Abstract
This 12‐week, prospective, randomised, controlled multi‐centre study compared the proportion of healed diabetic foot ulcers and mean healing time between patients receiving acellular matrix (AM) (study group) and standard of care (control group) therapies. Eighty‐six patients were randomised into study (47 patients) and control (39 patients) groups. No significant differences in demographics or pre‐treatment ulcer data were calculated. Complete healing and mean healing time were 69·6% and 5·7 weeks, respectively, for the study group and 46·2% and 6·8 weeks, respectively, for the control group. The proportion of healed ulcers between the groups was statistically significant (P = 0·0289), with odds of healing in the study group 2·7 times higher than in the control group. Kaplan–Meier survivorship analysis for time to complete healing at 12 weeks showed a significantly higher non healing rate (P = 0·015) for the control group (53·9%) compared with the study group (30·4%). After adjusting for ulcer size at presentation, which was a statistically significant covariate (P = 0·0194), a statistically significant difference in non healing rate between groups was calculated (P = 0·0233), with odds of healing 2·0 times higher in the study versus control group. This study supports the use of single‐application AM therapy as an effective treatment of diabetic, neuropathic ulcers.
Keywords: Acellular regenerative tissue matrix, Bioengineered matrix, Diabetes mellitus, Foot ulcer, Wound
Introduction
The United States consistently ranks third, behind India and China, on the list of countries with the highest diabetic populations, with an estimated 17·7 million cases in 2000 and a projected 30·3 million cases by 2030 (1). Persons with diabetes face a lifetime risk as high as 25% of developing a foot ulcer (2). Management of diabetic wounds of the lower extremity is critical, as ulcers that are slow to heal have a significant impact on patient quality of life 3, 4, 5, 6, 7, 8, 9. Conservative estimates suggest that the cost of treating a diabetic foot ulcer is approximately $28 000 during the 2 years after diagnosis (10). Given the high cost associated with treatment of lower extremity diabetic ulcers, Holzer et al. concluded that the development of better treatment strategies is warranted (11). Diabetic foot complications are the principal cause of non traumatic lower extremity amputations, accounting for up to 8 of every 10 non traumatic amputations 12, 13, 14. However, previous evidence suggests that up to 85% of diabetic foot and leg amputations may be prevented with the appropriate knowledge of risk factors and application of evidence‐based multidisciplinary treatment (12). Bioengineered skin grafts, including acellular matrix (AM) grafts, are promising alternatives for diabetic lower extremity wounds that often are unresponsive to traditional wound management modalities (15).
AM therapy has a long history of use in a wide variety of applications, including head and neck plastic and reconstructive surgery 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, abdominal wall reconstruction 33, 34, 35 and extremity reconstruction 36, 37, 38. AM grafts also have been effective in healing full‐thickness burn wounds 39, 40, 41, 42, 43, 44, 45. Multiple studies have shown graft integration, host cell infiltration and revascularisation of the AM, with no immune response 18, 32, 33, 44, 45. While several studies have reported outcomes in chronic wounds, most are not randomised controlled studies specifically evaluating AM therapy use in diabetic foot ulcers 15, 46, 47. The primary objective of the study was to compare healing rates at 12 weeks between patients receiving AM therapy and standard of care wound management, as measured by wound survivorship. ‘Wound survivorship’ refers to the duration of time that the wound persists and is synonymous with the term ‘not healed’. The secondary objective was to compare mean time to healing between treatment groups.
Methods
Study design
Ninety‐three patients were enrolled in a 12‐week prospective, randomised, parallel, controlled multicentre clinical trial involving 11 sites. Institutional review board approval was received for each site and informed consent was obtained from all patients before screening. Patients who were 18 years of age or older, with a diagnosis of type 1 ortype 2 diabetes, a University of Texas (UT) grade 1 or 2 diabetic foot ulcer ranging in size from 1 to 25 cm2, absence of infection based on Infectious Disease Society of America criteria, and adequate circulation to the affected extremity were eligible for inclusion 12, 48, 49. Adequate circulation was defined as the presence of at least one of three criteria within the previous 60 days: (i) transcutaneous oxygen measurement at the dorsum of the foot greater or equal to 30 mmHg, (ii) ankle brachial index ranging from 0·7 to 1·2, or (iii) at least biphasic Doppler arterial waveforms at the dorsalis pedis and posterior tibial arteries. Patients who were in poor metabolic control (HgA1c greater than 12% within the previous 90 days) were excluded, as were patients with serum creatinine levels of 3·0 mg/dl or greater. Patients with sensitivity to gentamicin, cefoxilin, linocmycin, polymyxin B or vancomycin also were excluded because of the broth composition in which the AM is processed. Additional exclusion criteria included non revascularable surgical sites, ulcers probing to bone (UT grades 3A to D), and wounds treated with biomedical or topical growth factors within the previous 30 days.
After surgical preparation of the recipient site, patients were randomised into one of two treatment groups. Patients in the study group received a single application of a human acellular dermal regenerative tissue matrix graft (4 × 4 cm GRAFTJACKET® Regenerative Tissue Matrix – Ulcer Repair; Wright Medical Technology, Inc., Arlington, TN). After suturing or stapling the AM graft into place, a silver‐based non adherent dressing (Silverlon; Argentum Medical, LLC, Chicago, IL) was applied. Secondary dressings (hydrogel bolsters or moist gauze) were applied routinely at the rate determined by the investigator until complete epithelialisation was achieved or 12 weeks of care had been provided.
Patients in the control group received standard‐of‐care wound management consisting of moist‐wound therapy with alginates, foams, hydrocolloids or hydrogels at the discretion of the treating physician. Alginates used in conjunction with foam typically were used for heavily exudative wounds. For wounds with minimal exudate, hydrocolloids or hydrogels commonly were used. Dressing changes occurred daily, unless recommended otherwise by the treating physician. Standard‐of‐care management continued until complete epithelialisation occurred or 12 weeks of care had been provided.
