Abstract
This study describes the significant correlation between the Braden Scale (BS) and the Palliative Performance Scale (PPS) in patients with advanced illness that has not been previously reported. The analysis was based on a prospective sequential case series of 664 patients suffering from advanced illness who were referred to a regional palliative medicine programme in Toronto, Canada. Baseline BS and PPS scores assessed within 24 hours of referral were considered for analysis. After controlling for age, gender, consult site and diagnosis (cancer versus non cancer), we observed a significant positive correlation between baseline PPS and BS scores (r = 0·885, P < 0·001). These findings suggest that for patients with advanced illness where BS is not routinely used, PPS could be considered as a proxy for pressure ulcer risk assessment.
Keywords: Advanced illness, Braden Scale, Palliative care, Palliative Performance Scale, Pressure ulcers
Introduction
Patients with advanced illness are in transition from curative care to supportive and palliative care (1). The overall management of such patients is complex as multiple management issues exist. Wound management is increasingly being recognised as a major domain in their overall care. Patients with advanced illness experience a wide range of wound‐related concerns (1) . Pressure ulcers represent up to 57% of all wounds seen in this clinical context (1) . Significant controversy exists regarding pressure ulcers; one position is that they are completely preventable and thus their occurrence reflects negligence and neglect, while the other position asserts that they are largely inevitable and represent part of the natural history of advanced illness. The truth lies somewhere in between these two extreme positions.
The Braden Scale (BS) (Figure 1), first developed in 1984 by Braden and Bergstrom (2), is a tool designed to assess the patient’s level of risk in developing pressure ulcers. The BS is comprised of six subscales that assess a patient’s sensory perception, the skin’s exposure to moisture, activity level, mobility, nutritional status, and friction and shear. For five of the subscales (sensory perception, mobility, activity, moisture and nutrition), the scores range from 1 to 4, with 4 representing the highest. The last subscale (friction and shear) ranges from 1 to 3. The sum of the six subscale scores yields the total BS score, which can range from 6 to 23. Lower total scores are associated with a higher risk of developing pressure ulcers. A number of studies have been conducted to determine the predictive validity of the BS 3, 4, 5. From these studies, five risk levels of developing pressure ulcers have been identified: 19–23 not at risk, 15–18 mild risk, 13–14 moderate risk, 10–12 high risk and ≤9 very high risk.
Figure 1.
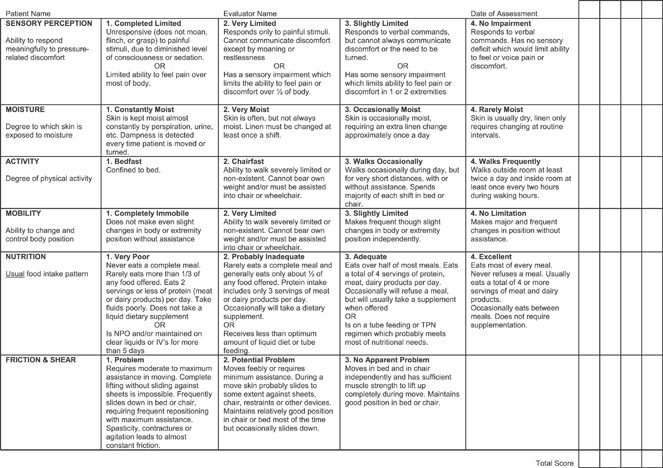
The Braden Scale (© Barbara Braden and Nancy Bergstrom, 1988. Reprinted with Permission).
The Palliative Performance Scale (PPS) (Figure 2) is used to assess the functional status of palliative care patients (6). The PPS has five dimensions including the patient’s ambulation, activity level and evidence of disease, self‐care, oral intake and level of consciousness. The PPS has 11 levels from PPS 0% to PPS 100% in 10% increments. A patient at PPS 0% is dead, while at PPS 100% is mobile and healthy. Since its introduction in 1996, the PPS has become a popular assessment tool used by clinicians to communicate the functional status of palliative care patients to aide in care planning and delivery (7). The PPS has also been found to be highly predictive in estimating the survival duration of critically ill patients in palliative care settings (8).
Figure 2.
