Abstract
Over the past 30 years as caregivers, clinicians have been exposed to a plethora of new advanced wound dressings. The moist wound care revolution began in the 1970s with the introduction of film and hydrocolloid dressings, and today these are the traditional types of dressings of the advanced dressing categories. Wound‐healing science has progressed significantly over the same period, as a result of intense clinical and scientific research around these product introductions. Today, the clinician understands moist wound healing, occlusion, cost effectiveness, wound bed preparation and MMP activity to name but a few of the many concepts in wound care that have flourished as a result of technology and product advancement. This review article presents a condensed history of dressing development over the past 30 years. However, in addition, such advancement is discussed in respect to its adoption in different parts of the world. The largest single markets of the world are generally the United States of America and Europe; as such, the development of both practice and technology generally begins there. Much has been written about these markets in previous review articles. For the purposes of this review, the development of wound care and the maturing of practice is discussed in respect to Canada, Japan and Australia representing smaller geographical areas where the development has been more recent but nonetheless significant.
Keywords: Dressing, Wound, Occlusive, Modern, Review, Australia, Japan, Canada
Introduction
Many articles have been written concerning the development of both product (1, 2, 3, 4) and technology over the past three decades 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25), often updates from the last. As such, these reviews have presented a regional perspective of the development of modern wound care during this period.
The development of the modern wound care concept and adoption of advanced products, however, are not universal (i.e. global) even today. Different markets develop at different times. Change of practice takes time and also differs by geographical region and culture.
Certain areas lead the way (e.g. the United States of America and United Kingdom), often as a result of market size and the globality of the English language. Other geographical areas follow, each learning from the other, which in turn results in a more rapid and focused development of practice and product.
Today, wound care is a global arena, with most geographical areas having some elements of technology and product, in addition to standards of practice, albeit at different stages of the evolutionary wound care path.
A dressing history
The management of wounds began in Egyptian times (26) with grease‐soaked gauze bandages – with little thought to wound management. Over the centuries, such care has become a little more sophisticated, but its primary goal – that of healing – remains the same. Traditional dressings such as gauze are non occlusive and dry out. Once this happens, they adhere to the wound bed. Even if they do not dry out, capillary loops (i.e. granulation tissue) can grow into the dressing structure (27), thereby resulting in dressing adherence. Such adherence leads to wound trauma, often noted with bleeding during dressing removal, and this can cause pain to the patient.
During the 1980s, wound care took a new direction with the widespread introduction of moist wound healing (28). In the next two decades, much has been proven around the benefits of moist healing.
Wound dressings have been designed to function in a specific manner. Often, their interaction with adjunct/complementary devices may also have been designed for or at least clinically evaluated. The choice of product for optimal wound management is not straightforward. This choice should not be for a single wound factor or indeed one specific function. The wound, the patient and their multiple needs should also be considered (29).
Optimal wound care wisdom understands and promotes the need for a moist interactive dressing in chronic wounds with the ability to heal (30). Unfortunately, practice does not necessarily follow and a large number of inappropriate dressings are still used today. Any treatment choice should be cognisant of other patient‐centred factors involved in the quality of their life in addition to healing.
Moist interactive wound care has been around for the last three decades. Therefore, the concept of moist wound healing is not new. Indeed, it is some 40 years since Winter (31) first published his findings regarding ‘keeping a wound moist’. But, even today, there is low use of this moist wound concept in regular wound care practice, albeit at a growing trend. Documentation of moist wound‐healing practices varies, depending on location and care setting, but it is generally accepted that less than 50% of chronic wounds receive modern moist wound dressings even when they are appropriate (32).
The main justifications are budget constraints (cost and unavailability) and lack of knowledge, particularly in routine health care providers, the result being that a large number of inappropriate dressings are applied to patients on a routine basis. These are mainly gauze‐based dressings, which do little for healing.
The basis for Winter's findings was faster healing, where a plastic cover created a moist environment. Others (33) went on to study this phenomenon in humans and demonstrated not only faster healing but better tissue quality [less scarring (34)] and reduced pain (35).
Dressing manufacturers have been cognisant of these findings for some time, and as a result, an evolutionary development process, through innovation, has provided the comprehensive range of moist interactive dressings available today.
The moist interactive dressings of today work on the same principle. By creating a moist environment, not only do they aid healing, they also soothe nerve endings, minimising or eliminating wound pain, allowing healing to progress more naturally.
During the evolutionary development of these products, manufacturers have become aware of product shortcomings and have designed better product variants. The modern wound care revolution truly began in the late 1980s and early 1990s, with an explosion of products and significant scientific/clinical research around the area of moist healing. It is now routinely accepted among key opinion leaders that moist wound healing has been shown to be superior with respect to wound management, when compared with dry dressings 35, 36, 37, 38, 39, 40, 41). This is not solely based on healing but a number of patient‐ and wound‐related factors as presented in the wound bed preparation paradigm (42).
The wound bed preparation paradigm discussed by Sibbald et al. (42) involves treating the cause, local wound care and patient‐centred concerns (Figure 1). Treating the cause revolves around the correct diagnosis of the wound aetiology. Patient‐centred concerns must focus on what the patient sees as the primary reasons for receiving treatment for their wound. Local wound care needs to revolve around the three pillars of local wound care practice: debridement, bacterial balance/prolonged inflammation and moisture balance.
Figure 1.
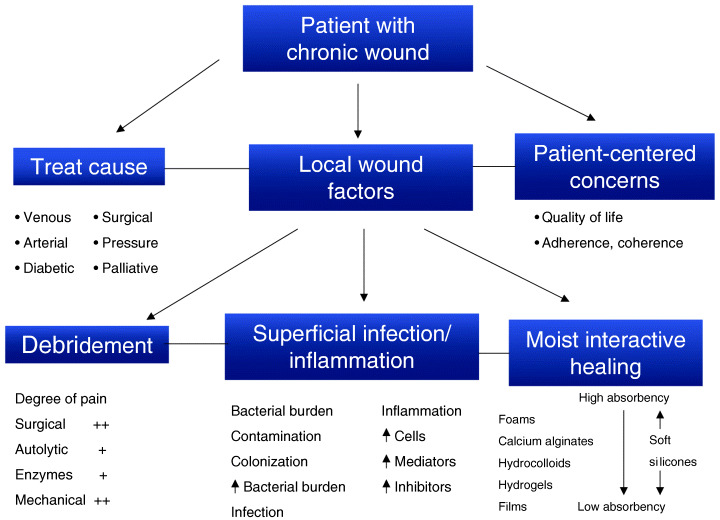
The chronic wound paradigm [adapted from Sibbald et al. (42)].
The three key considerations of local wound care, as outlined in Figure 1, are debridement, bacterial balance/prolonged inflammation and moist interactive healing.
Wound debridement can be achieved through different means, namely surgical, autolytic, enzymatic and mechanical (43). A number of factors come into play when choosing an appropriate debridement method (43). Care should be taken regarding the chosen method, as each can have a negative or positive impact on wound pain. More aggressive debridement regimes (e.g. surgical and mechanical) are initially detrimental to healing and more likely to be painful for the patient.
Wound infection (both in the superficial and in deep compartments) and prolonged inflammation can delay healing and may present with similar features clinically. Maintenance of wound bed bacterial balance can be of significant benefit in wound management, with increased local bacterial burden leading to the release of pro‐inflammatory mediators and modulators of inflammation that result in local pain (44) and delayed healing.
The healing revolution
The initial moist interactive dressings were polyurethane films that were designed around Winter's initial findings. They simply adhered to the surrounding skin and maintained moisture within the wound environment. These dressings provided some pain relief by preventing dehydration of the wound surface and bathing the exposed nerve endings in physiological wound secretions. The aggressive adhesive, however, sometimes caused trauma upon removal (45), although recently developed removal techniques help to minimise this issue (46). Strong adhesive bonds in these dressings are likely to cause skin tears on removal, unless the adhesive bond is weakened by stretching the dressing laterally and parallel to the wound surface before trying to remove the dressing by gently lifting at a 90° angle above the wound surface. Despite the precautions with removal, their non absorbency continues to be a problem. When fluid accumulates below the surface or leakage channels break the seal to the external environment, bacterial proliferation is facilitated.
Following the limitations of the film dressings, a number of more absorbent moist wound care categories were developed and a plethora of products followed:
-
•
hydrocolloids 47, 48, 49, 50, 51, 52) were shown to produce a moist environment by gelling with wound fluid over the wound bed and below the semi‐occlusive film covering;
-
•
foams 53, 54, 55, 56, 57, 58, 59, 60, 61) provided an easy‐to‐remove non adhesive contact surface (some newer products contain adhesive surfaces);
-
•
alginates (62, 63) transform from a fibre to a gel with wound fluid contact and therefore provide a non stick wound contact surface and a moist wound environment;
-
•
hydrogels 64, 65, 66, 67, 68, 69, 70, 71, 72, 73) provided high water content in a gel lattice that rendered them non adherent and soothing and provided the wound with the necessary moisture.
