Abstract
The prognosis of stage I pressure ulcers cannot be predicted; therefore, nursing interventions for preventing their deterioration have not been clearly established. This study describes the clinical course of stage I pressure ulcers and prospectively investigates the factors related to their deterioration. Thirty‐one stage I pressure ulcers in 30 patients in a long‐term care facility were studied, and morphological changes were assessed every day until the ulcers healed or deteriorated. The physiological changes were assessed by ultrasonography and thermography. Twenty ulcers healed, and 11 deteriorated. The characteristics of deterioration were as follows: (1) double erythema; (2) non blanchable erythema across the whole area determined by glass plate compression; (3) erythema away from the tip of the bony prominence; and (4) expanding erythema on the following day. We analysed the sensitivity, specificity, positive predictive value, negative predictive value and positive likelihood ratio for the diagnostic utility of the indicators of deterioration double erythema and distance from the tip of bony prominence, which can be instantly assessed without the use of any special device. The values were 36·4%, 95·0%, 80·0%, 73·1% and 7·28, respectively. These results suggest that clinicians can predict the prognosis of stage I pressure ulcers by initial assessment and provide appropriate care based on the assessment.
Keywords: Deep‐tissue injury, Descriptive study, Pressure ulcer
Introduction
Early identification of pressure ulcers leads to quicker healing. Therefore, it is important to identify stage I pressure ulcers according to the National Pressure Ulcer Advisory Panel (NPUAP) classification (1). Recently, it has been shown that stage I pressure ulcers may include deep‐tissue injury (DTI), which involves subcutaneous damage without skin breakdown, and some cases of DTI progress to full‐thickness ulcers 2, 3. On the other hand, some stage I pressure ulcers heal without skin breakdown. However, it is difficult to identify DTI at the early stages in the clinical setting; therefore, prevention and management strategies for stage I pressure ulcers are not well established. The distinction between stage I pressure ulcers that progress to full‐thickness ulcers and those that heal should be clarified to establish appropriate and intensive nursing care.
In 1991, Lyder stated that identification of stage I pressure ulcers should be based not only on visual examination but also on palpation, based on his study on coloured races (4). Sprigle et al. investigated the temperature difference between areas of erythema and the surrounding tissue and demonstrated the usefulness of monitoring temperatures of areas of erythema for assessing deep‐tissue damage (5). Sprigle et al. also showed that persistent erythema tended to become non blanchable, by investigating the clinical indicators of localised erythema, and also reported on the usefulness of spectroscopy for identifying stage I pressure ulcers in coloured patients (6). As stated, the methodology for identification of stage I pressure ulcers has been well documented; however, the prediction of prognosis of stage I pressure ulcers remains unclear.
Some cases of pressure‐induced superficial damage (stage I pressure ulcers) progress to the deeper tissues, including full‐thickness wounds (stage III or IV pressure ulcers), and this pathological condition is now regarded as DTI. DTI is usually described as purple or evolving ulcers 2, 7 with a mirror image (8). There is a risk for persistent erythema developing to DTI (6). One of main causes of DTI is prolonged pressure due to the body remaining in the same position for an extended period of time 9, 10. Although clinical knowledge is being gradually accumulated, obvious evidence of DTI has not been established, due to a lack of detailed descriptive studies of its prognosis and pathological examination. In consequence, the prognosis of stage I pressure ulcers, which are characterised by non blanchable erythema with intact skin, cannot be predicted; therefore, nursing interventions for preventing deterioration of stage I pressure ulcers are not clearly established.
The purpose of this study was to describe the clinical course of stage I pressure ulcers and prospectively investigate the factors related to their deterioration.
Materials and methods
Subjects
Patients in whom a stage I pressure ulcer was identified according to the NPUAP classification system between October 2002 and October 2003 in a long‐term care facility (Ishikawa, Japan) were included.
Design
This was a descriptive and prospective cohort study.