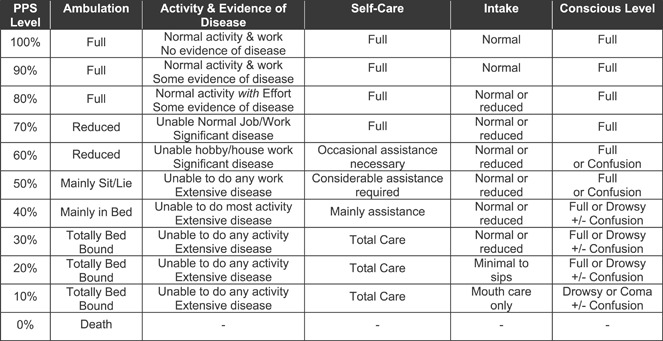
Palliative Performance Scale (© 2001 Victoria Hospice Society. 1996. Reprinted with Permission).
Presently, there is no risk assessment tool that is entirely exact. Furthermore, the success of any tool is predicated on whether health care professionals are actually tracking their results and trends and acting upon them. Regarding predictive validity, the BS has shown sensitivities that range from 70% to 100% and specificities ranging from 64% to 90% (3). Therefore, it tends to over predict the likelihood of developing pressure ulcers. Ultimately, the optimal mode of assessing pressure ulcer risk may involve the use of a composite assessment that uses multiple validated tools (BS, PPS, etc.) along with consideration of other risk factors such as comorbid illness (diabetes mellitus, paralysis, spinal deformity, etc.) and other parameters such as laboratory tests (haemoglobin, white cell count, erythrocyte sedimentation rate (ESR), C‐reactive protein (CRP), serum albumin, etc.).
Methods
Six hundred and sixty‐four sequential patients were eligible for the study. They represented patients referred to a consultative combined community and hospital‐based palliative medicine programme for consideration of supportive and palliative care. This programme serves an estimated population of 750 000 within the northwest quadrant of Metropolitan Toronto, Canada. Recruitment for this study was commenced with new referrals on 1 May 2005 and ended on 30 June 2006. All patients or their substitute decision makers provided consent to have their clinical data registered in a research database. The study protocol was approved by the research ethics board of the William Osler Health Centre in Toronto, Canada.
Each patient in this study had an initial PPS and BS assessment performed by a member of the palliative medicine consult team within 24 hours of the referral. Other data included in this study were the patient’s age, gender, first consult site (home or hospital) and diagnosis type (cancer or non cancer). Statistical analyses were performed with SPSS 16·0. Frequency distributions were examined overall and in two groups categorised by diagnosis type. Pearson chi‐squared test was used to examine the relationship between diagnosis type and each of the variables. General linear regression was used to test the correlation between PPS and BS and other variables.
Results
Patient characteristics
Of the 664 patients in this study, 465 (70·0%) were diagnosed with cancer and the remaining 199 (30·0%) were non cancer. Although male and female patients were distributed evenly overall, there were significantly more male patients in this cohort with cancer (χ 2 = 10·3, P = 0·001). Just over half of the patients (59·4%) had their first consult in the hospital, but patients with cancer were more likely to have their first consult at home (χ 2 = 103·2, P < 0·001). Compared with the median age of 77 years, patients with cancer were younger than those with non cancer: 72·5 years (cancer) versus 80·6 years (non cancer) (χ 2 = 54·6, P < 0·001). These patient characteristics are shown in Table 1.
Table 1.