All of these new products were designed with moist wound healing in mind, seeking the ‘Holy Grail’ of healing. Some products were designed to provide security with regard to adhesion, while maintaining a moist environment. Other products provide moisture balance by absorbing exudate. Finally, for optimal pain control, products needed to have a non stick, soothing nature (e.g. hydrogels). Continued and more widespread use of these products has provided insight into their advantages as well as their limitations.
Film and hydrocolloid dressings, with their aggressive adhesives, can result in skin stripping of the wound margins, if they are inappropriately removed (74, 75). Foams can stick to the wound bed if wound exudation is low or has decreased during use (76).
Some products rely heavily on secondary coverings for retention (e.g. hydrogels), but product combinations can sometimes be detrimental to wound healing. For example, an absorptive secondary covering may remove the hydrogel moisture in the secondary layer and dehydrate the wound bed. On the other hand, if an adhesive film is used over an amorphous hydrogel, the excess moisture delivered to the wound margins can cause maceration and wound deterioration.
Alginates suffer from a combination of the limitations experience by both foam and hydrogel dressings (77). Alginates absorb wound fluid onto their fibres, and if they become supersaturated in their gel transformation, they may cause maceration of the surrounding skin or strikethrough of excess exudate, through any secondary dressing. If a wound is too dry to transform an alginate fibre into a hydrogel‐like material, then the wound surface remains dry and the undissolved fibres do not provide moist interactive healing.
These findings led manufacturers to develop second‐ and third‐generation products of existing devices (e.g. better hydrocolloids or foams) (78, 79). Several variants of modern dressing classes appeared with wound contact surfaces to reduce adhesion (80). Absorptive fibres (e.g. hydrofibres) 81, 82, 83, 84, 85, 86) and next‐generation hydrocolloid dressings were developed to minimise adhesion to the wound, increase the absorption and permit painless removal of the dressings (87, 88).
These dressings were followed by the development of specialised wound contact materials that were purposely developed for pain management through easy non traumatic removal. Materials were specifically redesigned to have coatings that did not dry out and therefore remained non adherent. The most popular coating is silicone.
Silicones are not new to wound care; indeed, they have been around in the burns area for some 30 years (89, 90). The majority of their use has been as non adherent silicone gel sheets for the treatment of burns (91) and in the resolution of hypertrophic scars (92). Silicones provide painless removal (93). These materials have now progressed beyond simple gels and coatings, recently being redeveloped as soft silicone dressing technology.
Soft silicone dressings rely on a hydrophobic soft silicone layer that prevents the dressing from adhering to the wound surface. They do so by maintaining contact without causing friction and shear, thereby reducing the tear force on removal.
The soft silicones are among the first products to be specifically designed for atraumatic removal from the wound and surrounding skin, with a focus on pain management (94). A variety of product variants exist, providing a versatile technology for a number of clinical situations (95).
A biological approach
More sophisticated biological approaches have been around for the over the 30 years in which the development of advanced wound care has taken place. Such an approach began in the burns area with the use of artificial skin substitutes (96), although these have become significantly more advanced 97, 98, 99, 100) with the recent approvals of Dermagraft (101), Apligraf (102) and similar modalities.
Biologically active dressings, based on collagen (103, 104), chitosan (105), hyaluronic acid (106, 107), peptides (108) and growth factors (109, 110), have been developed and evaluated, but most still require pivotal data to substantiate widespread use in beyond‐difficult‐to‐heal wounds. This generally relates to their significant unit cost and the unavailability of good cost‐effective data.
Recently, clinicians have seen the reintroduction of maggots into medical practice. This is a novel biological approach to wound debridement and cleansing (111). A number of commercial organisations now make these available in a number of countries.
Recent developments around the antimicrobial dressing's category (112, 113) and regenerated cellulose‐collagen dressings (114) have given significant focus on the area of metalloproteases and other pro‐inflammatory mediators (cytokines/chemokines) in wound healing. Current research into the function of these products is greatly adding to the knowledge in this area.
Alternative approaches
Some non dressing approaches to wound healing exist. These generally centre on negative pressure therapy (115, 116), hyperbaric oxygen (117), topical oxygen delivery (118) and warm‐up therapies (119). Other approaches including transcutaneous electrical nerve stimulation, ultrasound and various skin grafting techniques (e.g. pinch grafts) have also been tried.
Some attempts have been made using systemic drugs to treat recalcitrant wounds (120) but with little success.
The future
As knowledge in this area progresses, more sophisticated dressings can and will be developed. However, there is still much to be discovered regarding the healing process itself.
Although new theories and concepts [e.g. wound bed preparation (121)] bring focus to this area of care, significant advances are still required, particularly in the area of wound diagnosis, to allow effective dressings and therapies to be more accurately targeted.
Summary
Wound therapies have evolved significantly over the past three decades, providing more effective and easily used dressings. Technology and product proliferation, however, varies by geography, usually as a function of economics and health care policy.
From the banana‐leaf (122, 123) and potato‐peel (124) approaches of the Third world to the sophisticated hyaluronates (125) and growth factors (126) of the developed world, much has advanced.
Significant opportunity still exists, however. Newer and better technologies can and will be developed. Diagnostic tools and products will become available.
Even today, however, much choice exists, and the choice of a particular product remains a guessing game (127, 128).
With the advent of care plans and the increase in clinical knowledge that these newer technologies and products bring to the wound care arena, as caregivers, we are better armed than ever before.
A well thought out local wound care regime, with the appropriate dressing or therapy choice (Table 1) that is patient, wound and disease specific, the healing outcome should be a vast improvement to that seen clinically three decades ago.
Table 1.
Classes of dressings [adapted from Reddy et al. (29)]
| Moisture balance (130) | ||||||
|---|---|---|---|---|---|---|
| Class/category (129) | Donates/absorbs Fluid (131) | Prevents maceration (132) | Debridement (autolytic) (133) | Bacterial balance (134) | Other patient‐centred concerns addressed | Examples |
| Wound contact layers (135) | None | No | No | None | None | Jelonet, NA Dressing, Adaptic, Urgotul |
| Films (136) | None – ambient moisture levels only | No | Minor – due to occlusion | Minor – due to barrier function (137) | None | Mefilm, Opsite, Tegaderm |
| Hydrogels (138) | Ability to donate; some have minor ability to absorb | No | High | None specifically, but they do not support bacterial growth. They may be used as vehicle for antibiotics | Generally comforting and soothing during use | Normlgel, Hypergel, Duoderm gel, IntaSite gel, Tegagel, Clearsite |
| Soft silicones (139) | Absorbency varies from minimal to high, depending on format | Yes | Minor – due to occlusion | None, except when occluded | Atraumatic removal (140) | Mepitel, Mepilex, Mepilex Border, Mepilex Transfer |
| Hydrocolloids (141) | Moderate absorption | No | High | Minor – due to acidic pH and barrier function (142) | Viral barrier | Duoderm, Tegasorb, Comfeel |
| Alginates and hydrofibres (143) | Moderate‐to‐high absorption | Yes | Moderate | None, except when occluded | Generally easily removed | Melgisorb, Kaltostat, Tegagen, Algisite M, Sorbsan, Aquacel |
| Foams and hydroactives (144) | Moderate‐to‐high absorption | Yes | Minor – due to occlusion | None, except through occlusion (137) | Cushioning of the wound site | Lyofoam, Allevyn, Tegafoam, Tielle, Biatain, Cutinova Hydro |
| Antimicrobials (145) | Minimal‐to‐moderate absorption | Yes | Depends on formulation | High | New formulations more effective | Acticoat, lodosorb, Aquacel Ag, Actisorb |
| Collagens (146) | Moderate absorption | Yes | Moderate | None, except when occluded | Provides needed wound factor | Promogran |
| Hyaluronates (147) | Moderate absorption | Yes | Moderate | None, except when occluded | Provides needed wound factor | Hyalofill |
| Skin replacements (148) | None | None | None | None | Provides a moist environment but mainly provides needed cells | Biobrane, Dermagraft, Trancyte, Integral, Apligraf, Orcel, OASIS |
| Growth factors (149) | None | None | None | None | Provides needed wound factor | Regranex |
| Adjuncts (150) | No | Yes | None | None | Useful for wounds stagnant with dressings | VAC, Warm‐up |
The remainder of this review focuses on the development of both dressings and practice from three geographical areas around the globe.
The development of dressing usage: an australian perspective
The use of wound dressings and wound products has been gradual in Australia, with a rapid increase over the past decade. To fully understand the issues involved, it is important to clarify the health systems in this country as it compares with Europe, USA and Asia.
The health system in Australia
Australia has a universal health system partly funded by a contribution by each taxpayer deducted from his/her weekly salary. This system provides payment of doctors' fees, pathology tests, radiology and other specialist services based on a schedule of fees. Patients are treated by their local general practitioner or by referral to a specialist. If there is a need for medication, this is prescribed by the doctor and dispensed by a pharmacist. The cost of medication is also reimbursed by the government, with the patient making a copayment of a few dollars if on a pension, or a maximum of $23 until a total of approximately $700 is spent; thereafter, the medication is at the concession rate. The list of drugs available is determined by the government on advice from an advisory committee. In general, wound dressings or wounds products such as bandages are not included on the schedule of products made available. The exceptions are some stomal products and a range of wound dressings, bandages and miscellaneous products available to veterans and their dependents. The schedule of these products is also determined by the government on advice of a reference committee. The veteran and their dependents are the only group who are able to obtain wound products subsidised by the government; all other patients regardless of their financial status must purchase their products themselves.