Procedure
Morphological characters of stage I pressure ulcers were prospectively observed in all patients. Physiological parameters were measured at the initial assessment point. After the final form of the ulcers was determined, ulcers were classified into a healed group and a deteriorated group. The healed group included ulcers in which erythema disappeared without progression to full‐thickness ulcers. The deteriorated group included ulcers that progressed to blister, eroded or deteriorated.
We compared the morphological characteristics and physiological parameters of the two groups and extracted the significant factors. The predictive prognostic value of these main characteristics for stage I pressure ulcers was assessed using diagnostic statistical methodology.
Survey contents
After receiving reports of erythema incidence from ward nursing staff, a wound ostomy and continence nurse assessed the lesion and determined whether it was a pressure ulcer by the glass plate compression method. After determination, the following parameters were investigated every day after the first sign of erythema.
Morphological characteristics
Morphological characteristics of ulcer included area, shape, presence of well‐demarcated areas, hardness and warmth of the central and marginal regions of erythema, sensation of pain in the erythema area and the presence or disappearance of erythema after glass plate compression. These morphological characteristics were obtained from detailed sketches of the wound. The distance between the central region of erythema and the top of the bony prominence was also measured. The area was measured using image analysis software (Scion Image Beta 4·02 for Windows 95 to XP, Scion Corporation, Frederick, MD).
Physiological parameters
The extent of deep‐tissue damage was assessed by ultrasonography (7·5 MHz, SSD‐500, Aloka, Tokyo, Japan). We scanned the entire lesion for the presence of a hypoechoic region, which is indicative of tissue damage or tissue fluid. The temperature of the central region of erythema and the surrounding skin, defined as 2 cm from the erythema region, was measured by thermography (TH5108ME, NEC san‐ei Instruments, Tokyo, Japan). We took an average of each temperature value in the erythema region and the surrounding skin.
Ethical considerations
Informed consent was obtained when the patients developed stage I pressure ulcers. In case of difficulty in communicating, consent was obtained from his/her family. When investigating the pressure ulcers, we confirmed the medical condition of the patients. The study protocol conformed to the ethical guidelines of the Japan Ministry of Health, Labour and Welfare.
Statistics
To compare each parameter between the healed and the deteriorated groups, the Mann–Whitney and Fisher exact probability tests were used. To assess the validity of the prognostic indicators, diagnostic accuracy was assessed. P < 0·05 was considered statistically significant.
Results
Patient characteristics
Thirty‐eight stage I pressure ulcers were detected by ward nurses. Seven ulcers were excluded (two due to withdrawal of patients from the study and five due to deterioration of medical conditions of the patients), giving a total of 31 ulcers from 30 patients. Twenty ulcers (64·5%) from 19 patients were categorised into the healed group and 11 ulcers (35·5%) from 11 patients into the deteriorated group. Patient characteristics are summarised in Table 1. The ulcer locations were distributed throughout the body (Table 2).
Table 1.
Patient characteristics*
| Healed group | Deteriorated group | |
|---|---|---|
| No. of patients | 19 | 11 |
| No. of ulcers | 20 | 11 |
| Age (years) | 83 (62–96) | 80 (68–96) |
| No. of women (%) | 8 (42·1) | 6 (54·5) |
| Braden score | 13 (7–20) | 10 (6–16) |
| Diagnosis (%) | ||
| Cerebrovascular disease | 12 (72·7) | 8 (72·7) |
| Diabetes mellitus | 1 (5·3) | 2 (18·2) |
| Spinal cord injury | 0 (0·0) | 1 (9·1) |
| Dementia | 3 (15·8) | 0 (0·0) |
| Low back pain | 2 (10·5) | 0 (0·0) |
| Pneumonia | 1 (5·3) | 0 (0·0) |
Values represent median with range.
Table 2.