Overall patient characteristics
| Variables | Overall | Diagnosis type | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of patients (%) | Mean | Median | Range | Number of patients with cancer (%) | Number of non cancer patients (%) | χ 2 | P value | |
| Gender | ||||||||
| Male | 330 (49·7) | 250 (37·7) | 80 (12·0) | 10·3 | 0·001 | |||
| Female | 334 (50·3) | 215 (32·4) | 119 (17·9) | |||||
| Site of first consult | ||||||||
| Home | 270 (40·7) | 248 (37·3) | 22 (3·3) | 103·2 | <0·001 | |||
| Hospital | 394 (59·3) | 217 (32·7) | 177 (26·7) | |||||
| Age in years | ||||||||
| <45 | 19 (2·9) | 74·9 | 77·0 | 19–103 | 16 (2·4) | 3 (0·5) | 54·6 | <0·001 |
| 45–64 | 107 (16·1) | 94 (14·2) | 13 (2·0) | |||||
| 65–74 | 143 (21·5) | 115 (17·3) | 28 (4·2) | |||||
| 75–84 | 247 (37·2) | 166 (25·0) | 81 (12·2) | |||||
| 85+ | 148 (22·3) | 74 (11·1) | 74 (11·1) | |||||
| Braden score | ||||||||
| 19–23 (not at risk) | 156 (23·5) | 14·3 | 14·0 | 6–22 | 154 (23·2) | 2 (0·3) | 236·5 | <0·001 |
| 15–18 (mild risk) | 171 (25·8) | 151 (22·7) | 20 (3·0) | |||||
| 13–14 (moderate risk) | 90 (13·6) | 68 (10·2) | 22 (3·3) | |||||
| 10–12 (high risk) | 131 (19·7) | 65 (9·8) | 66 (9·9) | |||||
| ≤9 (very high risk) | 116 (17·5) | 27 (4·1) | 89 (13·4) | |||||
| PPSV2 level | ||||||||
| PPS 10% | 27 (4·1) | PPS 44·7% | PPS 45% | PPS 10–80% | 3 (0·5) | 24 (3·6) | 269·0 | <0·001 |
| PPS 20% | 95 (14·3) | 20 (3·0) | 75 (11·3) | |||||
| PPS 30% | 129 (19·4) | 68 (10·2) | 61 (9·2) | |||||
| PPS 40% | 81 (12·2) | 63 (9·5) | 18 (2·7) | |||||
| PPS 50% | 111 (16·7) | 94 (14·2) | 17 (2·6) | |||||
| PPS 60% | 87 (13·1) | 84 (12·7) | 3 (0·5) | |||||
| PPS 70% | 107 (16·1) | 106 (16·0) | 1 (0·2) | |||||
| PPS 80% | 27 (4·1) | 27 (4·1) | 0 (0) | |||||
| Total | 664 | 465 (70·0) | 199 (30·0) | |||||
PPS, Palliative Performance Scale.
BS and PPS assessments
Overall, the initial BS scores in this study ranged from 6 to 22 with a median score of 14·0. The initial PPS scores ranged from PPS 10% to PPS 80% with a median of PPS 45%. The diagnosis type had a significant impact on the patients’ PPS and BS levels (χ 2 = 236·5 for PPS and χ 2 = 269·0 for BS, both P < 0·001). Specifically, non cancer patients had significantly higher occurrence of lower PPS and BS scores when compared against those with cancer. The patterns of PPS and BS scores are shown in Figure 3.
Figure 3.
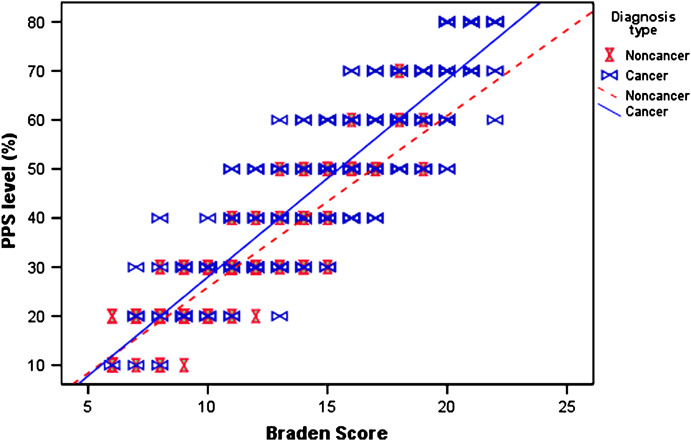
Correlation between BS and PPS. BS, Braden Scale; PPS, Palliative Performance Score.