This, however, does create a problem for the ongoing use by, in particular, the lower‐income groups such as pensioners who may not be in a position to afford the cost of wound dressings and products needed for their treatment. In general, local general practitioners will provide products for dressing wounds when the patient visits their clinic, and while treated in hospital, all products are provided.
Australia has a dual hospital system with public hospitals funded by the various State governments from grants by the federal government and private hospitals operated by various organisations and paid for by the patient or by a health insurance company if the patient has private health cover. Some private hospitals may insist on the patient paying extra to cover the costs of specialised dressings.
Product distribution in Australia
The distribution method for wound products has a strong impact on their use in Australia. Products are, in most cases, distributed directly to hospitals by the manufacturer or agent and via wholesalers to medical practices and pharmacies. Nursing home and special accommodation facilities obtain their supplies from a community pharmacy. The public usually obtain their dressings from one of over 5000 community pharmacies or a limited number of companies who supply treatment aids directly to the public. Apart from veterans and their dependents, all other patients pay for their supplies directly with no reimbursement, refund or tariff system in operation. This impacts on the ability of lower‐income groups such as pensioners to continue to use modern wound products.
The other factor that influences availability is pack size. In some cases, manufacturers provide products in pack sizes from 50 to 100, suitable for hospitals or medical practices but excessive for the individual patients. It will be important for greater penetration to occur for pack sizes to be consumer sensitive.
Wound management organisations in Australia
In March 1993, in Perth, Western Australia, during the Inaugural Australian Conference on Wound Care, Turning Wound Care Upside Down, a steering committee was convened to oversee the formation of the Australian Wound Management Association (AWMA). The association was formally recognised a year later in Melbourne at the Australian International Wound Management Conference, in March 1994.
The AWMA is a multidisciplinary, non profit association consisting of people who are committed to developing and improving wound management for all individuals through education, research, communication and networks.
The association acts as a parent body to the autonomous state wound management associations in New South Wales, Queensland, South Australia, Tasmania, Victoria, Australian Capital Territory and Western Australia. There are approximately 2000 members from the disciplines of nursing, medicine, pharmacy, podiatry, industry and the sciences. The New Zealand Wound Care Society is an affiliate of AWMA. Membership of the association is either through membership of state associations or directly through AWMA. Corporate membership is also welcomed.
Every second year, the association holds a national wound care conference with valuable assistance from the host state wound care association. On the alternate year, each state association holds a wound care conference.
Primary Intention – The Australian Journal of Wound Management is produced and published by the Association four times a year. The AWMA has forged strong links with other international wound healing societies. The Association hosted the First World Wound Healing Meeting in Melbourne in September 2000. At this meeting, the International Union of Wound Healing Societies was formed.
Guidelines and standards for wound management in Australia
The association aims to improve the community's understanding of wounds and wound management practices, and the association formed a Pressure Ulcer Interest Subcommittee in 1996. This committee has developed guidelines for the prediction and prevention of pressure ulcers.
The topics included in the clinical practice guidelines are:
-
•
staging of pressure ulcers
-
•
risk factors:
-
–
intensity and duration of pressure
-
–
tissue tolerance for pressure
-
–
extrinsic factors
-
–
intrinsic factors
-
•
risk assessment tools
-
•
skin care:
-
–
skin assessment
-
–
skin hygiene
-
–
skin moisture maintenance
-
–
maintenance of a suitable skin temperature
-
–
influence of nutrition on the skin
-
•
mechanical loading and support surfaces:
-
–
positioning and repositioning
-
–
eliminating shear and friction
-
–
reducing heel pressure
-
–
activity and mobilisation
-
–
support surfaces
-
–
basic hospital mattresses
-
–
foam pressure‐reducing devices
-
–
sheepskins, fibre‐filled overlays and gel pads
-
–
static air mattresses and overlays
-
–
alternating pressure devices
-
–
low‐air‐loss devices
-
–
high‐air‐loss or air‐fluidised beds
-
–
turning beds
-
–
evaluating support surfaces
-
–
selecting a support surface
-
•
documentation
-
•
summary of pressure ulcer preventative strategies.
The clinical practice guidelines are available in three forms: the full text, an abridged version and a pocket guide. They have been widely distributed around the country, and the full text version is available on the AWMA website (http://www.awma.com.au).
In 2002, the association also published standards for wound management.
The standards cover a broad range of practices:
Collaborative practice and interdisciplinary care
The optimal healing of the individual with a wound or potential wound is promoted by a collaborative and interdisciplinary approach to wound management.
Professional practice
The safety and wound healing potential of the individual is ensured by clinical practice in wound management that respects and complies with legislation, codes of practice, clinical practice guidelines and organisational policies.
Clinical decision‐making in wound management
The optimal healing of the individual with a wound is facilitated by an ongoing process of clinical decision‐making in order to determine the risk of wounding, wound aetiology and wound healing responses.
Best practice in wound healing
Wound management is practised according to the best available evidence for optimising healing in acute or chronic wounds.
Documentation
Documentation in the individual's record or management plan must facilitate communication and continuity of care between interdisciplinary team members and fulfil legal requirements.
Education
Education of individuals and their carers should facilitate better health care seeking behaviours. The clinician maximises opportunities for advancing self‐knowledge and skills in wound management.
Research
Wound healing is a dynamic process, and the clinician must anticipate that wound management practice will change as new scientific evidence becomes available.
Education
One of the most important and significant influences of practice has been the level of education of health professionals in Australia. Wound management is part of the undergraduate training, to some extent, in medicine, pharmacy, nursing, podiatry and veterinary science. Over the past 10–15 years, a number of training courses, mostly short courses, have become available. The interest is growing in seminars, tutorials, training days and conferences with many opportunities for health professionals to participate.
Postgraduate training and courses are available in particular for nurses with Masters of Clinical Nursing specialising in wound management from University of Central Queensland. Monash University in Melbourne has a range of postgraduate courses including graduate certificate, graduate diploma and masters, all delivered by distance education.
Overview
Modern wound management practice has been well accepted in Australia and, compared with many other countries, is well developed. There are a number of multidisciplinary wound clinics in Australia and research centres undertaking basic and clinical research.
A wide range of dressings are available in Australia. There is still wide use of the simple inert non stick dressings and modern gauze based dressings.
Moist wound products available for use include:
-
•
film dressings
-
•
hydrocolloid dressings
-
•
hydroactive dressings
-
•
alginate dressings
-
•
foam dressings
-
•
hydrogel dressings.
In addition, a number of miscellaneous products are available, for example:
-
•
cadexomer iodine dressings
-
•
various silver dressings
-
•
hypertonic saline dressings
-
•
silicone dressings
-
•
topical zinc
-
•
charcoal odour absorbing dressings.
The use of bandages and compression stockings is also well established, including the use of multilayer systems.
In general, wound management practice is moving forward steadily and will increase as practitioners gain more experience with modern products. There is still, however, considerable room for improvement, and this will be achieved by education, good communication between health professionals and governmental support of modern wound management practice.
Wound dressings: a canadian approach
The Canadian health care system
Canada is a large, unique and diverse country, of 31·6 million people, divided among 13 provinces and territories. Each province or territory has its own size (from 29 000 in Nunavut to 12 million in Ontario) and personality based on economics, cultural blend, resources, etc. (151).
Canada is known for its socialised medicine, which had its origins under the Canadian constitution, where the federal government was required to fund health care, and the provinces were delegated jurisdiction in the delivery of health care (152). However, many do not realise how much Canada has changed in its approach to health care over the last decade. The current national health system (Health Canada) began in the late 1950s, with a system of publicly funded hospital insurance, and completed in the late 1960s and early 1970s when comprehensive health insurance was put into place. The federal government finances about 40% of the costs, provided the provinces set up a system satisfying federal norms. The Canadian Health and Social Transfer Fund (post‐secondary education, social welfare programmes and health) gives the provinces a lump sum, and the provinces can allocate the money according to the provinces' needs (153, 154). All provincial systems are, thus, very similar, but to further identify the regional needs within each province or territory, multiple health regions have been created within the province or territory that are guided by their own board to deliver health care in the most effective way based on their regional needs. In recent years, the health care in Canada has been changing with the Canadian government delisting previously covered services and rationing of care. Wound care is an example for that change, with some provinces paying for the cost of wound care services and dressings, while some do not.
Canadian wound care practice
Canada has had a national wound care organisation since 1995 – the Canadian Association of Wound Care (CAWC). The CAWC is dedicated to the advancement of wound care in Canada by coordinating a collaborative, interdisciplinary effort among individuals and organisations involved with wound caring. The association's efforts are focused on five key areas: public policy, clinical practice, education, research and connecting with the international wound care community. The CAWC works to significantly improve patient care, clinical outcomes and the professional satisfaction of wound care clinicians (155).