Glycosylated haemoglobin measurements were obtained during screening and at study closure. Serum creatinine levels were measured at screening. Patients were evaluated by the investigators at least once every 7 days (±3 days) to obtain ulcer measurements and to perform dressing changes. For both groups, the study ulcer was cleansed with a sterile normal saline solution before wound dressing placement. Rinsing, swabbing or irrigating was acceptable. The surface area and depth of the ulcer were measured and recorded at each visit, in addition to degree of granulation. No granulation was defined as no observed signs of granulation tissue; mild granulation was defined as granulation beginning to fill in, but tissue may not be epithelialised; and marked granulation was defined as tissue that was filling in and was epithelialised. Acetate tracings of the wound and photographs were obtained after debridement and cleaning of the ulcer at the following intervals: after initial surgical preparation of recipient site, at 4‐, 8‐ and 12‐week post‐randomisation, and upon study exit or withdrawal. Although debridement is not routine standard of care after AM therapy, it was performed as part of this controlled study in an effort to keep the two treatment populations as homogenous as possible and to minimise possible bias regarding wound size measurements. Wound photographs were taken at a distance of 1 foot (30 cm) with a centimetre label placed so that markings were directly adjacent to the ulcer. If findings were remarkable, wounds were traced and photographed more frequently. Patients received the standard management protocols per group assignment for 12 weeks or until complete healing occurred. Offloading was performed using a removable cast walker (Active Offloading Walker; Royce Medical, Inc., Camarillo, CA). The device was converted to an ‘instant total contact cast’ to improve adherence to offloading if deemed necessary by the treating physician.
If clinical signs of infection were present, a wound culture was obtained via curettage at the ulcer base after aggressive sharp debridement. Appropriate systemic antibiotic treatment was administered until the infection resolved. Microbiology confirmation and choice of antibiotic therapy were recorded. All other adverse events also were recorded. Other complications possible with AM graft use include maceration, specific or non specific immune response, graft resorption or graft non integration.
The primary endpoint of the study was the proportion of ulcers that completely healed at 12 weeks. The secondary endpoint was mean time to healing. Complete healing was defined as 100% re‐epithelialisation without drainage.
AM description
The AM graft is processed from screened donated human skin supplied from United States tissue banks under the guidelines of the American Association of Tissue Banks and in accordance with Food and Drug Administration (FDA) requirements for procurement and processing of banked human tissues (CFR Title 21, Part 1270 and 1271). It is regulated by the FDA as human tissue for transplantation. The allograft skin is processed minimally to remove epidermal and dermal cells while preserving dermal structure. The resulting allograft serves as a scaffold to support cellular repopulation and revascularisation. Presence of an intact basement membrane complex, retention of collagen and absence of cells are confirmed through histology and immunohistochemistry. Absence of bacterial and fungal pathogens is assured through microbiological cultures. Residual moisture is less than 5%.
Statistical analysis
Statistical analysis was performed using SAS 9.1.3 Service Pack 4 (SAS Institute Inc., Cary, NC). Descriptive statistics, including mean, median, standard deviation, frequency and percentage, were used to describe numerical data. Demographic variables, diabetes type, wound size, time to complete healing for wounds that healed and change in wound size from baseline to most recent follow‐up evaluation for wounds that did not heal were compared between the study groups. Pearson’s chi‐square analysis was used to compare categorical information. For continuous variables that were normally distributed, one‐way analysis of variance was used to determine statistical significance. Using time to wound healing as the survival event, Kaplan–Meier survivorship analysis (product limit plot) was used to assess wound healing between treatment groups. Wounds were censored if they failed to heal by the 12‐week endpoint or at the latest available clinical assessment or if the patient withdrew from the study. Wound measurements obtained at the latest follow‐up evaluation were used for analysis. The log‐rank test was used to identify significant differences between survival curves. Cox proportional hazards model analysis was conducted to determine the relationship between wound healing and the following covariates: ulcer size at presentation, index ulcer duration, patient age, body mass index and diabetes type. In addition, hazard ratio estimation was performed to evaluate the association between time to wound healing and the significant covariates. Statistical differences were considered significant when the P ≤ 0·05 with a power of at least 0·80. Ninety‐five percent confidence intervals were used throughout the statistical analysis.
Results
The flow of study enrolment and participation through the clinical trial is diagramed in Figure 1. Of the 93 patients who were enrolled in the study, 7 did not meet the inclusion criteria and were deemed screen failures. The remaining 86 patients were randomised into two treatment groups, with 47 patients (47 ulcers) receiving AM therapy (study group) and 39 patients (39 ulcers) receiving standard of care therapy (control group). Four patients (two in the study group and two in the control group) were enrolled in the study despite having index ulcer sizes less than 1 cm2 and were considered protocol deviations. Because these patients were randomised and received treatment, they were included in the data analysis. Eight patients, six in the study group and two in the control group, did not complete the clinical trial. In the study group, one patient chose to withdraw from the study at the first week of evaluation and another was withdrawn during the ninth week of participation. Another patient in the study group was removed from study participation and statistical analysis because of patient non compliance that resulted in AM therapy removal. The remaining five patients (three in study group and two in control group) did not continue study participation due to adverse events.
Figure 1.
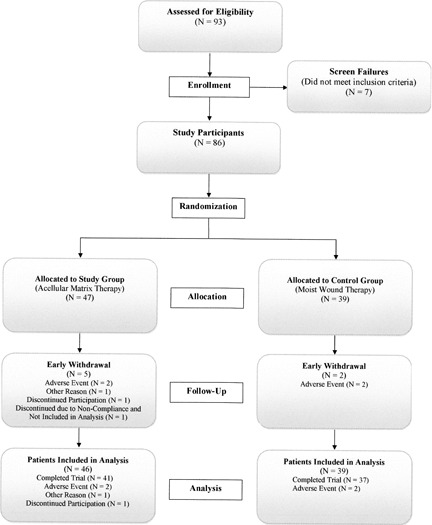
Flow chart of patient accounting for clinical trial.
