Abstract
This study aims to clarify (i) the incidence of pressure ulcers in neonates admitted to the neonatal intensive care units (NICUs) and (ii) risk factors of pressure ulcer development. All infants admitted to the NICU and kept in incubators from seven hospitals during the study period were recruited to the study. Each infant was given skin examination every day by nurses, and risk factors were collected three times a week by one researcher. The incidence of the pressure ulcers was calculated, and the risk factors for pressure ulcers were determined by using univariate and multivariate analysis. Eighty‐one infants were involved in the study. A total of 14 pressure ulcers occurred in 13 infants during the 11‐month study period, the incidence was 0·01 persons per day and cumulative incidence rate was 16·0%. Seven (50·0%) of 14 pressure ulcers were located on the nose. Multivariate analysis identified the following risk factors: skin texture (Dubowitz neonatal maturation assessment scale: skin texture score of 1 point or lower) [odds ratio 7·6; 95% confidence interval (CI) 1·58 –36·71, P = 0·012] and endotracheal intubation usage (odds ratio 4·0; 95% CI 1·04–15·42, P = 0·042).
Keywords: Incidence, Neonatal intensive care unit, Pressure ulcer, Risk factor
INTRODUCTION
Paediatric patients in the neonatal intensive care unit (NICU) or paediatric intensive care units (PICU) are at risk of pressure ulcers. Especially, the premature neonates nursed in incubators are high‐risk groups because of immaturity of the skin, new technologies such as high‐frequency ventilation, which restricts body movements (1), and warm and humid environment. Incidence rates of 26% and 27% in PICUs 2, 3 and 19% in NICU (4) have been reported. These incidence rates were higher than those of non critical hospitalised paediatric patients (0·29%) (5). The most common location where a pressure ulcer develops is the head, including the occipital region, nose and ears 2, 3, 6. However, incidence rates of the premature neonates nursed in incubators and the location of the pressure ulcer development are unknown.
Previous studies have reported various risk factors in PICU children; for instance, paediatric risk of mortality score and race (Caucasian) (2); respirator usage, average blood pressure of ≤50 mmHg and Braden Q scale scores of ≤16 points (3); and PICU stay of ≥96 hours, high positive end expiratory pressure, no body position changes, body position changes on low air‐loss beds and body weight loss (7). Although many studies have cited the risk factors for the PICU children, most of the previous studies have a very wide medical condition or paediatric age. We believe that there are specific risk factors for pressure ulcers of neonates who are nursed in incubators because extrinsic factors (incubator air temperature and humidity and support surfaces) and intrinsic factors (size and physical shape of an infant, physiological reactions and skin maturity) are different from those in children hospitalised in general units. However, specific data on the risk factors of pressure ulcers in neonates who are nursed in incubators have not been well documented.
This study aimed to (i) determine the incidence of pressure ulcers in neonates admitted to the NICU and nursed in incubators and (ii) determine risk factors that are associated with the occurrence of pressure ulcers to develop nursing and medical strategies to minimise this problem. In this study, a pressure ulcer was defined as a skin lesion resulting from pressure applied by the support surface or medical equipment.
METHODS
Design
This was a multisite prospective cohort study.
Settings and subjects
Seven NICUs participated in this study. Two of the seven NICUs are located in Tokyo, one is located in Fukui prefecture and four are located in Ishikawa prefecture in Japan. Three of the NICUs are contained within the university hospitals and four are contained within the public hospitals, with NICU beds ranging from 1 to 14· The study was conducted from January to November in 2006, and the length of the study at each hospital ranged from 2 to 4 months.
Subjects were neonates nursed in incubators at the NICU who did not have skin breakdown at the start of the study. The exclusion criteria were infants who were nursed in open cots, infants who had skin breakdowns prior to the beginning of the study and infants who were judged by physicians and nurses to be unsuitable for participation.
Data collection
Each infant was given a skin examination every day by nurses. Pressure ulcer information included location and initial stage. The stage of pressure ulcers was recommended by the National Pressure Ulcer Advisory Panel (8).