In 2000/2001, the CAWC published four pivotal articles to guide and support best practice in Canada. These articles, recently translated into French, are available in full text version on the CAWC website (http://www.cawc.net). This resource and their accompanying quick reference guides assist the health care professional in addressing patient‐specific concerns that support wound healing. The CAWC has also introduced a new publication Wound Care Canada that is dual language (French–English) and is also fully downloadable online.
Clinical enablers
Preparing the wound bed has been recently reviewed (44), which led to a modification in the wound‐healing paradigm including the epidermal edge effect to demonstrate healing of the wound (156). This paradigm supports the clinician, in not only best practice for wound management from a holistic perspective but supports dressing selection based on the guiding‐practice principles of wound management: wound aetiology, patient‐centred concerns and local wound care requirements (debridement requirements, infection control and moisture balance).
The CAWC has developed a longitudinal approach to wound care education with basic knowledge, skill and attitude development programme in a three‐part seminar series. This programme is offered yearly at various sites across Canada to all health care professionals in both English and French.
Canada is also fortunate to have a university‐based wound care programme, the International Interdisciplinary Wound Care Course (IIWCC), offered by University of Toronto that supports wound care knowledge development (157, 158).
By seeding not only our country with wound care leaders, but others, we support bedside clinicians in making best practice recommendations for wound management.
Bedside practice
A brief questionnaire was sent out to wound care experts (nurses) across Canada (British Columbia to Newfoundland) in an effort to understand regional differences in wound dressing practice.
-
1
Does your health region pay for wound care dressings in acute care, in community care and in long‐term care?
-
2
Who decides which dressing to use?
Acute care
It was clear by the responses that all hospitals covered the cost of dressings while the patients were in the hospital, and some had a form of high‐cost dressing control or review in place for some products (i.e. VAC or biologicals).
Home care
Caring for wounds in patients who had been discharged home for community‐based care was a bit different but still rang with similarities. Some regions had all dressings paid for regardless of where the care occurred, but the most common response was, as always in wound care, ‘it depends!’ Many provinces have a government programme that assists with the coverage of dressings for chronic wounds. Some provinces have a cost‐of‐living benchmark that assists low‐economic patients with the cost of dressings.
Care centres
Because care centres (nursing homes and long‐term care facilities) are often privately owned, there was less consistency. Some provided dressings to their residents but most seemed to look at insurance plans and families to cover costs. Some regions have programmes to support treatment that has been initiated by a wound specialist.
Who selects the dressing?
The nurse (wound care nurse or enterostomal therapist) was the most often mentioned clinician that selected the dressing; however, for surgical wounds, the surgeon was frequently mentioned. Many mentioned skilled teams that supported best practice through their skilled interventions. Care centres often relied on doctors and registered nurses for guidance. One factor that was important with dressing selection was agency inventories and product contracts; the ordering clinician needs to be aware of what is available.
Just as there is no such thing as ‘one dressing for all wounds’, there is no such thing as ‘one way to obtain and prescribe a dressing for a wound’. Canada is a large and diverse country with a variety of regionally specific needs, and each region addresses its wound‐related concerns according to its abilities.
Summary of the Canadian approach
It is good to remember that dressing is only one part of a complex treatment plan required to heal a wound. However, that one aspect of our wound care practice remains complicated with the ever‐increasing variety of wound care products. How do clinicians choose the correct dressing? This is the question many health care professionals want the answer to. Most dressings fall into a category that describes its benefits, indications and contraindications, and it is up to the wound clinicians to not only select the best product for our patient but to teach other clinicians the cost‐effective use of wound care dressings (159).
Education needs to revolve not only around the wound‐healing paradigm, removing the cause and patient centred concerns, but also around regional wound care practices and resources in order to give clinicians a framework for best practice in wound care.
The development of dressing usage and wound care guidelines in japan
Introduction of modern dressings
Prior to the introduction of a moist‐environment‐type dressing in 1987 by ConvaTec (a Bristol‐Myers Squibb Company), basic wound care involved the use of an antiseptic cleanser where the professionals' main objectives were to prevent infection and keep the wound dry to promote epithelialisation. Thus, the introduction of a hydrocolloid modern dressing (Duoactive or DuoDERM Varishesive, Granuflex) that provided a moist environment for wound healing sent reverberations throughout the medical community in Japan.
Impressed by the initial results, the enterostomal therapist (ET) nurses revolutionised the use of a moist or ‘modern’ dressing in Japan. This spurred on competition by other companies to introduce new types of modern dressings to Japan to meet the growing demand.
Polyurethane film dressings first received approval as an official medical supply for use in Japan in 1992. Subsequently, several dressing types followed, starting with alginate dressings in 1993, hydrogel dressings in 1995, polyurethane foam dressings in 1996, sulfadiazine silver‐lined dressings in 1997 and hydrofibre and hydropolymer dressings in 2000. At present, there are seven types of modern dressings currently used in Japan.
Modern dressings market
Uniquely characteristic to the Japanese market is that 44% of most of the chronic wound patients are still treated with gauze dressings, compared with only 16% who are treated with modern dressings.
The modern dressings market has been increasing yearly from $24·2 million in 1994 to $41·8 million in 2003 (exchange rate based on 1 US dollar = 110 yen). Some 70–80% of modern dressings are used for pressure ulcer treatment, with less than 10% being used for diabetic foot and venous ulcer patients. This ratio is the same as the chronic wound demographics in Japan.
Hereafter, all data will accordingly focus on pressure ulcers as the predominant group.
Actual conditions of modern dressing usage for pressure ulcers
Pressure ulcer care penalty system
The population of Japan's ageing society has reached an unprecedented number, and according to estimates, one‐quarter of the population will be 65 years of age, or older, by 2015. Along with an ageing society, the ratio of bedfast patients is also rapidly increasing to the point where in 2000, 13·0% of this demographic group were bedfast. The occurrence of pressure ulcers in a hospital setting is 4–9%, and 14% in a home setting has been reported. The fact that 70% are reported to be stage III or IV [National Pressure Ulcer Advisory Panel (NPUAP) classification] has led to a steady increase in pressure ulcer and medical treatment costs.
The concern over pressure ulcers has reached a level where the Ministry of Health, Labour and Welfare has introduced a penalty that is levied on hospitals that fail to comply with the recently implemented legislation that requires all hospitals to meet the following criteria based on risk assessment, wound assessment and treatment:
-
•
to establish a team of pressure ulcer specialists to prevent and treat pressure ulcers;
-
•
to establish a risk and wound assessment and management protocol for pressure ulcers;
-
•
to provide adequate support surfaces for pressure ulcer patients.
This legislation came into effect as of 1 October 2002, where hospitals that do not meet the above criteria, five points [55 cents (exchange rate based on 1 US dollar = 110 yen)] per patient will be excluded from the basic admittance insurance coverage that the hospital can claim. For example, this will amount to approximately $165 000 (exchange rate based on 1 US dollar = 110 yen) per year of lost revenue for an 800‐bed hospital.
Modern dressings and the insurance system
As of 1 April 2001, the cost of modern dressings, covered by the insurance system, which could be invoiced for reimbursement, was standardised nationwide. This reimbursement fee depends upon the amount of dressing used for the category of ‘Dressing for Skin Breakdown’ declared by the companies selling the dressings.
The type of dressing that the insurance system covers is determined by the depth of the pressure ulcer. The period in which the insurance covers the dressings is also an additional problem. At present, it is limited to only 3 weeks, after which the users must cover the full cost themselves.
Current use of modern dressings
In 1999, Ohura et al. (160) conducted a national survey to determine the extent of use of modern dressings in Japan. According to their findings, 159 of the 205 (78%) facilities that participated in the study used modern dressings.
Of all the modern dressings used, hydrocolloid dressings were the most widely used (all 159 facilities). Second were the polyurethane film dressings, used in 134 facilities, followed by alginate dressings that were used in 80 facilities, with hydrogel dressings being used in 24 facilities and polyurethane foam dressings in 19 facilities. From these results, it was found that hydrocolloid dressings, which were the first modern dressing to be introduced in Japan, were used by all the facilities surveyed (160).
Modern dressing and the national guideline
In 1998, the Ministry of Health and Welfare created a pressure ulcer prevention and treatment guideline (161). This guideline was developed by a panel of expert opinions based on their experience and not scientific‐based evidence. In this guideline, the proper selection and use of dressings are classified by the colour of the wound.
Developed by Fukui (162) in 1993, this colour system separately categorises shallow and deep pressure ulcers and classifies the healing process into four phases. The colour of a wound from an acute condition progressively changes from black‐to‐yellow to red‐to‐white phase.
According to the guideline, modern dressings are recommended for shallow pressure ulcers and deep pressure ulcers in the red‐to‐white phase. However, at present, there are no detailed selection standard criteria.
Modern dressings and prescription authority
In Japan, dressings are handled as prescription materials and can only be prescribed by physicians. In 1998, a survey conducted by Ohura et al. (163) revealed that pressure ulcer treatment is handled 40% by nurses, 40% by physicians and nurses and 7% by physicians only, and the remainder by others.
The actual state of the situation is that although the nurses do not have the authority to write prescriptions, they select most of the dressings to be used for the patients and the physicians only write the prescription.
Future outlook
Although the history of modern dressings in Japan is a mere 20 years old, 79% of the facilities began using them within the first 10 years after their introduction, and its demand still remains high.