A comparison of demographics between the two treatment groups is presented in Table 1. The majority of patients in both treatment groups had type 2 diabetes and were obese (body mass index of 30·0 or greater). Index ulcer location is compared between treatment groups in Figure 2. For both treatment groups, the most common location was the foot. Pre‐treatment ulcer data, including history of index ulcer and ulcer size at presentation, is compared between groups in Table 2. No statistically significant differences in demographic, ulcer location and pre‐treatment ulcer variables were observed between treatment groups. Glycosylated haemoglobin levels obtained pre‐treatment and at study completion are compared between treatment groups in Table 3 and indicate that metabolic control was maintained throughout the course of study. The differences between treatment groups were not statistically significant at either time interval.
Table 1.
Comparison of demographic variables between treatment groups
| Demographic variable | Study group (n = 46) | Control group (n = 39) |
|---|---|---|
| Age (years) | ||
| Mean | 55·4 | 58·9 |
| Median | 55·0 | 58·0 |
| Standard deviation | 9·6 | 11·6 |
| Range | 32·0–78·0 | 35·0–93·0 |
| Number of patients | 46 | 39 |
| Body mass index (lbs/in2) | ||
| Mean | 33·1 | 34·6 |
| Median | 32·1 | 33·5 |
| Standard deviation | 6·7 | 8·5 |
| Range | 24·3–52·8 | 20·9–61·1 |
| Number of patients | 45 | 38 |
| Diabetes mellitus type | ||
| Type 1 | 5 (10·9%) | 2 (5·1%) |
| Type 2 | 41 (89·1%) | 37 (94·9%) |
| Number of patients | 46 | 39 |
Figure 2.
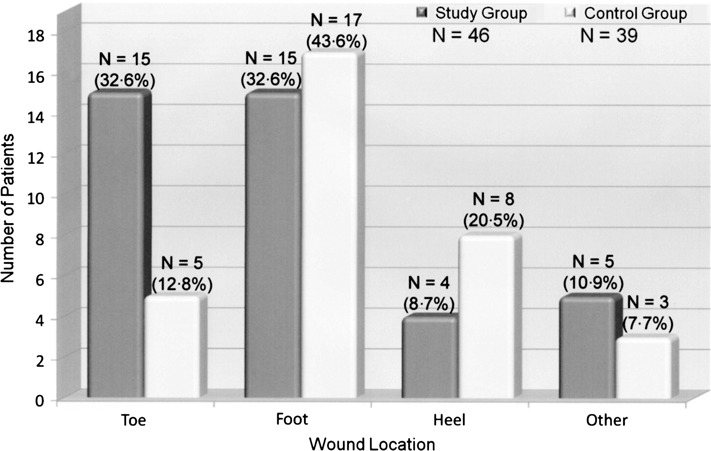
Comparison of index ulcer location between treatment groups.
Table 2.
Comparison of pre‐treatment ulcer data between treatment groups
| Index ulcer duration (weeks) | Ulcer size at presentation (cm2) | |||
|---|---|---|---|---|
| Study group (n = 46) | Control group (n = 39) | Study group (n = 46) | Control group (n = 39) | |
| Mean | 23·3 | 22·9 | 3·6 | 5·1 |
| Median | 16·0 | 12·0 | 2·2 | 3·2 |
| Standard deviation | 22·4 | 29·8 | 4·3 | 4·8 |
| Range | 0·00–96·00 | 3·00–139·00 | 0·6–23·3 | 0·4–18·9 |
Table 3.
Comparison of glycosylated hemoglobin levels obtained at pre‐treatment and study completion between treatment groups
| Pre‐treatment level (%) | Level at study completion (%) | |||
|---|---|---|---|---|
| Study group (n = 46) | Control group (n = 39) | Study group (n = 25) | Control group (n = 22) | |
| Mean | 8·2 | 7·6 | 8·0 | 7·1 |
| Median | 8·5 | 7·5 | 7·8 | 6·7 |
| Standard deviation | 2·0 | 1·6 | 1·6 | 1·5 |
| Range | 2·0–11·9 | 5·6–11·9 | 5·2–11·0 | 5·3–11·2 |
Of the patients completing the clinical trial, complete healing occurred in 32 (69·6%) of the 46 patients in the study group and 18 (46·2%) of the 39 patients in the control group. The time to complete healing is presented in Table 4. There was a statistically significant difference in proportion of healed ulcers between the treatment groups (P = 0·0289, OR = 2·7). Based on the odds ratio, the odds of healing in the study group were 2·7 times higher than in the control group. No statistically significant difference in mean time to wound healing was observed between treatment groups.
Table 4.
Comparison of time to complete healing of ulcers that healed on or before 12 weeks between treatment groups
| Time to complete healing (weeks) | ||
|---|---|---|
| Study group (n = 32) | Control group (n = 18) | |
| Mean | 5·7 | 6·8 |
| Median | 4·5 | 7·0 |
| Standard deviation | 3·5 | 3·3 |
| Range | 1·0–12·0 | 2·0–12·0 |
Complete healing did not occur in 14 (30·4%) of the 46 patients in the study group or in 21 (53·8%) of the 39 patients in the control group. For this non healing subset, no statistically significant differences were calculated between treatment groups in mean final ulcer size, percent of ulcer area completely healed or change from ulcer size at presentation (Table 5). Despite not healing within the study timeframe of 12 weeks, 12 (85·7%) patients in the study group experienced a decrease in ulcer size and the other 2 (14·3%) patients had ulcers that did not increase or decrease in size. Fifteen (71·4%) patients in the control group experienced a decrease in ulcer size, while five (23·8%) increased in size (mean healing percent = −30·37 ± 19·89, median −25·74, range, −64·11 to −13·71). Three (21·4%) patients in the study group and six (28·6%) patients in the control group had ulcers that were at least 90% healed at the final follow‐up evaluation. All but one patient in the control group exhibited mild or marked granulation at the final follow‐up evaluation.