Location of pressure ulcers, n (%)
| Region | Healed group (n= 20) | Deteriorated group (n= 11) |
|---|---|---|
| Greater trochanter | 8 (40·0) | 2 (18·2) |
| Sacrum | 5 (25·0) | 2 (18·2) |
| Outside of dorsum pedis | 2 (10·0) | 1 (9·1) |
| Planta pedis | 2 (10·0) | 0 (0·0) |
| Heel | 1 (5·0) | 3 (27·2) |
| Posterior iliac crest | 1 (5·0) | 0 (0·0) |
| Vertebra | 1 (5·0) | 0 (0·0) |
| Coccyx | 0 (0·0) | 2 (18·2) |
| Fibula | 0 (0·0) | 1 (9·1) |
Morphological changes in the healed group
The ulcers in the healed group were of circular or oval form, and they had an appearance of non homogeneous erythema. Some of the ulcers showed non blanchable erythema in their entirety, and others showed partially blanchable erythema determined by glass plate compression. Fourteen ulcers (70·0%) were located just above the bony prominence. The median distance between the centre of the erythema region and the tip of the bony prominence was 0 cm (range 0–3·2 cm) and the median area was 2·9 cm2 (range 1·0–23·7 cm2).
Three ulcers (15·0%) had double erythema, defined as an area of strong erythema, and the redness faded away and the ulcers became reduced in size on the fifth or sixth day after detection. On the eighth day, the redness faded further, and the double erythema region presented as brown discolouration. Finally, on the 13th or 14th day, stage I pressure ulcer area was totally pigmented and the skin did not appear to breakdown. Seventeen ulcers (85·0%) without double erythema faded across the whole area and their size was reduced on the fifth or sixth day. On the seventh or eighth day, pigmentation occurred at the outer edge and in the erythema region, and it disappeared with glass plate compression (Figure 1).
Figure 1.
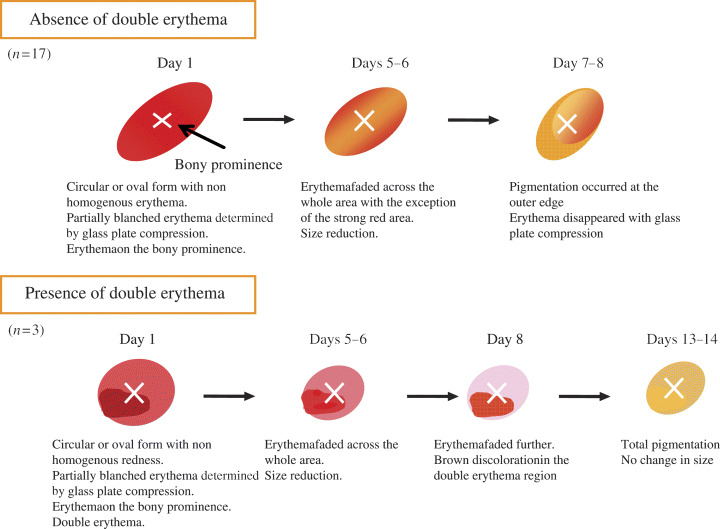
Morphological changes in the healed group (n= 20).
Morphological changes in the deteriorated group
The ulcers were irregularly shaped, and non homogeneous erythema was detected in the deteriorated group. Double erythema was observed in nine ulcers (81·8%) and appeared purple with induration. The erythema area represented such strong swelling that the skin texture could not be observed. The redness did not disappear with glass plate compression. The median distance between the centre of redness and the tip of the bony prominence was 2·7 cm (range 2.0–3·0 cm) and the median of area was 3·2 cm2 (range 1·2–7·4 cm2). On the second day, the colour of the erythema region became intense, and erythema expanded in size. The ulcers developed blistering (n= 4, 36·4%), erosion (n= 6, 54·5%) or bone exteriorisation (n= 1, 9·1%). In the blistering group, the central region was blistered with pigmentation at the edge of lesion, between the fourth and seventh days. In the erosion group, abrasion was observed in the double erythema area between the third and sixth days. One ulcer developing bone exteriorisation presented brown discolouration, with a bluish region, and increased in size on the 20th day. A black eschar was observed between the bluish and double erythema regions (Figure 2).
