provide controlled negative pressure. Tubing attached to the TRAC Pad® connects to a fluid collection canister contained within a programmable, portable computer‐controlled vacuum pump, creating a negative pressure at the wound surface interface 11, 12, 13, 14, 15.
Inclusion criteria for the study consisted of individuals aged ≥18 years, presence of a diabetic foot amputation wound up to the transmetatarsal level of the foot and evidence of adequate perfusion. Adequate perfusion was defined as either transcutaneous oxygen measurements on the dorsum of the foot ≥30 mmHg or ankle brachial indices ≥0·7 and ≤1·2 and toe pressure ≥30 mmHg.
Exclusion criteria included active Charcot arthropathy of the foot, wounds resulting from burns or venous insufficiency. Also excluded were patients presenting with untreated cellulitis or osteomyelitis (following amputation), collagen vascular disease, malignancy in the wound or uncontrolled hyperglycaemia (haemoglobin A1c > 12%). Patients were excluded if they were being treated with corticosteroids, immunosuppressive medications or chemotherapy. Previous treatment with the VAC System™ within the past 30 days, present or previous treatment with growth factors, normothermic therapy, hyperbaric medicine or bioengineered tissue products within the past 30 days also constituted exclusionary criteria.
Patients randomised to NPWT received therapy delivered through the VAC System™ with dressing changes every 48 hours according to standardised treatment guidelines. Wounds were treated with NPWT until the wound was closed or until the completion of the 112‐day period of assessment. Patients randomised to the SWT arm were treated with moist wound therapy using alginates, hydrocolloids, foams or hydrogels according to standardised guidelines at the discretion of the attending clinician (16).
All patients received off‐loading therapy, preventatively and therapeutically, as indicated. A pressure relief walker or sandal (Active Offloading Walker; Royce Medical, Camarillo, CA) was provided for all patients. Contraindications and considerations associated with off‐loading therapy were evaluated and determined by the principal investigator at each study site.
To assess sensory neuropathy, subjects were evaluated with the 10 g (5·07) Semmes‐Weinstein, monofilament test or vibration perception threshold (VPT) testing using previously published criteria for diagnosis of loss of protective sensation 2, 17, 18, 19. Sharp debridement was used to excise non viable tissue within 2 days of randomisation and as deemed necessary by the treating physician throughout the course of the study. Likewise, the decision for surgical closure of amputation wounds was determined individually by the physician investigator.
Evaluations were based on data collected from wound assessments and photographs performed by the treating clinician. Wound assessment included the wound location, date of onset, size (including depth), base colour, peri‐wound colour and condition, presence of oedema, drainage type/amount and granulation tissue assessment. Wound photographs were taken using a digital camera on days 0, 7, 14, 28, 42, 56, 84 and 112. In addition, a bi‐layered wound tracing was made for planimetric assessment, and granulation tissue was estimated in percentages and recorded as 0–25%, 26–50%, 51–75% or 76–100%. Complete wound closure was defined as 100% reepithelisation without drainage. Wound size and healing closure were confirmed by independent, blinded wound evaluators who were not involved in the care of the study patients and did not know the patient’s treatment assignment.
Statistical analysis
The sample size for this study was predicated on detecting a 20% difference in complete wound closure. Based on a power of 80% and a significance level of 5%, a minimum sample size of 122 subjects was established. To ensure adequate power for evaluating the individual response parameters and to account for subject attrition, enrolment continued until the minimum target sample size was exceeded by approximately 30%. Subjects were randomised in a 1:1 ratio (NWPT:control) using permuted blocks, balanced intra‐centre.
The primary analytical method presented in this report is based on a time‐to‐event strategy using Kaplan–Meier estimates, followed by a log‐rank test. This statistical procedure provides a comparison of the distribution of events between the two treatment groups. In addition to the event rates calculated for the post‐baseline time points, the median time to 100% closure was also calculated. This same methodology was also used to compare the overall duration of treatment and within the individual patient subsets defined by their outcome status.
Continuous demographic parameters, such as the patient’s age at the time of enrolment, were summarised for the population using descriptive statistics (n, mean, median, standard deviation, minimum and maximum value and 95% two‐sided confidence limits) and compared between groups using a two‐sample t‐test. Categorical demographic parameters, such as gender, were summarised as a proportion of the intent to treat (ITT) population and compared using a two‐tailed Fisher’s exact test. Comorbid risk factors were summarised for the ITT population by treatment assignment and according to the type of variable (categorical and continuous) and compared between groups.