Table 5.
Comparison of ulcers that did not completely heal at or on 12 weeks between treatment groups
| Final wound size (cm2) | Percent healed (presentation versus final wound size) (%) | |||
|---|---|---|---|---|
| Study group (n = 14) | Control group (n = 20) | Study group (n = 14) | Control group (n = 20) | |
| Mean | 1·9 | 3·5 | 49·1 | 47·2 |
| Median | 1·1 | 1·1 | 46·3 | 65·2 |
| Standard deviation | 2·3 | 5·2 | 35·9 | 52·0 |
| Range | 0·01–9·0 | 0·02–19·5 | 0·00–99·9 | −64·1 to 99·4 |
Summarising the six adverse events that occurred during the course of the study, one patient in the control group was admitted to the hospital for altered mental status and hypotension during the first week of study participation. A hallux amputation that was deemed not to be related to study treatment was performed 26 days after admission. This patient was censored at week 0. A study group patient was censored at week 1 because of infection that required a hallux amputation. Another study patient was censored at week 2 after the investigator discovered that the AM therapy was no longer on the wound. At the fifth‐week evaluation, a patient in the control group presented with an abscess secondary to the study wound and was removed from the study because treatment outside the study protocol was necessary. This patient was censored at week 5. After the eighth week of evaluation, a patient in the study group required vascular surgery to treat a blocked artery, which was unrelated to the AM therapy, and was censored from the analysis at week 8. After repeated incidences of non compliance by a study patient, including use of an offloading device, AM therapy completely dislodged from a plantar wound during the second week of participation. Because of the deviation in management, this patient was not included in the analysis.
The Kaplan–Meier survivorship analysis for time to complete healing is presented in Figure 3. A statistically significant difference in non healing rate was calculated between treatment groups (P = 0·0075). At the 12‐week endpoint, the non healing rate of 53·8% in the control group was significantly higher than the 30·4% non healing rate observed in the study group. The proportion of healed ulcers at weekly evaluation intervals between treatment groups is presented in Figure 4. Beginning at the 3‐week follow‐up evaluation, the proportion of healed ulcers in the study group was at least 15% higher than the control group. The Cox proportional hazards model analysis indicated that ulcer size at presentation was statistically significant covariates that should be considered when analysing non healing rate (P = 0·0194). Index ulcer duration, age, body mass index and diabetes type were not significant covariates. After adjusting for ulcer size at presentation, there was a statistically significant difference in non healing rate between treatment groups (P = 0·0233). The corresponding hazard ratio of 2·0 (95% CI, 1·0–3·5) indicated that the probability of healing is approximately two times greater in the study group than in the control group.
Figure 3.
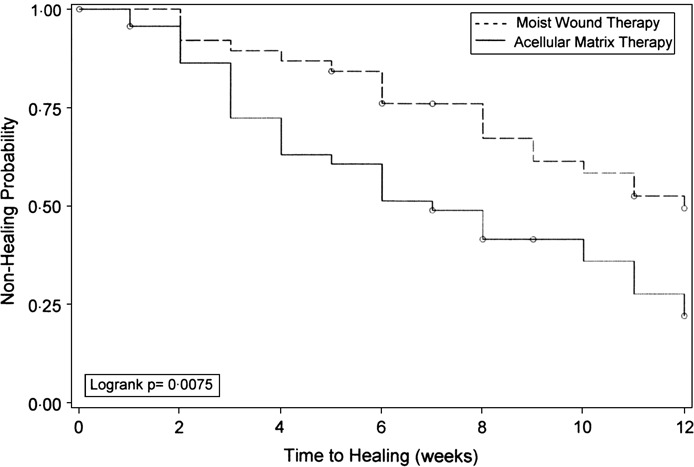
Kaplan–Meier survivorship analysis for time to complete healing by treatment.
Figure 4.
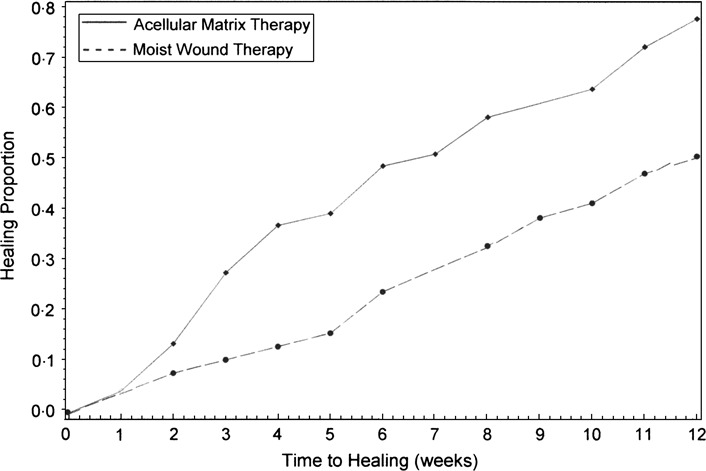
Healing proportion curve by treatment.
Discussion
The results of this study support previous literature documenting successful use of AM therapy 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50. In addition, this study provides further evidence that bioengineered skin grafts such as AM grafts are effective treatment options for diabetic lower extremity wounds. The use of AM therapy offers several advantages compared with other bioengineered skin grafts. First, the graft is an immunologically inert, acellular, replacement scaffold derived from human cadaver tissue and comprised of collagen and extracellular protein matrices. It provides a favorable microenvironment for bioingrowth by promoting nutritional diffusion and cellular proliferation at the graft site 51, 52, 53, 54. These properties result in the rapid revascularisation and cellular repopulation of the matrix scaffold 55, 56, 57, 58. The cells then respond to local growth factor and biomechanical stimuli, transitioning the scaffold into nascent, vital dermal tissue. Because AM therapy provides a scaffold onto which the body may build dermal tissue, a single application often is sufficient for complete healing. In the current study, the ulcers that healed in the study group received only one application of the AM therapy. Although many other bioengineered grafts contain growth factors, they do not offer the scaffold onto which dermal tissue may be built. Multiple applications of these grafts, therefore, may be required for the body to build a sufficient base to achieve complete healing.