Figure 2.
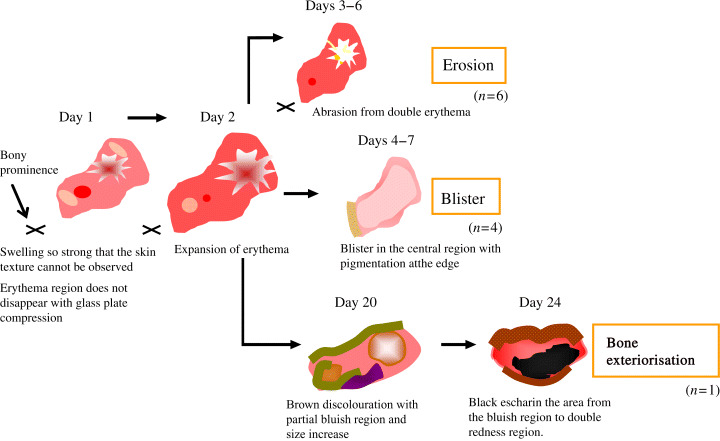
Morphological changes in the deteriorated group (n= 11).
Morphological characteristics of the deteriorated group
By comparing the morphological changes in the healed and deteriorated groups on the first and second days, four characteristics were noted: (1) double erythema; (2) non blanchable erythema across the whole area determined by glass plate compression; (3) erythema away from the tip of the bony prominence; and (4) an expanding erythema on the following day.
The size of double erythema in the deteriorated group was significantly larger than that in the healed group (χ2= 16·79, P < 0·001; Table 3).
Table 3.
Presence of double erythema, n (%)*
| Healed group (n= 20) | Deteriorated group (n= 11) | χ2 | P value | |
|---|---|---|---|---|
| Positive | 3 (15·0) | 10 (90·9) | ||
| 16·79 | <0·001 | |||
| Negative | 17 (85·0) | 1 (9·1) | ||
Fisher exact probability test.
Non blanchable erythema across the whole area, with glass plate compression, was observed more frequently in the deteriorated group (χ2= 8·957, P = 0·007; Table 4).
Table 4.
Presence of non blanchable erythema across the whole area determined by glass plate compression, n (%)*
| Healed group (n= 20) | Deteriorated group (n= 11) | χ2 | P value | |
|---|---|---|---|---|
| Positive | 7 (35·0) | 10 (90·9) | ||
| 8·957 | 0·007 | |||
| Negative | 13 (65·0) | 1 (9·1) | ||
Fisher exact probability test.
To compare the distance between the erythema region and the tip of the bony prominence, we excluded the ulcers occurring in the foot because there was little subcutaneous tissue in the foot; therefore, skin pouches or creases were less likely to occur. After five ulcers in the healed group and four in the deteriorated group were excluded, the difference reached statistical significance (U= 12·00, P= 0·003; Table 5).
Table 5.
Erythema away from the tip of the bony prominence (cm)*
| Healed group (n= 15) | Deteriorated group (n= 7) | U | P value | |
|---|---|---|---|---|
| Distance | 0 (0–3·2) | 2·7 (2·0–3·0) | 12·00 | 0·003 |
Values represent median (range). Mann–Whitney test.
The expanded erythema region was determined by calculating the difference between the area of redness on the day of detection and that on the following day. The median difference was −0·77 cm2 (range −14·1 to 1·08 cm2) in the healed group and 0·44 cm2 (range 0·15–6·02 cm2) in the deteriorated group, indicating significant expansion in the ulcer area in the deteriorated group (U= 1·00, P < 0·001; Table 6).
Table 6.
Change in area of redness on the following day (cm2)*
| Healed group (n= 20) | Deteriorated group (n= 11) | U | P value | |
|---|---|---|---|---|
| Difference in area | −0·77 (−14·1 to 1·08) | 0·44 (0·15–6·02) | 1·00 | <0·001 |
Values represented median (range). Mann–Whitney test.