Risk factors based on review of the literature were collected through observations, and medical and nursing records were reviewed three times a week by one researcher. These data included demographic information (gender, birthweight and gestational age), Apgar score (1‐ and 5‐minute score), diagnoses, data on the course of NICU hospitalisation including length of stay, nutritional status, haemodynamic responses (heart rate, blood pressure and haemoglobin), body temperature, urine output, O2 saturation, use of endotracheal intubation and amount of positive expiratory pressure, use of nasal continuous positive airway pressure (CPAP) and nasal directional positive airway pressure (DPAP), use of vasoactive sedatives, narcotics and paralytic medications, limited number of position changes, use of support surfaces, temperature and humidity in the incubator, Braden Q score, Dubowitz neonatal maturation assessment scale and others. The endpoints of the follow‐up survey were (i) occurrence of pressure ulcers, (ii) the infant's transference from an incubator to a cot and (iii) discharge from the NICU.
Instruments
The Apgar score (9) is an index to evaluate the immediate postnatal health of a neonate and consists of five categories: breathing, heart rate, muscle tone, reflex irritability and skin colour. Each category contains a subscale in which the neonate can be rated from 0 (most risk) to 2 (normal). Postnatal 1‐ and 5‐minute Apgar scores can range from 0 to 10, with more than 7 indicating normal health.
The Braden Q score (10) is a tool used to assess a paediatric patient's risk of developing pressure ulcers and consists of seven categories: mobility, activity, sensory perception, moisture, nutrition, friction and shear, and tissue perfusion and oxygenation. Each category contains a subscale in which the patient can be rated from 1 (most risk) to 4 (least risk). Possible scores can range from 7 to 28, with the lower scores indicating a higher risk of developing pressure ulcers.
The Dubowitz neonatal maturation assessment scale (11) is a tool to assess gestational age in the newborn infant and consists of 10 neurologic and 11 external criteria. In this study, we selected ‘skin texture’ from the external criteria. This item contains a subscale in which the infant can be rated from 0 (most immature) to 4 (normal).
Data analysis
The incidence of pressure ulcers was calculated, and the risk factors for pressure ulcers were determined by using univariate and multivariate analysis. Incidence density and cumulative incidence were calculated using the following formulas:
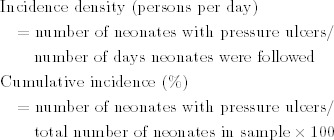 |
Statistical Package for the Social Sciences for Windows version 10.1 (Chicago, IL, USA) was used to conduct statistical analyses using the χ 2 test, Fisher's direct probability test and Mann–Whitney test. When significant differences were found, multivariate analysis was conducted to extract the risk factors for pressure ulcer. First, a correlation matrix was prepared for variables with significant differences and then Pearson's correlation coefficients were used.
Using pressure ulcer as a dependent variable, independent variables were analysed by logistic regression analysis. In all tests, the level of significance was set at P < 0·05.
Ethical considerations
This study was conducted with approval from the Ethics Review Board of the Department of Medical Sciences, Kanazawa University, and approval was obtained from the ethics review boards of all participating hospitals. The study purpose and contents were explained orally and in writing to the parents of infants who were admitted to the NICU and written consent to participate in this study was obtained.
RESULTS
Of the 211 neonates admitted to the NICU during the study period, those with skin breakdowns (n = 6) and those who were judged by physicians and nurses to be unsuitable for participation (n = 3) were excluded from the study. Of the remaining 202 neonates, consent was obtained for 81 neonates (38·2%), and the data obtained from these 81 neonates were analysed. None of the neonates were withdrawn from the study because of exacerbated physical condition. There were 39 (48·1%) males and 42 (51·9%) females. The mean of the Braden Q score was 22·2 points. Low‐birthweight was the most common reason for hospitalisation (Table 1). Most gestational age groups were less than 30 weeks (Figure 1).
Table 1.