However, concerning pressure ulcer, these dressings that have been introduced face the following problems:
-
•
Insurance plan only covers it for 3 weeks. Thus, stage III or IV pressure ulcers cannot receive full coverage, as they normally take 6 months to 1 year to heal.
-
•
Nurses only select dressings and do not have the authority to issue prescriptions. This authority still remains the physicians' sole responsibility.
-
•
In Japan, owing to strict standards implemented by the government, at present, there are no modern dressings that are effective for treating infected wounds. As a result, traditional topical ointments must be used in combination with gauze during the entire healing period.
-
•
We do not have adequate guidelines for pressure ulcer dressing usage. The present guideline is a based upon a panel of expert opinions and does not necessarily use the best scientific‐based evidence.
Recent studies and approaches have been developed to rectify this situation. In order to promote the proper use of modern dressing, Sanada et al. (164) performed a cost‐effectiveness analysis between traditional topical ointments with gauze and modern dressing.
The results provide evidence that for stage II and stage III pressure ulcers, there was a significant reduction in the overall cost of treatment using modern dressings.
Based on this evidence, the Japanese Society of Pressure Ulcers, realising the need to ease the modern dressing restrictions, submitted a petition to the Ministry of Health, Labour and Welfare.
The society also increased the courses to educate nurses to become wound care specialists and is currently revising the national guideline using scientific‐based evidence.
Conclusion
Wound care dressings and practice are slowly but surely becoming a global practice. Technologies and practice do change – but change takes time! This review has provided insight into the development of three different geographical areas, with three different health care systems, but the ultimate outcome remains the same, that is the proliferation of moist wound healing and best practice.
References
- 1. Birdsell DC, Hein KS, Lindsay RL. The theoretically ideal donor site dressing. Ann Plast Surg 1979;2(6):535–7. [DOI] [PubMed] [Google Scholar]
- 2. Eaglstein WH, Mertz PM. New methods for assessing epidermal wound healing: the effects of triamcinolone acetonide and polyethelene film occlusion. J Invest Dermatol 1978;71(6):382–4. [DOI] [PubMed] [Google Scholar]
- 3. James JH, Watson AC. The use of Opsite, a vapour permeable dressing, on skin graft donor sites. Br J Plast Surg 1975;28(2):107–10. [PubMed] [Google Scholar]
- 4. Tracy GD, Lord RS, Kibel C, Martin M, Binnie M. Varihesive sealed dressing for indolent leg ulcers. Med J Aust 1977;1(21) (777):780. [DOI] [PubMed] [Google Scholar]
- 5. Harding KG, Morris HL, Patel GK. Science, medicine and the future: healing chronic wounds. BMJ 2002;324(7330):160–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alvarez OM, Mertz PM, Eaglstein WH. The effect of occlusive dressings on collagen synthesis and re‐epithelialization in superficial wounds. J Surg Res 1983;35(2):142–8. [DOI] [PubMed] [Google Scholar]
- 7. Berardesca E, Maibach HI. Skin occlusion: treatment or drug‐like device? Skin Pharmacol 1988; 1(3):207–15. [DOI] [PubMed] [Google Scholar]
- 8. Trueman P, Boothman S. TIELLE Plus fluid handling capacity can lead to cost savings. Br J Community Nurs 2003;8(9 Suppl):S22. [DOI] [PubMed] [Google Scholar]
- 9. Sibbald RG, Orsted H, Schultz GS, Coutts P, Keast D. Preparing the wound bed: focus on infection and inflammation. Ostomy Wound Manage 2003;49(11):23–51. [PubMed] [Google Scholar]
- 10. Derbyshire A. A case study to demonstrate the role of proteases in wound healing. Prof Nurse 2003;19(2):108–11. [PubMed] [Google Scholar]
- 11. Morris HL, Jones V, Harding KG. Wound care: putting theory into practice – The Cardiff Wound Healing Research Unit in the United Kingdom. In: Krasner DL, Rodeheaver GT, Sibbald, RG , editors. Chronic wound care: a clinical source book for healthcare professionals, 3rd edition. Wayne, PA: HMP Communications, 2001: 135–44. [Google Scholar]
- 12. Teot L, Meaume S, Desmouliere A. Putting theory into practice: wound healing in France. In: Krasner DL, Rodeheaver GT, Sibbald RG, editors. Chronic wound care: a clinical source book for healthcare professionals, 3rd edition. Wayne, PA: HMP Communications, 2001: 155–62. [Google Scholar]
- 13. Motta G. Regulatory issues and reimbursement. In: Krasner DL, Rodeheaver GT, Sibbald RG, editors. Chronic wound care: a clinical source book for healthcare professionals, 3rd edition. Wayne, PA: HMP Communications, 2001: 199–207. [Google Scholar]
- 14. Eaglstein WH. Moist wound healing with occlusive dressings: a clinical focus. Dermatol Surg 2001;27(2):175–81. [DOI] [PubMed] [Google Scholar]
- 15. Field FK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg 1994;167(1A):2S–6S. [DOI] [PubMed] [Google Scholar]
- 16. Findlay D. Modern dressings: what to use. Aust Fam Physician 1994;23(5):824–9, 832. [PubMed] [Google Scholar]
- 17. Jeter KF, Tintle TE. Wound dressings of the nineties: indications and contraindications. Clin Podiatr Med 1991;8(4):799–816. [PubMed] [Google Scholar]
- 18. Kannon GA, Garrett AB. Moist wound healing with occlusive dressings. A clinical review. Dermatol Surg 1995;21(7):583–90. [DOI] [PubMed] [Google Scholar]
- 19. Lionelli GT, Lawrence WT. Wound dressings. Surg Clin North Am 2003;83(3):617–38. [DOI] [PubMed] [Google Scholar]
- 20. Ovington LG. Wound care products: how to choose. Home Healthc Nurse 2001;19(4) (224–31):240. [DOI] [PubMed] [Google Scholar]
- 21. Park GB. Burn wound coverings – a review. Biomater Med Devices Artif Organs 1978;6(1):1–35. [DOI] [PubMed] [Google Scholar]
- 22. Provan A, Phillips TJ. An overview of moist wound dressings: the under cover story. Dermatol Nurs 1991;3(6):393–6. [PubMed] [Google Scholar]
- 23. Queen D, Evans JH, Gaylor JD, Courtney JM, Reid WH. Burn wound dressings – a review. Burns Incl Therm Inj 1987;13(3):218–28. [DOI] [PubMed] [Google Scholar]
- 24. Rovee DT. Evolution of wound dressings and their effects on the healing process. Clin Mater 1991;8(3–4):183–8. [DOI] [PubMed] [Google Scholar]
- 25. Seaman S. Dressing selection in chronic wound management. J Am Podiatr Med 2002;92(1):24–33. [DOI] [PubMed] [Google Scholar]
- 26. Sipos P, Gyory H, Hagymasi K, Ondrejka P, Blazovics A. Special wound healing methods used in ancient Egypt and the mythological background. World J Surg 2004;28(2):211–6. [DOI] [PubMed] [Google Scholar]
- 27. Rogers AA, Walmsley RS, Rippon MG, Bowler PG. Adsorption of serum‐derived proteins by primary dressings: implications for dressing adhesion to wounds. J Wound Care 1999;8(8):403–6. [DOI] [PubMed] [Google Scholar]
- 28. Alvarez O. Moist environment for healing: matching the dressing to the wound. Ostomy Wound Manage 1988;21: 64–83. [PubMed] [Google Scholar]
- 29. Reddy M, Kohr R, Queen D, Keast D, Sibbald RG. Practical treatment of wound pain and trauma: a patient‐centered approach. An overview. Ostomy Wound Manage 2003;49(4 Suppl):2–15. [PubMed] [Google Scholar]
- 30. Keast DH, Orsted H. The basic principles of wound care. Ostomy Wound Manage 1998; 44(8):24–8, 30–1. [PubMed] [Google Scholar]
- 31. Winter GD. Formation of the scab and the rate of epithelialisation of superficial wounds in the skin of the young domestic pig. J Wound Care 1995;4(8):366–7. [PubMed] [Google Scholar]
- 32. Eaglstein WH. Moist wound healing with occlusive dressings: a clinical focus. Dermatol Surg 2001;27(2):175–81. [DOI] [PubMed] [Google Scholar]
- 33. Gates JL, Holloway GA. A comparison of wound environments. Ostomy Wound Manage 1992; 38(8):34–7. [PubMed] [Google Scholar]