Physiological characteristics of the deteriorated group
Ultrasonography indicated the presence of a hypoechoic region between the epidermis and dermis in 18 ulcers (90·9%) in the healed group. A hypoechoic region in the deep tissues, which indicated DTI, was observed in two ulcers (10·0%) in the healed group and six (54·5%) in the deteriorated group (Figure 3, Table 7, [link]). The median difference between temperatures in the erythema and healthy regions was 0·2°C (range −0·7 to 1·3°C) in the healed group and −0·1°C (range −0·9 to 0·6°C) in the deteriorated group. The significant decrease was seen in the deteriorated group (U= 55·50, P= 0·049; Table 8).
Figure 3.
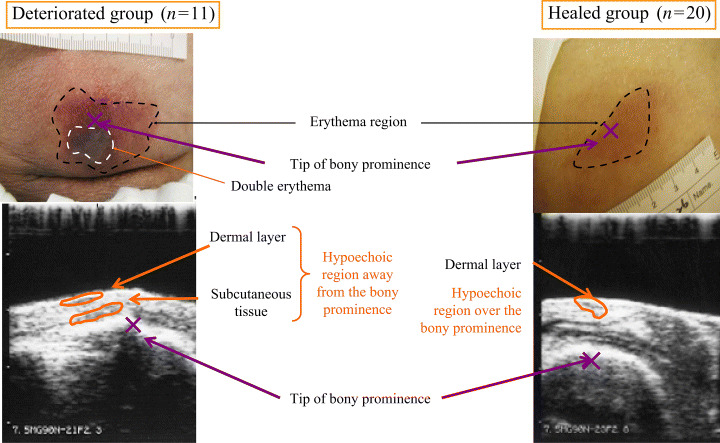
Ultrasonography of the deteriorated and healed stage I pressure ulcer.
Table 7.
Presence of hypoechoic regions in the deep tissues, n (%)*
| Healed group (n= 20) | Deteriorated group (n= 11) | χ2 | P value | |
|---|---|---|---|---|
| Positive | 2 (10·0) | 6 (54·5) | ||
| 7·355 | .012 | |||
| Negative | 18 (90·0) | 5 (45·5) | ||
Fischer exact probability test.
Table 8.
Comparison of temperature difference between erythema and healthy regions (°C)*
| Healed group (n= 20) | Deteriorated group (n= 11) | U | P value | |
|---|---|---|---|---|
| Difference in temperature | 0·2 (−0·7 to 1·3) | −0·1 (−0·9 to 0·6) | 55·50 | 0·049 |
Values represent median (range). Mann–Whitney test.
Prediction of prognosis of stage I pressure ulcers
Four of the above‐mentioned characteristics of deteriorated erythema were suggested in the present study. For clinical implications, we suggest that the feasibility of predicting deterioration is of importance. Therefore, we used two characteristics presence or absence of double erythema and erythema away from the tip of the bony prominence (0 cm or farther) as parameters because the other two characteristics require special devices for assessment or prospective assessment. We defined the lesion as showing erythema away from the tip of the bony prominence if lesion was >0 cm. We analysed the sensitivity, specificity, positive predictive value, negative predictive value, and positive likelihood ratio for the diagnostic value of the indicators for deterioration, double erythema and distance from the tip of the bony prominence. The values were 36·4%, 95·0%, 80·0%, 73·1%, and 7·28, respectively (Table 9).
Table 9.
Prediction of stage I pressure ulcer deterioration (n)*
| Prognosis | ||||
|---|---|---|---|---|
| Healed | Deteriorated | Total | ||
| Test | Positive | 1 | 4 | 5 |
| Negative | 19 | 7 | 26 | |
| Total | 20 | 11 | 31 | |
Test parameters included double erythema and erythema away from the tip of the bony prominence.