Demographic characteristics
| N (%) | |
|---|---|
| Gender | |
| Male | 39 (48·1) |
| Female | 42 (51·9) |
| Gestational age (week), mean (range) | 32·5 (24–41) |
| Under 30 | 23 |
| 30–32 | 18 |
| 33–35 | 18 |
| 36–38 | 14 |
| Over 39 | 8 |
| Birthweight (g), mean (range) | 1745 (478–4122) |
| Apgar score | |
| 1 minutes, mean (range) | 8 (0–10) |
| 5 minutes, mean (range) | 9 (0–10) |
| Braden Q score, mean (range) | 22·2 (19–24) |
| Reason for admission and disease | |
| Low‐birthweight | 60 (74·1) |
| Asphyxia neonatorum | 6 (7·4) |
| Sepsis pneumonitis | 4 (4·9) |
| Transient tachypnea of the newborn | 3 (3·7) |
| Surgery * | 3 (3·7) |
| Others | 5 (6·2) |
*Congenital esophageal atresia, congenital duodenal atresia and meconium peritonitis.
Figure 1.
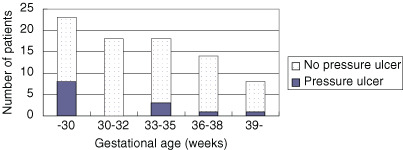
Distribution of gestational age.
A total of 13 neonates had pressure ulcers, and the neonates were followed for a total of 1723 days. Incidence density was 0·01 persons per day, and during the entire study period, the cumulative incidence was 16·0%. A total of 14 pressure ulcers occurred in 13 neonates, and 6 of these occurred within 1 week and 3 occurred over 3 weeks (Figure 2). The most common location was the nose (n = 7, 50·0%), followed by the labrum and dorsum of the foot (n = 2, 14·2%). Six patients who developed pressure ulcers on the nose used nasal CPAP or DPAP. Pressure ulcers were classified as stage I (n = 3, 21·4%) and stage II (n = 11, 78·6%) (Table 2).
Figure 2.
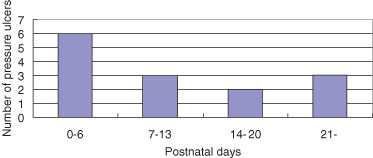
Day of development of pressure ulcer.
Table 2.
Location and stage of pressure ulcers
| N (%) | |
|---|---|
| Location | |
| Nose | 7 (50·0) |
| Labrum | 2 (14·2) |
| Dorsum of foot | 2 (14·2) |
| Back | 1 (7·1) |
| Occiput | 1 (7·1) |
| Leg | 1 (7·1) |
| Stage of pressure ulcer | |
| Stage I | 3 (21·4) |
| Stage II | 11 (78·6) |
The risk factors that were significant at P < 0·05 were birthweight, skin texture, incubator temperature, incubator humidity, support surface, limited number of position changes and use of endotracheal intubation. Of the 13 neonates with pressure ulcers, the gestational ages of 8 neonates were less than 33 weeks. Pearson correlation coefficients were calculated, and when analysing two variables with r≥|0·4|, one was used as an independent variable. The following parameters were subjected to multivariate analysis: birthweight, skin texture, endotracheal intubation, nasal CPAP and DPAP, and support surface. Moreover, ‘hospital’ was added to the independent variables. The variables were considered in a stepwise‐reduction logistic regression analysis, and we found that skin texture and endotracheal intubation were risk factors for pressure ulcers in the NICU (Table 3).
Table 3.
Risk factors for pressure ulcer *
| β | P | Odds ratio | 95% confidence interval | |
|---|---|---|---|---|
| Skin texture | 2·023 | 0·012 | 7·6 | 1·58–36·71 |
| Endotracheal intubation | 1·376 | 0·047 | 4·0 | 1·04–15·42 |
| Constant | ||||
| −2·709 | 0·000 | 0·1 |
*Logistic regression analysis (stepwise reduction).
DISCUSSION
This is the first study to focus on the incidence and risk factors for pressure ulcer development in the NICU. We clarified the characteristics and risk factors for pressure ulcers in the NICU by directly observing the skin of neonates and determining the physical condition of neonates and incubator settings. Moreover, a multisite prospective study yielded highly reliable results.