Results
The study population had many descriptive characteristics that reached statistical significance including age, gender, current alcohol use, albumin level, ankle brachial index (ABI) measurement, wound area and wound duration (Table 1). There was a significantly higher proportion of acute wounds (75·3%) than chronic wounds (24·7%), evaluated in this clinical trial (P < 0·001). The individuals with acute wounds were younger and more frequently men and had poorer glucose control, larger wounds, higher ABI, lower albumin and a higher proportion of current alcohol use than their counterparts with chronic wounds.
Table 1.
Descriptive characteristics of the study population
| Acute wound (n = 122) | Chronic wound (n = 40) | P value | |
|---|---|---|---|
| Age (years) | 56 (12·3) | 65 (12·2) | <0·001* |
| Sex (male) | 105 (86%) | 27 (68%) | 0·017† |
| Ethnic origin | |||
| Non Hispanic white | 59 (48%) | 18 (20%) | 0·651† |
| African‐American | 20 (16%) | 8 (20%) | |
| Mexican‐American | 38 (31%) | 14 (35%) | |
| Native‐American | 5 (4%) | 0 (0%) | |
| Body mass index (kg/m2) | 31·0 (8·4) | 30·6 (8·2) | 0·494‡ |
| Haemoglobin A1c (%) | 8·4% (1·8) | 7·4% (1·6%) | 0·001‡ |
| Wound area (cm2) | 22·8 (21·0) | 14·1 (17·9) | 0·004‡ |
| Wound duration (months) | 0·40 (0·22) | 5·0 (9·3) | <0·001‡ |
| Currently use alcohol | 37 (30%) | 3 (8%) | 0·003† |
| Currently use tobacco | 10 (8%) | 5 (12%) | 0·529† |
| Type 2 diabetes | 107 (88%) | 39 (98%) | 0·122† |
| Pre‐albumin (g/l) | 0·181 (0·08) | 0·206 (0·11) | 0·196‡ |
| Albumin (g/l) | 32 (6·0) | 36 (6·2) | 0·001* |
| Ankle brachial systolic pressure index (mmHg) | 1·1 (0·22) | 0·99 (0·12) | 0·009‡ |
| Transcutaneous oxygen tension (TcPO2; mmHg) | 41·3 (9·0) | 42·3 (10·2) | 0·810‡ |
Two‐sample t‐test.
Fisher’s exact test.
Wilcoxon rank sum test.
Figures in parentheses refer to either percentages (as labeled) or standard deviation.
Acute versus chronic wounds were evaluated across the study population without regard to treatment group. The evaluation analysed the proportion that achieved complete closure and the time to complete closure. For the proportion of acute and chronic patients achieving complete wound closure, no significant difference was found (P = 0·716). For time to complete closure, Kaplan–Meier curves were prepared. The results from the log‐rank test, comparing the time to closure revealed that there was no significant difference in time to complete closure between the acute and the chronic wounds (P = 0·979) (Figure 1).
Figure 1.
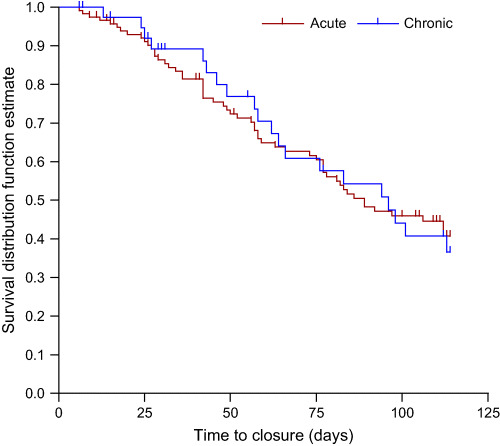
Acute versus chronic wound survival. Log‐rank test comparing the time‐to‐event profiles revealed no significant difference in time to complete closure (P = 0·979).
In the treatment groups (NPWT versus SWT), the proportion of patients and time to complete closure was compared for acute and chronic wounds (Figure 2). There was not a significant difference in the proportion of acute or chronic wounds that achieved complete closure between NPWT and SWT groups, (acute P = 0·072, chronic P = 0·320).
Figure 2.
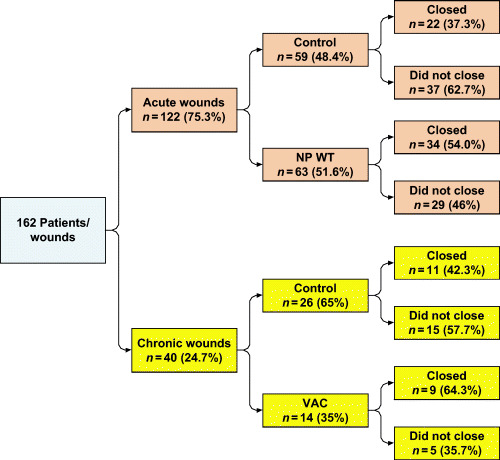
Comparison between the proportion of patients and the proportion of time to complete closure for acute and chronic wounds in the treatment groups. NPWT,negative pressure wound therapy; VAC, vacuum‐assisted closure.