Discussion
Knowing the clinical course of stage I pressure ulcers is essential for their appropriate management and prevention of their deterioration. This study is the first attempt to prospectively describe the chronological changes in the erythema region immediately after its development. Morphological and physiological differences between the healed and deteriorated ulcers were observed, and four characteristics of deteriorated erythema were newly established: (1) double erythema; (2) non blanchable erythema across the whole ulcer area determined by glass plate compression; (3) redness away from the tip of the bony prominence; and (4) expanding redness on the following day. We now discuss these phenomena as they occur in the deep tissues and propose suggestions for nursing care to prevent deterioration of stage I pressure ulcers.
Causes of deterioration of erythema
Double erythema indicating DTI
Double erythema appeared purple with induration. It was reported that erythema with a purple region was indicative of a full‐thickness ulcer (2). Hypoechoic regions in the deep tissues were observed under the purple area with induration, which indicated the accumulation of exduate, resulting from an inflammation response. Therefore, this ultrasonographic feature suggests that DTI under the intact skin occurs in cases of deteriorated erythema. Histopathological examination of non blanchable redness revealed red blood cell engorgement of the capillaries and venules, and perivascular and diffuse haemorrhage (11). These findings suggest that the purple region is indicative of red blood cell leakage following vessel disruption, and the red region indicates red blood cell engorgement of the capillaries. In consequence, double erythema indicates dermal injury and DTI without epidermal damage.
Non blanchable erythema across the whole area determined by glass plate compression, indicates reduced blood flow
From a study of the histopathological aspects of non blanchable erythema, Witkowski and Parish reported that prolonged compression leads to the development of red blood cell engorgement of capillaries and venules, followed by perivascular and later diffuse haemorrhage (11). From a physiological standpoint, Sprigle et al. reported that there were temperature differences between the erythema and healthy regions (5). Our results also showed that there was a lower temperature in the erythema region in the deteriorated group. This could have been caused by blood flow obstruction in the capillaries and venules; therefore, the non blanchable erythema was indicative of reduced blood flow.
Erythema away from the tip of the bony prominence indicating skin shearing
Erythema in the deteriorated group was located away from the tip of the bony prominence. This could have been due to deep‐tissue ischaemia caused by skin shearing during off‐balance posture. Pressure ulcers are caused by ischaemia due to compression, and shear and friction are regarded as related local factors for pressure ulcer development 12, 13. Horizontal shear force occurs when two materials in contact with each other move in opposite directions, producing distortion of the skin surface and/or deep tissue (14). Reichel stated that shear force twists the capillaries, resulting in tissue deformation and ischaemia in the deep tissues (15). Therefore, erythema away from the tip of the bony prominence indicates shear force production and causes severe tissue ischaemia, resulting in deterioration of stage I pressure ulcers.
Expanding erythema indicating persistent reduced blood flow
In the present study, the ulcer size expanded significantly on the day after detection in the deteriorated group, indicating that the presence of persistent erythema likely leads to skin breakdown, a finding also reported by Sprigle et al. (6). Expansion of the erythema area suggests that increased vascular endothelial damage, due to blood flow obstruction of capillaries and venules, increases platelet accumulation, inflammation and vascular destruction (16). Expanding erythema may be caused by persistent reduced blood flow due to vascular obstruction by shear force, as well as compression of the deep tissue beneath the bony prominence.
Prediction of stage I pressure ulcer deterioration
The present study suggested the four above‐mentioned characteristics of deteriorated erythema. With regard to the implications for clinical practice, we suggest that the feasibility of predicting deterioration is of importance. Therefore, we used two characteristics double erythema and erythema away from the tip of the bony prominence as parameters because other two characteristics requires special device for assessment or prospective assessment. The predictive value is considered to be high enough for clinical practice. These results indicate that initial assessment of stage I pressure ulcers is so important that clinicians can predict lesion prognosis. Therefore, observation of these two parameters by nurses when detecting stage I pressure ulcers shows that the ulcers have DTI with reduced blood flow, so pressure reduction or avoidance of shear force should be carried out.