Because the graft is derived from human tissue and its structural and biochemical integrity are retained during processing, it is easily recognised by the body’s immune system and, therefore, does not provoke a foreign body or inflammatory tissue response 44, 55, 59. In addition, as all cellular components are extracted during manufacturing, the scaffold does not contain immune response targets. No specific or non specific immune responses were observed in the current study. Conversely, many of the other commercially available bioengineered grafts are xenografts, produced from either bovine or porcine sources, and have been shown to induce an inflammatory reaction in both animal models and in humans 60, 61.
The purpose of this study was to compare the proportion of healed ulcers and the mean time to healing between patients receiving AM therapy and standard of care management. The study population was comprised of patients with UT grade 1 or 2 diabetic foot ulcers ranging in size from 0·4 to 25 cm2 and most commonly located on the foot. There was not a significant difference in demographics, ulcer location and pre‐treatment ulcer data between treatment groups, reinforcing the randomisation process and also suggesting that any differences in outcomes are more likely the result of differences in treatment. Complete healing was determined by the proportion of wounds that healed at or within the 12‐week study duration. At the end of the study, complete healing and mean time to healing were 69·6% and 5·7 weeks, respectively, for the study group and 46·2% and 6·8 weeks, respectively, for the control group. The odds of healing in the study group were 2·7 times higher than in the control group. Ulcers in the study group also healed on average 1 week sooner than those receiving moist therapy, although this trend did not reach significance.
Factoring in time, the rate of wound healing was assessed by performing a Kaplan–Meier survivorship. At the 12‐week endpoint, the non healing rate of 53·8% in the control group was significantly higher than the 30·4% non healing rate observed in the study group. This finding is of clinical significance because of the importance of healing a wound as quickly as possible. Because wound healing is influenced by multiple factors, including ulcer size, a Cox proportional hazards model analysis was performed. After adjusting for ulcer size at presentation, which was determined to be a clinically and statistically significant covariate, the probability of healing was approximately two times greater in the study group than in the control group.
Two patients in the control group experienced adverse events during the course of study. One was hospitalised during the first week of study participation and subsequently required a hallux amputation. The other was removed from the study after 5 weeks to administer treatment for an abscess that developed secondary to the study wound. Of the study group patients completing the clinical trial, no complications possible with AM therapy, such as maceration, immune response, graft resorption or graft non integration were reported. However, one study patient required a hallux amputation because of an infection that may or may not have been related to AM therapy use. Two AM therapy failures were noted in the study group. In the first case, the investigator discovered that the AM therapy no longer was on the wound when the patient returned for the second‐week evaluation, with no explanation provided by the patient. After repeated incidences of patient non compliance, including weight‐bearing ambulation, AM therapy completely dislodged from a plantar wound in another patient during the second week of participation. Despite the investigator’s attempt to gain patient compliance by prescribing a wheelchair, the adverse event occurred because the patient used the index foot to manoeuvre in the wheelchair.
Three other published studies report the results of AM therapy for diabetic lower extremity ulcers at specific endpoints 46, 47, 50. Although direct comparison between these studies and the current one is difficult because of differences in demographics, co‐morbidities, wound complexities and study endpoints, the proportions of ulcers healed and corresponding mean times to healing are summarised in Table 6. The other prospective study involved full‐thickness Wagner grade 2 ulcers (50). The other two were 20‐week studies comprised of more complex wounds, with the Winters et al. study involving all UT grades, including infected and/or ischemic wounds 46, 47. The proportion of wounds healed at 12 weeks and an analysis of UT grade 2A wounds at 20 weeks were provided in the discussion portion of the Winters et al. study (47). Given the difference in wound complexity, the current study had an approximately three times higher proportion of healed wounds with a two times shorter mean time to healing compared with the 12‐week data reported by Winters et al. (47). Graft failure rates were similar between studies, with lack of patient adherence to care being a factor potentially influencing these rates. The proportion of UT grade 2A ulcers healed at 20 weeks ranged from 50·0% to 82·4% in the Winters et al. (47) and Martin et al. studies (46).
Table 6.