Incidence of pressure ulcers in the NICU
In our study, the cumulative incidence rate was 16·0%, which was similar to that found in a previous study (4). In contrast, the incidence of pressure ulcers in Japanese University Hospital inpatients is 2·5% (12). We thought that the higher incidence of pressure ulcers in the NICU might be a burgeoning health care cost associated with pressure ulcer management.
The most common location for pressure ulcers was the nose (50·0%). Previous study (2) reported that the most common location of skin breakdowns in the PICU was the nose. Another study conducted in a PICU (3) reported that the occipital region was the most common location for pressure ulcers. However, in this study, only one pressure ulcer occurred in the occipital region (7·1%). The low incidence of pressure ulcers in the occipital region may be because of the positive effect of kangaroo mother care (13). This relieves pressure from the occipital region. Even in neonates with respirators, gel pillows and foam mattresses were used to redistribute pressure as much as possible.
Risk factors for pressure ulcers in NICU
Multivariate analysis extracted the following risk factors: skin texture and endotracheal intubation. Skin texture was objectively assessed using the ‘skin texture’ test of the Dubowitz neonatal maturity assessment scale (11). In this study, we defined skin immaturity as having a score of 0 (extremely thin and gelatin‐like) or 1 (thin and smooth). The odds ratio for skin immaturity was 7·6. Skin immaturity is believed to be influenced by gestational age and age after birth. It is generally accepted that skin functions mature around the 33rd week of gestation, but at this stage, the stratum corneum is thin and the papillary layer is prone to damage. In neonates with gestational ages of less than 33 weeks, the dermoepidermal junction remains immature. It has also been reported that, irrespective of gestational age, the skin rapidly develops within 2 or 3 weeks of birth and matures to the skin of full‐term neonates (14). In our study, of the 13 neonates with pressure ulcers, the gestational ages of 8 neonates were less than 33 weeks (Figure 1). In addition, of the 14 pressure ulcers, 11 occurred within 21 days of birth. For these neonates, even small external forces can cause skin damage.
In neonates with unstable respiration, endotracheal intubation is used. However, in this study, no significant difference was seen in oxygen saturation and oxygen concentration between the pressure ulcer and non pressure ulcer groups, thus negating the role of hypoxia in pressure ulcer development. Pender and Frazier (15) studied the prevalence of dermal pressure ulcers in mechanically ventilated patients and described that no relationship was identified between the perfusion and oxygenation variables evaluated in this study and development of dermal pressure ulcers. However, compared with non pressure ulcer groups, pressure ulcer groups were with limited number of position changes. Six patients who developed pressure ulcers on the nose used nasal CPAP or DPAP. Therefore, nasal CPAP or DPAP was thought to be an independent risk factor for nasal pressure ulcers. It has also been reported that the nasal CPAP cannula causes compression necrosis (14) and nasal deformations (16).
Study limitations
Informed consent for this study was obtained for 38·2% of the 211 neonates. There was only one extremely immature neonate weighing less than 500 g, and none of the neonates had genetic diseases, congenital abnormities or congenital heart diseases that were likely to have exacerbated circulation. Subsequently, the results of this study cannot be applied to such neonates. A researcher directly visited the hospitals to observe and assess the skin of the subjects. This may have altered the awareness of hospital staff with regard to skin breakdown, and Hawthorne effects could have lowered its incidence.
Implementation to nursing
The results of this study confirmed that the most common location of pressure ulcer is the nose and that pressure ulcers are caused by skin texture immaturity and endotracheal intubation usage. This study used the ‘skin texture’ test of the Dubowitz neonate maturity assessment scale, which has been used to estimate gestational age at birth, and the results suggest that it is possible to assess the risk of pressure ulcers using this instrument periodically. It is also desirable to establish preventative care as well as to improve the endotracheal intubation materials to suit various skin conditions.
CONCLUSION
A multisite prospective study was conducted on 81 neonates who were placed in incubators at the NICU of seven hospitals in Japan.
-
1
Incidence density was 0·01 persons per day and cumulative incidence was 16·0%.