- 34. Foertsch CE, O'Hara MW, Stoddard FJ, Kealy GP. Treatment‐resistant pain and distress during pediatric burn‐dressing changes. J Burn Care Rehabil 1998;19(3):219–24. [DOI] [PubMed] [Google Scholar]
- 35. Nemeth AJ, Eaglstein WH, Taylor JR, Peerson LJ, Falanga V. Faster healing and less pain in skin biopsy sites treated with an occlusive dressing. Arch Dermatol 1991;127(11):1679–83. [PubMed] [Google Scholar]
- 36. Rubio PA. Use of semiocclusive, transparent film dressings for surgical wound protection: experience in 3637 cases. Int Surg 1991;76(4):253–4. [PubMed] [Google Scholar]
- 37. Eisenberg M. The effect of occlusive dressings on re‐epithelializations of wounds in children with epidermolysis bullosa. J Pediatric Surg 1986; 21(10):892–4. [DOI] [PubMed] [Google Scholar]
- 38. Eaglstein WH, Mertz PM, Falanga V. Occlusive dressings. Am Fam Physician 1987;35(3):211–6. [PubMed] [Google Scholar]
- 39. Eaglstein WH. Experiences with biosynthetic dressings. J Am Acad Dermatol 1985;12(2 pt 2): 434–40. [DOI] [PubMed] [Google Scholar]
- 40. Ehleben CM, May SR, Still JM. Pain associated with an adherent polyurethane wound dressing. Burns Incl Therm Inj 1985;12(2):122–6. [DOI] [PubMed] [Google Scholar]
- 41. Harding KG, Jones V, Price P. Topical treatment: which dressing to choose. Diabetes Metab Res Rev 2000;16 Suppl 1: S47–50. [DOI] [PubMed] [Google Scholar]
- 42. Sibbald RG, Williamson D, Orsted HL, Campbell K, Keast D, Krasner D, Sibbald D. Preparing the wound bed – debridement, bacterial balance and moisture balance. Ostomy Wound Manage 2000;46(11):14–22, 24–8, 30–5. [PubMed] [Google Scholar]
- 43. Foster L, Moore P. Acute surgical wound care. 3. Fitting the dressing to the wound. Br J Nurs 1999;8(4):200–2, 204, 206. [DOI] [PubMed] [Google Scholar]
- 44. Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11 Suppl 1: S1–28. [DOI] [PubMed] [Google Scholar]
- 45. Campbell K, Woodbury MG, Whittle H, Labate T, Hoskin A. A clinical evaluation of 3M no sting barrier film. Ostomy Wound Manage 2000;46(1):24–30. [PubMed] [Google Scholar]
- 46. Burge TS. Removing adhesive retention dressings. Br J Plast Surg 2004;57(1):93. [DOI] [PubMed] [Google Scholar]
- 47. Baxter H. A comparison of two hydrocolloid sheet dressings. Br J Community Nurs 2000;5(11):572, 574, 576–7. [DOI] [PubMed] [Google Scholar]
- 48. Chang KW, Alsagoff S, Ong KT, Sim PH. Pressure ulcers – randomised controlled trial comparing hydrocolloid and saline gauze dressings. Med J Malaysia 1998;53(4):428–31. [PubMed] [Google Scholar]
- 49. Williams C. Granuflex. Br J Nurs 1994;3(14):730–3. [DOI] [PubMed] [Google Scholar]
- 50. Tracey GD, Lord RS, Kibel C, Martin M, Binnie M. Varihesive sealed dressing for indolent leg ulcers. Med J Aust 1977;1(21):777, 780. [DOI] [PubMed] [Google Scholar]
- 51. Friedman SJ, Su WP. Management of leg ulcers with hydrocolloid occlusive dressings. Arch Dermatol 1984;120(10):1329–36. [PubMed] [Google Scholar]
- 52. Heffernan A, Martin AJ. A comparison of a modified form of Granuflex (Granuflex Extra Thin) and a conventional dressing in the management of lacerations, abrasions and minor operation wounds in an accident and emergency department. J Accid Emerg Med 1994;11(4):227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martini L, Reali UM, Borgognoni L, Brandani P, Andriessen A. Comparison of two dressings in the management of partial‐thickness donor sites. J Wound Care 1999;8(9):457–60. [DOI] [PubMed] [Google Scholar]
- 54. Ashford RL, Freear ND, Shippen JM. An in‐vitro study of the pressure‐relieving properties of four wound dressings for foot ulcers. J Wound Care 2001;10(2):34–8. [DOI] [PubMed] [Google Scholar]
- 55. Ballard K. The Tielle family of dressings: overview of the product range. Br J Nurs 2001;10(12):808–14. [DOI] [PubMed] [Google Scholar]
- 56. Carter K. Hydropolymer dressings in the management of wound exudate. Br J Community Nurs 2003;8(9 Suppl):S6. [DOI] [PubMed] [Google Scholar]
- 57. Fletcher J. The application of foam dressings. Nurs Times 2003;99(31):59. [PubMed] [Google Scholar]
- 58. Kammerlander G, Eberlein T. Use of Allevyn heel in the management of heel ulcers. J Wound Care 2003;12(8):313–5. [DOI] [PubMed] [Google Scholar]
- 59. Thomas S, Banks V, Bale S, Fear‐Price M, Hagelstein S, Harding KG, Orpin J, Thomas N. A comparison of two dressings in the management of chronic wounds. J Wound Care 1997;6(8):383–6. [DOI] [PubMed] [Google Scholar]
- 60. Ballard K. Clinical and scientific data of a hydropolymer range of dressings. Br J Nurs 2002;11(20 Suppl):S37–43. [DOI] [PubMed] [Google Scholar]
- 61. Viamontes L, Temple D, Wytall D, Walker A. An evaluation of an adhesive hydrocellular foam dressing and a self‐adherent soft silicone foam dressing in a nursing home setting. Ostomy Wound Manage 2003;49(8):48–52, 54–6, 58. [PubMed] [Google Scholar]
- 62. Bale S, Baker N, Crook H, Rayman A, Rayman G, Harding KG. Exploring the use of an alginate dressing for diabetic foot ulcers. J Wound Care 2001;10(3):81–4. [DOI] [PubMed] [Google Scholar]
- 63. Piacquadio D, Nelson DB. Alginates. A “new” dressing alternative. J Dermatol Surg Oncol 1992;18(11):992–5. [DOI] [PubMed] [Google Scholar]
- 64. Whittle H, Fletcher C, Hoskin A, Campbell K. Nursing management of pressure ulcers using a hydrogel dressing protocol: four case studies. Rehabil Nurs 1996;21(5):239–42. [DOI] [PubMed] [Google Scholar]
- 65. Misterka S. Clinical evaluation of a hydrogel type dressing materials after their 8 years use. Polim Med 1991;21(1–2):23–30. [PubMed] [Google Scholar]
- 66. Staniszewska‐Kus J, Paluch D, Solski L. Experimental testing of the hydrogel dressing Geliperm. Polim Med 1988;18(4):197–210. [PubMed] [Google Scholar]
- 67. Sdlarik KM, Vacik J, Wichterle O, Hajek M. Modern dressings. Hydrogels. Rozhl Chir 1995;74(1):3–7. [PubMed] [Google Scholar]
- 68. Pajewski LA, Pantaleoni GC, Rosiak J. Hydrogels in medicine. Origin and clinical use. Clin Ter 1994;145(11):373–82. [PubMed] [Google Scholar]
- 69. Agren MS. An amorphous hydrogel enhances epithelialisation of wounds. Acta Derm Venereol 1998;78(2):119–22. [DOI] [PubMed] [Google Scholar]
- 70. Eisenbud D, Hunter H, Kessler L, Zulkowski K. Hydrogel wound dressings: where do we stand in 2003? Ostomy Wound Manage 2003;49(10):52–7. [PubMed] [Google Scholar]
- 71. Kickhofen B, Wokalek H, Scheel D, Ruh H. Chemical and physical properties of a hydrogel wound dressing. Biomaterials 1986;7(1):67–72. [DOI] [PubMed] [Google Scholar]
- 72. Mandy SH. A new primary wound dressing made of polyethylene oxide gel. J Dermatol Surg Oncol 1983;9(2):153–5. Vernon T.Intrasite Gel and Intrasite Conformable: the hydrogel range. Br J Nurs 2000;9(16):1083–8. [DOI] [PubMed] [Google Scholar]
- 73. Yates DW, Hadfield JM. Clinical experience with a new hydrogel wound dressing. Injury 1984;16(1):23–4. [DOI] [PubMed] [Google Scholar]
- 74. Finnie A. Hydrocolloids in wound management: pros and cons. Br J Community Nurs 2002;7(7):338, 340, 342. [DOI] [PubMed] [Google Scholar]
- 75. Dykes PJ, Heggie R, Hill SA. Effects of adhesive dressings on the stratum corneum of the skin. J Wound Care 2001;10(2):7–10. [DOI] [PubMed] [Google Scholar]
- 76. Malone WD. Wound dressing adherence: a clinical comparative study. Arch Emerg Med 1987;4(2):101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Agren MS. Four alginate dressings in the treatment of partial thickness wounds: a comparative experimental study. Br J Plast Surg 1996;49(2):129–34. [DOI] [PubMed] [Google Scholar]
- 78. Daniels S, Sibbald RG, Ennis W, Eager CA. Evaluation of a new composite dressing for the management of chronic leg ulcer wounds. J Wound Care 2002;11(8):290–4. [DOI] [PubMed] [Google Scholar]