Summary of ulcer healing proportion and mean time to healing for published AM therapy diabetic ulcer studies
| Study | Study design | Study endpoint (weeks) | Ulcer type | Ulcer location | Number of cases | Proportion of healed ulcers, n (%) | Mean time to healing (weeks) | AM therapy failures |
|---|---|---|---|---|---|---|---|---|
| Current study | Prospective | 12 | UT grades 1A and 2A | Foot | 46 | 32 (69·6%) | 5·7 | 2 (4·3%) (1 non compliance, 1 unknown) |
| Brigido (50) | Prospective | 16 | Full‐thickness, Wagner grade 2 | Lower extremity (85·7% foot) | 14 | 12 (85·7%) | 11·9 | 0 (0/0%) |
| Martin et al. (46) | Retrospective | 20 | UT grade 2A | Foot | 17 | 14 (82·4%) | 8·9 | 0 (0/0%) |
| Winters et al. (47) | Retrospective | 12 | UT grades 1–3 | Lower extremity (86·0% foot) | 100 | 22 (22·0%) | 9·6 | |
| 20 | UT grade 2A | 18 | 9 (50·0%) | 9·8 | ||||
| 20 | UT grades 1–3 | 100 | 91 (91·0%) | 13·8 | 1 (1%) (non compliance) |
In addition to comparing the current study results with previous AM therapy studies, a historical control group also is available for comparison. Margolis et al. performed a meta‐analysis that assessed the percentage of neuropathic diabetic foot ulcers that healed after receiving good standard of care (62). The meta‐analysis was limited to control arms from randomised controlled trials of non infected wounds that reported the percentage of healed wounds after a set treatment duration (62). Margolis et al. defined good care as a standard care regimen consisting of debridement, wound care with either saline‐moistened gauze or placebo gel and gauze, and instructions to patients regarding the importance of avoiding weight‐bearing ambulation, which were components of the wound management protocol in the current study (62). Based on group‐level or study‐level data, 24·2% of diabetic neuropathic ulcers healed after 12 weeks of good treatment, with 30·9% of ulcers healing after 20 weeks of good wound care (62). Despite the lack of heterogeneity between the studies included in the meta‐analysis, the percentage of wounds healed at 12 and 20 weeks remained relatively constant among the studies (62). Margolis et al. concluded by recommending that their data be used as a benchmark by physicians to shape patient expectations regarding chances of healing and by regulatory agencies and managed care corporations to determine what percentage of wounds should be healed within a set time period (62). The 12‐week healing rates of 69·6% and 46·2% for the study and control groups, respectively, in the current study are two to three times above this recommended benchmark. These successful results support the treatment of diabetic, neuropathic ulcers using the wound management protocols employed in this study.
Strengths of this study include the prospective, randomised, controlled design and the multicentre involvement. Although 12 weeks is a common assessment endpoint in wound studies, continued follow‐up would be beneficial. Among the ulcers that did not heal, 21·4% and 28·6% in the study and control groups, respectively, were at least 90% healed compared with their measurements at presentation. In addition, 85·7% patients in the study group experienced a decrease in ulcer size, whereas the remaining 14·3% had ulcers that did not increase or decrease in size. Although 71·4% patients in the control group experienced a decrease in ulcer size, the remaining 23·8% had ulcers that increased in size. Therefore, because a large percentage of ulcers were improving in size at the conclusion of the study, observing the ulcers for another 4–12 weeks would be beneficial in determining how many more ulcers would heal and in identifying any further differences in healing proportion or mean complete healing time between treatment groups. The additional data also would allow more comparisons to other studies in the literature.
The results of this prospective, randomised, multicentre study indicate that diabetic foot ulcers treated with AM therapy have a two to three times higher probability of healing compared with those with standard of care management. The 69·6% proportion of healed ulcers in the study group was significantly higher than the 46·2% proportion of healed ulcers in the study group. In addition, at the 12‐week endpoint, the non healing rate of 53·8% in the control group was significantly higher than the 30·4% non healing rate observed in the study group. The study also suggests that the probability of healing in 12 weeks may be affected by initial wound size. This study lends further support to the safety of AM therapy, as infection and possible graft‐related complications did not occur. The proportion of wounds healed also exceeds a recommended benchmark for assessing wound treatment methods. Based on the successful results obtained with AM therapy and the clinical importance of prompt wound healing in overall outcome, the use of AM therapy for the treatment of diabetic, neuropathic ulcers is supported.
Acknowledgements
The authors thank Jodi F. Hartman, MS, and Michelle L. Wright, MPH, of Orthopaedic Research & Reporting, Ltd, for assistance with data interpretation and writing.
This clinical trial was supported by Wright Medical Technology, Inc. (Arlington, TN). Support included compensation to study personnel, providing AM therapy at no charge and statistical analysis. DGA and Orthopaedic Research & Reporting, Ltd received research funding from Wright Medical Technology, Inc.
References
- 1. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–53. [DOI] [PubMed] [Google Scholar]
- 2. Singh H, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005; 293:217–28. [DOI] [PubMed] [Google Scholar]