Kaplan–Meier curves were prepared based on time to complete closure, and the log‐rank test comparing the time‐to‐event profiles was significant in favour of the NPWT group in both acute (P = 0·030) and chronic wounds (P = 0·033) (3, 4.
Figure 3.
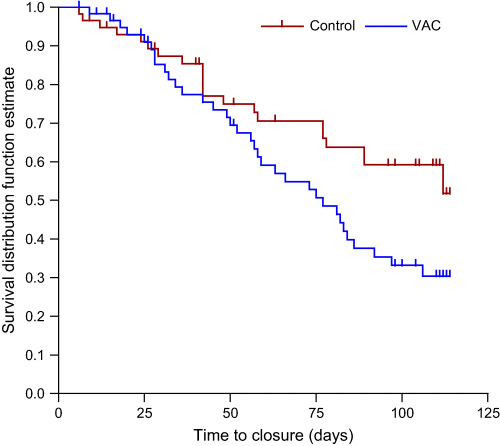
Acute wounds:negative pressure wound therapy (NPWT) versusstandard wound therapy (SWT). Log‐rank test comparing the time‐to‐event profiles was significant in favour of the NPWT group over the SWT group for acute wounds (P = 0·030).
Figure 4.
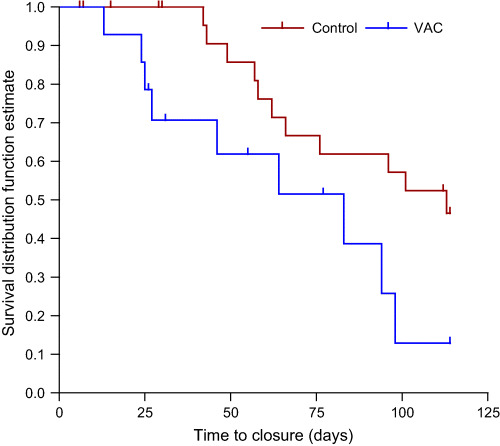
Chronic wounds:negative pressure wound therapy (NPWT) versusstandard wound therapy. Log‐rank test comparing the time‐to‐event profiles was significant in favour of the NPWT group for acute wounds (P = 0·033).
We also evaluated the response to granulation tissue among individuals presenting with minimal granulation tissue. When taking into account only those patients who started with 0–10% wound granulation at baseline, a total of 34 patients fell into this category. The vast majority of these patients (88·2%) had acute wounds.
With the numbers available, there was no significant difference in the proportion of patients who achieved 76–100% wound granulation in patients with acute versus chronic wounds (60% of 30 patients with acute versus 100% of 4 patients with chronic wounds, P = 0·273). Kaplan–Meier curves based on time to 76–100% wound granulation revealed a significant difference in acute and chronic wounds (P < 0·001).
The proportion of patients who achieved 76–100% wound granulation and time to 76–100% wound granulation was compared for acute and chronic wounds across treatment groups. There was no significant difference in the proportion of acute wounds (P = 0·264) in patients who experienced 76–100% wound granulation between NPWT and control. The outcome was similar when considering chronic wounds. All wounds that fell into this particular wound classification were able to achieve 76–100% wound granulation, and the proportions were the same for both the NPWT group and the SWT group.
Among patients with acute wounds, the log‐rank test comparing the time‐to‐granulation profiles was significant in favour of NPWT (P < 0·001). The log‐rank test comparing the time‐to‐event profiles for chronic wounds did not show significance; however, even with four patients, it did appear to be trending in that direction in favour of the NPWT group (P = 0·090).
Discussion
The results of this study suggest that there was not a difference in the rate of healing of large acute or chronic open foot amputation wounds treated with NPWT. To our knowledge, this is the first study to evaluate this association in a randomised controlled trial. As in the previously published RCT evaluating the entire population, there was a strong association in rate of granulation of acute wounds favouring NPWT, and a trend suggesting the same in the chronic wounds. Overall, NPWT healing outcomes was superior to SWT in both the acute and the chronic arms. A further strength of the current observations is that the precise duration of the wound is much more accurately recorded, unlike studies of chronic neuropathic foot ulcers where patients and clinicians are more frequently unaware of the true duration of the lesions.