- 79. Goodhead A. Clinical efficacy of Comfeel Plus transparent dressing. Br J Nurs 2002;11(4):284, 286–7. [DOI] [PubMed] [Google Scholar]
- 80. Martinez Cuervo F, Franco Gutiez T, Lopez Rebordinos MT, Menendez S, Rodriguez B. [Treatment of chronic skin ulcers in the elderly. Descriptive study on the use of hydrocellular dressing]. Rev Enferm 1998;21(244):51–60. [PubMed] [Google Scholar]
- 81. Bowler PG, Jones SA, Davies BJ, Coyle E. Infection control properties of some wound dressings. J Wound Care 1999;8(10):499–502. [DOI] [PubMed] [Google Scholar]
- 82. Brunner U, Eberlein T. Experiences with hydrofibres in the moist treatment of chronic wounds, in particular of diabetic foot. Vasa 2000;29(4):253–7. [DOI] [PubMed] [Google Scholar]
- 83. Russell L, Carr J. New hydrofibre and hydrocolloid dressings for chronic wounds. J Wound Care 2000;9(4):169–72. [DOI] [PubMed] [Google Scholar]
- 84. Garcia CF, Salvador Moran MJ, Roman Garcia MJ. [Treatment of skin lesions combining hydrofiber and extra‐fine hydrochloride dressings]. Rev Enferm 2002;25(2):50–4. [PubMed] [Google Scholar]
- 85. Legarra MS, Vidallach Ribes MS, Esteban GM. [Non‐comparative evaluation of a new hydrofiber dressing in the treatment of vascular ulcers]. Rev Enferm 1997;20(231):59–63. [PubMed] [Google Scholar]
- 86. Vloemans AF, Soesman AM, Kreis RW, Middelkoop E. A newly developed hydrofibre dressing, in the treatment of partial‐thickness burns. Burns 2001;27(2):167–73. [DOI] [PubMed] [Google Scholar]
- 87. Legarra Muruzaba S, Vidallach Ribes MS, Estanban Gonzalo M. Non‐comparative evaluation of a new hydrofiber dressing in the treatment of vascular ulcers. Rev Enferm 1997;20(231):59–63. [PubMed] [Google Scholar]
- 88. Rosello Ruiz A. Case report about the combined use of 2 moist wound dressings in the treatment of chronic diabetic foot ulcers. Rev Enferm 1998;21(242 Suppl):9–12. [PubMed] [Google Scholar]
- 89. Mollard P. Penile dressings of CMH silicone elastomer foam. Chir Pediatr 1984;25(2):117–9. [PubMed] [Google Scholar]
- 90. Brossy JJ. Foam elastomer dressings in surgery. S Afr Med J 1981;59(16):559–60. [PubMed] [Google Scholar]
- 91. Bugmann P, Taylor S, Gyger D, Lironi A, Genin B, Vunda A, La Scala O, Birraux J, Le Coultre C. A silicone‐coated nylon dressing reduces healing time in burned paediatric patients in comparison with standard sulfadiazine treatment: a prospective randomized trail. Burns 1998;24(7):609–12. [DOI] [PubMed] [Google Scholar]
- 92. Perkins K, Davey RB, Wallis KA. Silicone gel: a new treatment for burn scars and contractures. Burns Incl Therm Inj 1983;9(3):201–4. [DOI] [PubMed] [Google Scholar]
- 93. Bail DH, Schneider W, Khalighi K, Seboldt H. Temporary wound covering with a silicon sheet for the soft tissue defect following open fasciotomy. Technical note. J Cardiovasc Surg (Torino) 1998;39(5):587–91. [PubMed] [Google Scholar]
- 94. Romanelli M, Van De Looverbosch D, Heyman H, Meaume S, Ciangherotti A, Charpin S. An open multi‐center randomised study comparing a self‐adherent soft silicone foam dressing versus a hydropolymer dressing, in patients with pressure ulcer stage II according to the EPUAP guidelines. Oral presentation at the EWMA meeting in Granada, May 2002.
- 95. Williams C. Mepitel. Br J Nurs 1995;4(1):51–2, 54–5. [DOI] [PubMed] [Google Scholar]
- 96. Schwope AD, Wise DL, Sell KW, Dressler DP, Skornick WA. Evaluation of wound‐covering materials. J Biomed Mater Res 1977;11(4):489–502. [DOI] [PubMed] [Google Scholar]
- 97. Phillips TJ, Gilchrest BA. Cultured epidermal grafts in the treatment of leg ulcers. Adv Dermatol 1990;5: 33–48. [PubMed] [Google Scholar]
- 98. Cooper ML, Hansbrough JF. Use of a composite skin graft composed of cultured human keratinocytes and fibroblasts and a collagen‐GAG matrix to cover full‐thickness wounds on athymic mice. Surgery 1991;109(2):198–207. [PubMed] [Google Scholar]
- 99. Hansbrough JF, Morgan J, Greenleaf G, Underwood J. Development of a temporary living skin replacement composed of human neonatal fibroblasts cultured in Biobrane, a synthetic dressing material. Surgery 1994;115(5):633–44. [PubMed] [Google Scholar]
- 100. Barret JP, Dziewulski P, Ramzy PI, Wolf SE, Desai MH, Herndon DN. Biobrane versus 1% silver sulfadiazine in second‐degree pediatric burns. Plast Reconstr Surg 2000;105(1):62–5. [DOI] [PubMed] [Google Scholar]
- 101. Gentzkow GD, Iwasaki SD, Hershon KS, Mengel M, Prendergast JJ, Ricotta JJ, Steed DP, Lipkin S. Use of Dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care 1996;19(4):350–4. [DOI] [PubMed] [Google Scholar]
- 102. Curran MP, Plosker GL. Bilayered bioengineered skin substitute (Apligraf): a review of its use in the treatment of venous leg ulcers and diabetic foot ulcers. Biodrugs 2002;16(6):439–55. [DOI] [PubMed] [Google Scholar]
- 103. Balleste TJ, Blanco BJ. [Collagen powder dressings in the healing of secondary intention wounds. Report of a case]. Rev Enferm 2002;25(12):50–4. [PubMed] [Google Scholar]
- 104. Bou Torra JE, Soldevilla Agreda JJ, Martinez CF, Rueda LJ. [Collagen powder dressing in the treatment of pressure ulcer. Multicenter comparative study assessing effectiveness and cost]. Rev Enferm 2002;25(9):50–7. [PubMed] [Google Scholar]
- 105. Ishihara M, Nakanishi K, Ono K, Sato M, Kikuchi M, Saito Y, Yura H, Matsui T, Hattori H, Venoyama M, Kurita A. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials, 2002;23(3):833–40. [DOI] [PubMed] [Google Scholar]
- 106. Doillon CJ, Silver FH. Collagen‐based wound dressing: effects of hyaluronic acid and fibronectin on wound healing. Biomaterials 1986;7(1):3–8. [DOI] [PubMed] [Google Scholar]
- 107. Davidson JM, Nanney LB, Broadley KN, Whitsett JS, Aquino AM, Beccaro M, Rastrelli A. Hyaluronate derivatives and their application to wound healing: preliminary observations. Clin Mater 1991;8(1–2):171–7. [DOI] [PubMed] [Google Scholar]
- 108. Hashimoto T, Suzuki Y, Tanihara M, Kakimaru Y, Suzuki K. Development of alginate wound dressings linked with hybrid peptides derived from laminin and elastin. Biomaterials 2004;25(7–8):1407–14. [DOI] [PubMed] [Google Scholar]
- 109. Skokan SJ, Davis RH. Principles of wound healing and growth factor considerations. J Am Podiatr Med 1993;83(4):223–7. [DOI] [PubMed] [Google Scholar]
- 110. Henderson JL, Cupp CL, Ross EV, Shick PC, Keefe MA, Wester DC, Hannon T, McConnell D. The effects of autologous platelet gel on wound healing. Ear Nose Throat J 2003;82(8):598–602. [PubMed] [Google Scholar]
- 111. Drisdelle R. Maggot debridement therapy: a living cure. Nursing 2003;33(6):17. [DOI] [PubMed] [Google Scholar]
- 112. Dowsett C. An overview of Acticoat dressing in wound management. Br J Nurs 2003;12(19 Suppl):S44–49. [DOI] [PubMed] [Google Scholar]
- 113. Lansdown AB. Silver‐containing dressings: have we got the full picture? J Wound Care 2003; 12(8):317–8. [DOI] [PubMed] [Google Scholar]
- 114. Cullen B, Smith R, McCulloch E, Silcock D, Morrison L. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen 2002;10(1):16–25. [DOI] [PubMed] [Google Scholar]
- 115. Evans D, Land L. Topical negative pressure for treating chronic wounds: a systematic review. Br J Plast Surg 2001;54(3):238–42. [DOI] [PubMed] [Google Scholar]
- 116. Eginton MT, Brown KR, Seabrook GR, Towne JB, Cambria RA. A prospective randomized evaluation of negative‐pressure wound dressings for diabetic foot wounds. Ann Vasc Surg 2003;17(6):645–9. [DOI] [PubMed] [Google Scholar]
- 117. Rowe K. Hyperbaric oxygen therapy: what is the case for its use? J Wound Care 2001;10(4):117–21. [DOI] [PubMed] [Google Scholar]
- 118. Onouye T, Menaker G, Christian M, Moy R. Occlusive dressing versus oxygen mist therapy following CO2 laser resurfacing. Dermatol Surg 2000;26(6):572–6. [DOI] [PubMed] [Google Scholar]