- 3. Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers. N Engl J Med 2004;351:48–55. [DOI] [PubMed] [Google Scholar]
- 4. Houghton PE, Kincaid CB, Lovell M, Campbell KE, Keast DH, Woodbury MG, Harris KA. Effect of electrical stimulation on chronic leg ulcer size and appearance. Phys Ther 2003;83:17–28. [PubMed] [Google Scholar]
- 5. Meijer JW, Trip J, Jaegers SM, Links TP, Smits AJ, Groothoff JW, Eisma WH. Quality of life in patients with diabetic foot ulcers. Disabil Rehabil 2001;23:336–40. [DOI] [PubMed] [Google Scholar]
- 6. Phillips T, Stanton B, Provan A, Lew R. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol 1994;31:49–53. [DOI] [PubMed] [Google Scholar]
- 7. Tennvall GR, Apelqvist J. Health‐related quality of life in patients with diabetes mellitus and foot ulcers. J Diabetes Complications 2000;14:235–41. [DOI] [PubMed] [Google Scholar]
- 8. Vileikyte L. Diabetic foot ulcers: a quality of life issue. Diabetes Metab Res Rev 2001;17:246–9. [DOI] [PubMed] [Google Scholar]
- 9. Vileikyte L, Boulton AJM. Psychological/behavioral issues in diabetic neuropathic foot ulceration. Wounds 2000;12(6 Suppl B):43B‐7. [Google Scholar]
- 10. Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, Wagner EH. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999;22:382–7. [DOI] [PubMed] [Google Scholar]
- 11. Holzer SE, Camerota A, Martens L, Cuerdon T, Crystal‐Peters J, Zagari M. Clin Ther 1998;20:169–81. [DOI] [PubMed] [Google Scholar]
- 12. Armstrong DG, Lavery LA, Harkless LB. Who is at risk for diabetic foot ulceration? Clin Podiatr Med Surg 1998;15:11–9. [PubMed] [Google Scholar]
- 13. Armstrong DG, Lavery LA, Quebedeaux TL, Walker SC. Surgical morbidity and the risk of amputation due to infected puncture wounds in diabetic versus nondiabetic adults. J Am Podiatr Med Assoc 1997;87:321–6. [DOI] [PubMed] [Google Scholar]
- 14. Reiber GE. The epidemiology of diabetic foot problems. Diabet Med 1996;13 Suppl 1:S6–11. [PubMed] [Google Scholar]
- 15. Brigido SA, Boc SF, Lopez RC. Effective management of major lower extremity wounds using an acellular regenerative tissue matrix: a pilot study. Orthopedics 2004;27 Suppl 1:s145–49. [DOI] [PubMed] [Google Scholar]
- 16. Achauer BM, VanderKam VM, Celikoz B, Jacobson DG. Augmentation of facial soft‐tissue defects with Alloderm dermal graft. Ann Plast Surg 1998;41:503–7. [DOI] [PubMed] [Google Scholar]
- 17. Benecke JE Jr. Tympanic membrane grafting with alloderm. Laryngoscope 2001;111:1525–7. [DOI] [PubMed] [Google Scholar]
- 18. Clark JM, Saffold SH, Israel JM. Decellularized dermal grafting in cleft palate repair. Arch Facial Plast Surg 2003;5:40–5. [DOI] [PubMed] [Google Scholar]
- 19. Costantino PD, Govindaraj S, Hiltzik DH, Buchbinder D, Urken ML. Acellular dermis for facial soft tissue augmentation: preliminary report. Arch Facial Plast Surg 2001;3:38–43. [PubMed] [Google Scholar]
- 20. Fisher E, Frodel JL. Facial suspension with acellular human dermal allograft. Arch Facial Plast Surg 1999;1:195–9. [DOI] [PubMed] [Google Scholar]
- 21. Govindaraj S, Cohen M, Genden EM, Costantino PD, Urken ML. The use of acellular dermis in the prevention of Frey's syndrome. Laryngoscope 2001;111:1993–8. [DOI] [PubMed] [Google Scholar]
- 22. Gryskiewicz JM, Rohrich RJ, Reagan BJ. The use of Alloderm for the correction of nasal contour deformities. Plast Reconstr Surg 2001;107:561–71. [DOI] [PubMed] [Google Scholar]
- 23. Jackson IT, Yavuzer R. AlloDerm for dorsal nasal irregularities. Plast Reconstr Surg 2001;107:553–60. [DOI] [PubMed] [Google Scholar]
- 24. Kridel RW. Septal perforation repair. Otolaryngol Clin North Am 1999;32:695–724. [DOI] [PubMed] [Google Scholar]
- 25. Rhee PH, Friedman CD, Ridge JA, Kusiak J. The use of processed allograft dermal matrix for intraoral resurfacing: an alternative to split‐thickness skin grafts. Arch Otolaryngol Head Neck Surg 1998;124:1201–4. [DOI] [PubMed] [Google Scholar]
- 26. Rohrich RJ, Muzaffar AR. Rhinoplasty in the African‐American patient. Plast Reconstr Surg 2003;111:1322–41. [DOI] [PubMed] [Google Scholar]
- 27. Romo T 3rd, Sclafani AP, Sabini P. Reconstruction of the major saddle nose deformity using composite allo‐implants. Facial Plast Surg 1998;14:151–7. [DOI] [PubMed] [Google Scholar]
- 28. Rubin PA, Fay AM, Remulla HD, Maus M. Ophthalmic plastic applications of acellular dermal allografts. Ophthalmology 1999;106:2091–7. [DOI] [PubMed] [Google Scholar]
- 29. Shorr N, Perry JD, Goldberg RA, Hoenig J, Shorr J. The safety and applications of acellular human dermal allograft in ophthalmic plastic and reconstructive surgery: a preliminary report. Ophthal Plast Reconstr Surg 2000;16:223–30. [DOI] [PubMed] [Google Scholar]
- 30. Sinha UK, Chang KE, Shih CW. Reconstruction of pharyngeal defects using AlloDerm and sternocleidomastoid muscle flap. Laryngoscope 2001;111:1910–6. [DOI] [PubMed] [Google Scholar]
- 31. Sinha UK, Saadat D, Doherty CM, Rice DH. Use of AlloDerm implant to prevent Frey syndrome after parotidectomy. Arch Facial Plast Surg 2003;5:109–12. [DOI] [PubMed] [Google Scholar]
- 32. Terino EO. Alloderm acellular dermal graft: applications in aesthetic soft‐tissue augmentation. Clin Plast Surg 2001;28:83–99. [PubMed] [Google Scholar]
- 33. Buinewicz B, Rosen B. Acellular cadaveric dermis (AlloDerm): a new alternative for abdominal hernia repair. Ann Plast Surg 2004;52:188–94. [DOI] [PubMed] [Google Scholar]
- 34. Girard S, Sideman M, Spain DA. A novel approach to the problem of intestinal fistulization arising in patients managed with open peritoneal cavities. Am J Surg 2002;184:166–7. [DOI] [PubMed] [Google Scholar]
- 35. Guy JS, Miller R, Morris JA Jr, Diaz J, May A. Early one‐stage closure in patients with abdominal compartment syndrome: fascial replacement with human acellular dermis and bipedicle flaps. Am Surg 2003;69:1025–9. [PubMed] [Google Scholar]