In this study, we were surprised by the lack of differences in time to healing between the acute and the chronic wound groups. While the acute wounds were larger, with a higher proportion of alcohol use and poorer nutritional status, patients still healed at a similar rate. Other large cohort studies have suggested differences in response based on wound duration 20, 21. There may be overt biological differences between wounds based on this criterion that may affect matrix deposition, coagulation, inflammation and remodelling (3). While these may be important differentiating factors when comparing surgical wounds in healthy adults with diabetic foot wounds in impaired hosts, it might equally stand to reason that these differences may not be as profound or cut and dry in the population we evaluated. Certainly, all these patients received their partial foot amputation because of a pre‐existing wound/infection. It is this factor that may have been a great equaliser and may play a role in clinical decision making when deciding when to use an advanced wound healing modality.
In conclusion, wound duration does not initially appear to play an overt role in the efficacy of NPWT delivered through the VAC System™ in individuals with large wounds secondary to partial foot amputation. It is our initial contention that this single criterion should not be a barrier to use of this technology in this population. We look forward to further studies that might further elaborate on this potentially intriguing finding.
Acknowledgement
This study has been registered with ClinicalTrials.gov (number NCT00224796).
References
- 1. Boulton AJ, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–24. [DOI] [PubMed] [Google Scholar]
- 2. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. [DOI] [PubMed] [Google Scholar]
- 3. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366:1736–43. [DOI] [PubMed] [Google Scholar]
- 4. Gupta S, Cho T. A literature review of negative pressure wound therapy. Ostomy Wound Manage 2004;50 11A Suppl:2–4S. [PubMed] [Google Scholar]
- 5. Armstrong DG, Lavery LA. Negative pressure wound after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–10. [DOI] [PubMed] [Google Scholar]
- 6. Peters EJ, Lavery LA, Armstrong DG, Fleischli JG. Electric stimulation as an adjunct to heal diabetic foot ulcers: a randomized clinical trial. Arch Phys Med Rehabil 2001;82:721–5. [DOI] [PubMed] [Google Scholar]
- 7. Steed DL. Clinical evaluation of recombinant human platelet‐derived growth factor for the treatment of lower extremity diabetic ulcers. Diabetic Ulcer Study Group. J Vasc Surg 1995;21:71–8. [DOI] [PubMed] [Google Scholar]
- 8. Gentzkow GD, Iwasaki SD, Hershon KS. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care 1996;19:350–4. [DOI] [PubMed] [Google Scholar]
- 9. Veves A, Falanga V, Armstrong DG, Sabolinski ML. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Apligraf Diabetic Foot Ulcer Study. Diabetes Care 2001;24:290–5. [DOI] [PubMed] [Google Scholar]
- 10. Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg 2002;137:822–7. [DOI] [PubMed] [Google Scholar]
- 11. Thoner B, Fleischmann W, Moch D. Wound treatment by vacuum sealing. Krankenpfl J 1998;36:78–82. [PubMed] [Google Scholar]
- 12. Banwell PE. Topical negative pressure therapy in wound care. J Wound Care 1999;8:79–84. [DOI] [PubMed] [Google Scholar]
- 13. Banwell PE, Teot L. Topical negative pressure (TNP): the evolution of a novel wound therapy. J Wound Care 2003;12:22–8. [DOI] [PubMed] [Google Scholar]
- 14. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 15. Mullner T, Mrkonjic L, Kwasny O, Vecsei V. The use of negative pressure to promote the healing of tissue defects: a clinical trial using the vacuum sealing technique. Br J Plast Surg 1997;50:194–9. [DOI] [PubMed] [Google Scholar]
- 16. Wound Ostomy Continence Nursing Society . Guideline for management of wounds in patients with lower‐extremity neuropathic disease, Guideline Number 3. Glenview, IL: WOCN, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Young MJ, Breddy JL, Veves A, Boulton AJ. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. A prospective study. Diabetes Care 1994;17:557–60. [DOI] [PubMed] [Google Scholar]
- 18. Boulton AJ, Vinik AI, Arezzo JC, Sosenko JM. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28:956–62. [DOI] [PubMed] [Google Scholar]
- 19. Armstrong DG, Lavery LA, Vela SA, Quebedeaux TL, Fleischli JG. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med 1998;158:289–92. [DOI] [PubMed] [Google Scholar]
- 20. Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: predicting which ones will not heal. Am J Med 2003;115:627–31. [DOI] [PubMed] [Google Scholar]
- 21. Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Healing diabetic neuropathic foot ulcers: are we getting better? Diabet Med 2005;22:172–6. [DOI] [PubMed] [Google Scholar]