-
2
Multivariate analysis identified only two risk factors for pressure ulcer: skin texture (odds ratio: 7·6) and endotracheal intubation usage (odds ratio: 4·0).
From these results, we believe that ‘skin texture’ scores as determined by the Dubowitz neonatal maturation assessment scale might be used to assess the risk of pressure ulcers, and new protective nasal materials for neonates using endotracheal intubation must be developed.
REFERENCES
- 1. Lund C. Prevention and management of infant skin breakdown. Nurs Clin North Am 1999;34:907–20. [PubMed] [Google Scholar]
- 2. Zollo MB, Gostisha ML, Berens RJ, Schmidt JE, Weigle CG. Altered skin integrity in children admitted to a pediatric intensive care unit. J Nurs Care Qual 1996;11:62–7. [DOI] [PubMed] [Google Scholar]
- 3. Curley MA, Quigley SM, Lin M. Pressure ulcers in pediatric intensive care: incidence and associated factors. Pediatr Crit Care Med 2003;4:284–90. [DOI] [PubMed] [Google Scholar]
- 4. Huffines B, Logsdon MC. The neonatal skin risk assessment scale for predicting skin breakdown in neonates. Issues Compr Pediatr Nurs 1997;20:103–14. [DOI] [PubMed] [Google Scholar]
- 5. Baldwin KM. Incidence and prevalence of pressure ulcers in children. Adv Skin Wound Care 2002;15:121–4. [DOI] [PubMed] [Google Scholar]
- 6. McLane KM, Bookout K, McCord S, McCain J. The 2003 national pediatric pressure ulcer and skin breakdown prevalence survey. A multisite study. J Wound Ostomy Continence Nurs 2004;31:168–78. [DOI] [PubMed] [Google Scholar]
- 7. McCord S, McElvain V, Sachdeva R, Schwartz P. Risk factors associated with pressure ulcers in the pediatric intensive care unit. J Wound Ostomy Continence Nurs 2004;31:179–83. [DOI] [PubMed] [Google Scholar]
- 8. National Pressure Ulcer Advisory Panel. Pressure ulcer prevalence, cost, and risk assessment. Consensus development conference statement. Decubitis 1989;2:23–8. [PubMed] [Google Scholar]
- 9. Jepson HA, Talashek ML, Tichy AM. The Apgar score: evolution, limitations, and scoring guidelines. Birth 1991;18:83–92. [DOI] [PubMed] [Google Scholar]
- 10. Curley MA, Razmus IS, Roberts KE, Wypij D. Predicting pressure ulcer risk in pediatric patients: the Braden Q scale. Nurs Res 2003;52:22–31. [DOI] [PubMed] [Google Scholar]
- 11. Dubowitz LM, Dubowitz V, Goldberg C. Clinical assessment of gestational age in the newborn infant. J Pediatr 1970;77:1–10. [DOI] [PubMed] [Google Scholar]
- 12. Sanada H, Miyachi Y, Ohura T, Moriguchi T, Tokunaga K, Shido K, Nakagami G. The Japanese pressure ulcer surveillance study: a retrospective cohort study to determine prevalence of pressure ulcers in Japanese hospitals. Wounds 2008;20:176–82. [PubMed] [Google Scholar]
- 13. Charpak N, Ruiz‐Pelaez JG, Figueroa de CZ, Charpak Y. A randomized, controlled trial of kangaroo mother care: results of follow‐up at 1 year of corrected age. Pediatrics 2001;108:1072–9. [DOI] [PubMed] [Google Scholar]
- 14. Irving V. Caring for and protecting the skin of pre‐term neonates. J Wound Care 2001;10:253–6. [DOI] [PubMed] [Google Scholar]
- 15. Pender LR, Frazier SK. The relationship between dermal pressure ulcers, oxygenation and perfusion in mechanically ventilated patients. Intens Crit Care Nurs 2005;21:29–38. [DOI] [PubMed] [Google Scholar]
- 16. Friedman J. Plastic surgical problems in the neonatal intensive care unit. Clin Plastic Surg 1981;25:599–617. [PubMed] [Google Scholar]