- 36. Sinha UK, Shih C, Chang K, Rice DH. Use of AlloDerm for coverage of radial forearm free flap donor site. Laryngoscope 2002;112:230–4. [DOI] [PubMed] [Google Scholar]
- 37. Wax MK, Winslow CP, Andersen PE. Use of allogenic dermis for radial forearm free flap donor site coverage. J Otolaryngol 2002;31:341–5. [DOI] [PubMed] [Google Scholar]
- 38. Witt PD, Cheng CJ, Mallory SB, Lind AC. Surgical treatment of pseudosyndactyly of the hand in epidermolysis bullosa: histological analysis of an acellular allograft dermal matrix. Ann Plast Surg 1999;43:379–85. [DOI] [PubMed] [Google Scholar]
- 39. Lattari V, Jones LM, Varcelotti JR, Latenser BA, Sherman HF, Barrette RR. The use of a permanent dermal allograft in full‐thickness burns of the hand and foot: a report of three cases. J Burn Care Rehabil 1997;18:147–55. [DOI] [PubMed] [Google Scholar]
- 40. Munster AM, Smith‐Meek M, Shalom A. Acellular allograft dermal matrix: immediate or delayed epidermal coverage? Burns 2001;27:150–3. [DOI] [PubMed] [Google Scholar]
- 41. Sheridan RL, Choucair RJ. Acellular allogenic dermis does not hinder initial engraftment in burn wound resurfacing and reconstruction. J Burn Care Rehabil 1997;18:496–9. [DOI] [PubMed] [Google Scholar]
- 42. Sheridan R, Choucair R, Donelan M, Lydon M, Petras L, Tompkins R. Acellular allodermis in burns surgery: 1‐year results of a pilot trial. J Burn Care Rehabil 1998;19:528–30. [DOI] [PubMed] [Google Scholar]
- 43. Tsai CC, Lin SD, Lai CS, Lin TM. The use of composite acellular allodermis‐ultrathin autograft on joint area in major burn patients – one year follow‐up. Kaohsiung J Med Sci 1999;15:651–8. [PubMed] [Google Scholar]
- 44. Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full‐thickness burns. Burns 1995;21:243–8. [DOI] [PubMed] [Google Scholar]
- 45. Wainwright D, Madden M, Luterman A, Hunt J, Monafo W, Heimbach D, Kagan R, Sittig K, Dimick A, Herndon D. Clinical evaluation of an acellular allograft dermal matrix in full‐thickness burns. J Burn Care Rehabil 1996;17:124–36. [DOI] [PubMed] [Google Scholar]
- 46. Martin BR, Sangalang M, Wu S, Armstrong DG. Outcomes of allogenic acellular matrix therapy in treatment of diabetic foot wounds: an initial experience. Int Wound J 2005;2:161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Winters CL, Brigido SA, Liden BA, Simmons M, Hartman JF, Wright ML. A multi‐center study involving the use of a human acellular dermal regenerative tissue matrix for the treatment of diabetic lower extremity wounds. Adv Skin Wound Care 2008;21:375–81. [DOI] [PubMed] [Google Scholar]
- 48. Lavery LA, Armstrong DG, Harkless LB. Classification of diabetic foot ulcerations. J Foot Ankle Surg 1996;35:528–31. [DOI] [PubMed] [Google Scholar]
- 49. Lavery LA, Armstrong DG, Murdoch DP, Peters EJ and Lipsky BA. Validation of the Infectious Diseases Society of America’s diabetic foot infection classification system. Clin Infect Dis 2007;44:562–5. [DOI] [PubMed] [Google Scholar]
- 50. Brigido SA. The use of an acellular dermal regenerative tissue matrix in the treatment of lower extremity wounds: a prospective 16‐week pilot study. Int Wound J 2006;3:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eyre‐Brook AL. The periosteum: its function reassessed. Clin Orthop Relat Res 1984;189:300–7. [PubMed] [Google Scholar]
- 52. Gugala Z, Gogolewski S. Regeneration of segmental diaphyseal defects in sheep tibiae using resorbable polymeric membranes: a preliminary study. J Orthop Trauma 1999;13:187–95. [DOI] [PubMed] [Google Scholar]
- 53. Macnab I, De Haas WG. The role of periosteal blood supply in the healing of fractures of the tibia. Clin Orthop Relat Res 1974;105:27–33. [PubMed] [Google Scholar]
- 54. Malmquist JP. Successful implant restoration with the use of barrier membranes. J Oral Maxillofac Surg 1999;57:1114–6. [DOI] [PubMed] [Google Scholar]
- 55. Chaplin JM, Costantino PD, Wolpoe ME, Bederson JB, Griffey ES, Zhang WX. Use of an acellular dermal allograft for dural replacement: an experimental study. Neurosurgery 1999;45:320–7. [DOI] [PubMed] [Google Scholar]
- 56. Eppley BL. Experimental assessment of the revascularization of acellular human dermis for soft‐tissue augmentation. Plast Reconstr Surg 2001;107:757–62. [DOI] [PubMed] [Google Scholar]
- 57. Harris RJ. Root coverage with a connective tissue with partial thickness double pedicle graft and an acellular dermal matrix graft: a clinical and histological evaluation of a case report. J Peridontol 1998;69:1305–11. [DOI] [PubMed] [Google Scholar]
- 58. Livesey SA, Herndon DN, Hollyoak MA, Atkinson YH, Nag A. Transplanted acellular allograft dermal matrix. Potential as a template for the reconstruction of viable dermis. Transplantation 1995;60:1–9. [PubMed] [Google Scholar]
- 59. Sclafani AP, Romo T III, Jacono AA, McCormick S, Cocker R, Parker A. Evaluation of acellular dermal graft in sheet (AlloDerm) and injectable (Micronized AlloDerm) forms for soft tissue augmentation. Clinical observations and histological analysis. Arch Facial Plast Surg 2000;2:130–6. [DOI] [PubMed] [Google Scholar]
- 60. Malcarney HL, Bonar F, Murrell GA. Early inflammatory reaction after rotator cuff repair with a porcine small intestine submucosal implant: a report of 4 cases. Am J Sports Med 2005;33:907–11. [DOI] [PubMed] [Google Scholar]
- 61. Zheng MH, Chen J, Kirilak Y, Willers C, Xu J, Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater 2005;73:61–7. [DOI] [PubMed] [Google Scholar]
- 62. Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta‐analysis. Diabetes Care 1999;22:692–5. [DOI] [PubMed] [Google Scholar]