Limitations and future issues
Participants in this study were aged 65 years or more, so we could not assess age‐related factors for deterioration. We consider that some age‐related differences can be seen in pressure ulcer development and natural history because the structure of the dermal and epidermal layers, and the mechanical properties of the subcutaneous tissues, may alter as age increases. In addition, this study included a small number of patients, which made it difficult to estimate the risk factors for deterioration by multiple logistic regression analysis. Investigation for patients with different pressure ulcer risks should be conducted. Moreover, nursing intervention studies should also be conducted to clarify the effectiveness of prevention of deterioration of stage I pressure ulcers with double redness and skin shear.
Authorship
Miwa Sato: concept and design, acquisition of subjects and data, analysis and interpretation of data, preparation of manuscript. Hiromi Sanada, Chizuko Konya and Junko Sugama: concept and design, analysis and interpretation of data, preparation of manuscript. Gojiro Nakagami: analysis and interpretation of data, preparation of manuscript.
References
- 1. The National Pressure Ulcer Advisory Panel . Pressure ulcers prevalence, cost and risk assessment: consensus development conference statement – the National Pressure Ulcer Advisory Panel. Decubitus 1989;2:24–8. [PubMed] [Google Scholar]
- 2. Black JM. Unusual wounds: deep tissue injury. Wounds 2003;15:380. [Google Scholar]
- 3. Russell L. Pressure ulcer classification: defining early skin damage. Br J Nurs 2002;11:S33–41. [DOI] [PubMed] [Google Scholar]
- 4. Lyder CH. Conceptualization of the stage 1 pressure ulcer. J ET Nurs 1991;18:162–5. [DOI] [PubMed] [Google Scholar]
- 5. Sprigle S, Linden M, McKenna D, Davis K, Riordan B. Clinical skin temperature measurement to predict incipient pressure ulcers. Adv Skin Wound Care 2001;14:133–7. [DOI] [PubMed] [Google Scholar]
- 6. Sprigle S, Linden M, Riordan B. Analysis of localized erythema using clinical indicators and spectroscopy. Ostomy Wound Manage 2003;49:42–52. [PubMed] [Google Scholar]
- 7. Witkowski JA. Purple ulcers. J ET Nurs 1993;20:132. [PubMed] [Google Scholar]
- 8. Donnelly J.; National Pressure Ulcer Advisory Panel. Should we include deep tissue injury in pressure ulcer staging systems? The NPUAP debate. J Wound Care 2005;14:207–10. [DOI] [PubMed] [Google Scholar]
- 9. Vermillion C. Operating room acquired pressure ulcers. Decubitus 1990;3:26–30. [PubMed] [Google Scholar]
- 10. Aronovitch SA. Intraoperatively acquired pressure ulcer prevalence: a national study. J Wound Ostomy Continence Nurs 1999;26:130–6. [DOI] [PubMed] [Google Scholar]
- 11. Witkowski JA, Parish LC. Histopathology of the decubitus ulcer. J Am Acad Dermatol 1982;6:1014–21. [DOI] [PubMed] [Google Scholar]
- 12. Agency for Health Care Policy and Research. Pressure ulcers in adults: prediction and prevention. Quick Reference Guide for Clinicians. 1992. (AHCPR 92.0050) [PubMed]
- 13. Braden B, Bergstrom N. A conceptual schema for the study of the etiology of pressure sores. Rehabil Nurs 1987;12:8–12. [DOI] [PubMed] [Google Scholar]
- 14. Fontaine R, Risley S, Castellino R. A quantitative analysis of pressure and shear in the effectiveness of support surfaces. J Wound Ostomy Continence Nurs 1998;25:233–9. [PubMed] [Google Scholar]
- 15. Reichel SM. Shearing force as a factor in decubitus ulcers in paraplegics. JAMA 1958;166:762–3. [DOI] [PubMed] [Google Scholar]
- 16. Barton AA, Barton M. The management and prevention o pressure sores. London: Faber, 1981. [Google Scholar]