- 119. Alvarez OM, Rogers RS, Booker JG, Patel M. Effect of noncontact normothermic wound therapy on the healing of neuropathic (diabetic) foot ulcers: an interim analysis of 20 patients. J Foot Ankle Surg 2003;42(1):30–5. [DOI] [PubMed] [Google Scholar]
- 120. Quirinia A. Ischemic wound healing and possible treatments. Drugs Today (Barc) 2000;36(1):41–53. [DOI] [PubMed] [Google Scholar]
- 121. Hess CT, Kirsner RS. Orchestrating wound healing: assessing and preparing the wound bed. Adv Skin Wound Care 2003;16(5):246–57. [DOI] [PubMed] [Google Scholar]
- 122. Gore MA, Akolekar D. Evaluation of banana leaf dressing for partial thickness burn wounds. Burns 2003;29(5):487–92. [DOI] [PubMed] [Google Scholar]
- 123. Gore MA, Akolekar D. Banana leaf dressing for skin graft donor areas. Burns 2003;29(5):483–6. [DOI] [PubMed] [Google Scholar]
- 124. Keswani MH, Vartak AM, Patil A, Davies JW. Histological and bacteriological studies of burn wounds treated with boiled potato peel dressings. Burns 1990;16(2):137–43. [DOI] [PubMed] [Google Scholar]
- 125. Colletta V, Dioguardi D, Di Lonardo A, Maggio G, Torasso F. A trial to assess the efficacy and tolerability of Hyalofill‐F in non‐healing venous leg ulcers. J Wound Care 2003;12(9):357–60. [DOI] [PubMed] [Google Scholar]
- 126. Nagai MK, Embil JM. Becaplermin: recombinant platelet derived growth factor, a new treatment for healing diabetic foot ulcers. Expert Opin Biol Ther 2002;2(2):211–8. [DOI] [PubMed] [Google Scholar]
- 127. Hansson C. Interactive wound dressings. A practical guide to their use in older patients. Drugs Aging 1997;11(4):271–84. [DOI] [PubMed] [Google Scholar]
- 128. Casey G. Wound dressings. Paediatr Nurs 2001;13(4):39–42. [DOI] [PubMed] [Google Scholar]
- 129. Benbow M. Mixing and matching dressing products. Nurs Stand 2000;14(49):56–8, 60, 62. [DOI] [PubMed] [Google Scholar]
- 130. Fletcher J. Exudate theory and the clinical management of exuding wounds. Prof Nurse 2002;17(8):475–8. [PubMed] [Google Scholar]
- 131. Anderson I. Practical issues in the management of highly exuding wounds. Prof Nurse 2002;18(3):145–8. [PubMed] [Google Scholar]
- 132. Cutting KF, White RJ. Avoidance and management of peri‐wound maceration of the skin. Prof Nurse 2002;18(1):33, 35–6. [PubMed] [Google Scholar]
- 133. O'Brien M. Exploring methods of wound debridement. Br J Community Nurs 2002: 10–18. [PubMed]
- 134. Sibbald RG, Williamson D, Orsted HL, Campbell K, Keast D, Krasner D, Sibbald D. Preparing the wound bed – debridement, bacterial balance and moisture balance. Ostomy Wound Manage 2000;46(11):14–22, 24–8, 30–5. [PubMed] [Google Scholar]
- 135. Terrill PJ, Varughese G. A comparison of three primary non‐adherent dressings applied to hand surgery wounds. J Wound Care 2000;9(8):359–63. [DOI] [PubMed] [Google Scholar]
- 136. Knauth A, Gordin M, McNelis W, Baumgrat S. Semi‐permeable polyurethane membrane as an artificial skin for the premature neonate. Pediatrics 1989;83(6):945–50. [PubMed] [Google Scholar]
- 137. Ameen H, Moore K, Lawrence JC, Harding KG. Investigating the bacterial barrier properties of four contemporary wound dressings. J Wound Care 2000;9(8):385–8. [DOI] [PubMed] [Google Scholar]
- 138. Sprung P, Hou Z, Ladin DA. Hydrogels and hydrocolloids: an objective product comparison. Ostomy Wound Manage 1998;44(1):36–42, 44, 46. [PubMed] [Google Scholar]
- 139. O'Dovovan DA, Mehdi SY, Eadie PA. The role of Mepitel silicone net dressings in the management of fingertip injuries in children. J Hand Surg [Br] 1999;24(6):727–30. [DOI] [PubMed] [Google Scholar]
- 140. Collier M, Hollingworth H. Pain and tissue trauma during dressing change. Nurs Stand 2000;14(40):71–3. [DOI] [PubMed] [Google Scholar]
- 141. Limova M, Troyer‐Caudle J. Controlled, randomized clinical trial of 2 hydrocolloid dressings in the management of venous insufficiency ulcers. J Vasc Nurs 2002;20(1):22–32. [DOI] [PubMed] [Google Scholar]
- 142. Mertz PM, Marshall DA, Eaglstein WH. Occlusive wound dressings to prevent bacterial invasion and wound infection. J Am Acad Dermatol 1985;12(4):662–8. [DOI] [PubMed] [Google Scholar]
- 143. Fowler E, Papen JC. Evaluation of an alginate dressing for pressure ulcers. Decubitus 1991;4(3):47–8, 50, 52. [PubMed] [Google Scholar]
- 144. Young T. Reaping the benefits of foam dressings. Community Nurse 1998;4(5):47–8. [PubMed] [Google Scholar]
- 145. Campton‐Johnston S, Wilson J. Infected wound management: advanced technologies, moisture‐retentive dressings, and die‐hard methods. Crit Care Nurs Q 2001;24(2):64–77. [DOI] [PubMed] [Google Scholar]
- 146. Purna SK, Babu M. Collagen based dressings – a review. Burns 2000;26(1):54–62. [DOI] [PubMed] [Google Scholar]
- 147. Ballard K, Baxter H. Developments in wound care for difficult to manage wounds. Br J Nurs 2000;9(7):405–8, 410, 412. [DOI] [PubMed] [Google Scholar]
- 148. Hansbrough JF, Franco ES. Skin replacements. Clin Plast Surg 1998;25(3):407–23. [PubMed] [Google Scholar]
- 149. Moore K. Compromised wound healing: a scientific approach to treatment. Br J Community Nurs 2003;8(6):274–8. [DOI] [PubMed] [Google Scholar]
- 150. Cullum N, Nelson EA, Flemming K, Sheldon T. Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy. Health Technol Assess 2001;5(9):1–221. [DOI] [PubMed] [Google Scholar]
- 151.Statistics Canada., authors http://www.statcan.ca/start.html
- 152.Health Canada., authors http://www.hc‐sc.gc.ca/english
- 153. Canada's Health Care System. Health Canada: health system and policy division [data file: H39‐502/1999], Ottawa, Ontario, 1999.
- 154. Health Canada. First ministers accord on health care renewal. Retrieved September 10, 2003, from http://www.hc‐sc.gc.ca/english/hca2003/accord.html
- 155.Canadian Association of Wound Care website., authors http://www.cawc.net
- 156. Sibbald RG, Orsted HL, Schultz G, Coutts P, Keast D. International Wound Bed Preparation Advisory Board; Canadian Chronic Wound Advisory Board. Preparing the wound bed 2003: Focus on infection and inflammation. Ostomy Wound Manage 2003;49(11):23–51. [PubMed] [Google Scholar]
- 157.International Interdisciplinary Wound Care Course website., authors doi: 10.1111/j.1742-4801.2004.00005.x. http://www.twhc.ca [DOI] [PMC free article] [PubMed]
- 158. Sibbald RG, Orsted HL. The International Interdisciplinary Wound Care Course at the University of Toronto: a 4‐year evolution. Int Wound J 2004; 1(1):34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ovington L. Wound care products: How to choose. Home Healthcare Nurse 2001;19(4):224–32. [DOI] [PubMed] [Google Scholar]
- 160. Ohura T, Sanada H, Mino Y. The cost effectiveness of using of modern dressing and traditional dressing in pressure ulcer or management vs. activity based costing, clinical comparison research. Japan Aging Med J. In press. [DOI] [PubMed] [Google Scholar]
- 161. Ministry of Health and Welfare Bureau for the Elderly. Supervisor for Elderly Insurance. Pressure ulcer prevention/treatment guideline. Tokyo: Shorinsha, 1998:. 77–89. [Google Scholar]
- 162. Fukui M. Pressure ulcer healing manual. Tokyo: Shorinsha, 1993. [Google Scholar]
- 163. Ohura T, Fujii T, Moriguchi T et al. Ministry of Health, Labor and Welfare Longevity General Scientific Research Enterprise – research into the prevention and evaluation of pressure ulcer treatment /nursing/home care/home care devices (H10‐Longevity‐012). Ministry of Health, Labor and Welfare, 1998, Longevity Research Report, 1999:. 87–102.
- 164. Sanada H. Current issues in pressure ulcer management of bedfast elderly in Japan. J Tissue Viability 2001;11(1):35–6. [DOI] [PubMed] [Google Scholar]