Correlation between BS and PPS
A strong linear trend was shown between the initial BS and the initial PPS scores, but it appeared to differ between cancer and non cancer patients. This correlation was examined further using the partial correlation test before and after taking into account the effects of age, gender, consult site and diagnosis type. The significant correlation between PPS and BS persisted whether the effect of other factors were removed or not (r = 0·936 before and r = 0·885 after, both P < 0·001). Furthermore, our linear regression model that included all the variables showing diagnosis type and consult site had a significant impact on the correlation of PPS and BS (F = 1700·9, P < 0·001). However, age and gender had no effect on this relationship. Based on the distribution of the PPS scores for each BS risk levels shown in Table 2 and the computed PPS scores from the linear regression model, a suggested conversion table between BS and PPS levels is shown in Table 3.
Table 2.
Distribution of PPS over BS levels
| BS level | PPS level | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | Total | |
| 19–23 (not at risk) | 0 | 0 | 0 | 0 | 7 | 30 | 92 | 27 | 156 |
| 15–18 (mild risk) | 0 | 0 | 4 | 21 | 78 | 53 | 15 | 0 | 171 |
| 13–14 (moderate risk) | 0 | 1 | 22 | 43 | 20 | 4 | 0 | 0 | 90 |
| 10–12 (high risk) | 0 | 24 | 85 | 16 | 6 | 0 | 0 | 0 | 131 |
| ≤9 (very high risk) | 27 | 70 | 18 | 1 | 0 | 0 | 0 | 0 | 116 |
| Total | 27 | 95 | 129 | 81 | 111 | 87 | 107 | 27 | 664 |
BS, Braden Scale; PPS, Palliative Performance Scale.
Table 3.
Suggested conversion between BS and PPS levels*
| BS | PPS |
|---|---|
| 19–23 (not at risk) | PPS 60%–PPS 80% |
| 15–18 (mild risk) | PPS 50%–PPS 60% |
| 13–14 (moderate risk) | PPS 40% |
| 10–12 (high risk) | PPS 30%–PPS 40% |
| ≤9 (very high risk) | PPS 10%–PPS 20% |
BS, Braden Scale; PPS, Palliative Performance Scale.
PPS = −8·99 + 3·77 (BS) − 3·25 site + 2·35 Diagnosis, where site = 0 for home and 1 for hospital and Diagnosis = 0 for non cancer and 1 for cancer.
Discussion
The development of pressure ulcers is a common clinical problem for patients with advanced illness, especially in the palliative care setting. The BS has been shown to be a validated and reliable tool for assessing the risk of developing pressure ulcers. Yet, unlike the PPS that is widely used to assess one’s functional status in palliative care, the BS tool is not commonly applied to these patients. In this study, a significant correlation between the initial BS and the initial PPS scores in a cohort of 664 patients with advanced illness referred to a regional palliative medicine programme that has never been reported until now.
In particular, patients with lower BS scores who were at higher risks of developing pressure ulcers also had lower PPS scores with reduced functional status. Furthermore, non cancer patients had greater risks of pressure ulcers when compared against those with cancer, but this difference diminished with decreasing function. Given this strong positive correlation, it would seem that PPS could be considered as a proxy measure in pressure ulcer risk assessment within the palliative care setting. Thus, for seriously ill patients with low PPS scores, clinicians should advocate for prescribed turning schedules and the use of special support surfaces and closely monitor for the development of early stage pressure lesions. The observed correlation is intuitive as patients spending more time in bed in one position because of fatigue, weakness, etc. may also have somewhat reduced fluid intake and poor nutritional status with decreased tissue turgor and cachexia.
Furthermore, because BS scores are often used as specific criteria by health authorities to determine whether progressively more expensive support surfaces (air fluidised, low air loss, alternating air, static flotation, etc.) need to be provided for the patient, the relationship of functional status could also be used for cost efficiency. In a case report of a patient with dementia who became bed bound, the Functional Assessment Staging Tool (FAST ) criteria of Stage 7(c) used by the National Hospice Pallative Care Organization (NHPCO) for 6‐month prognosis has shown to be much less predictive than PPS (PPS was 80% accurate in predicting <6 months versus FAST of 0% versus Mortality Rating Index 57%) (9). Thus, a low PPS combined with low BS score may improve accuracy, and the development of pressure ulcers is associated with decreased survival 10, 11, 12.
In spite of the striking correlation noted between the BS and the PPS scores, the findings from this study were from one regional palliative care programme only. As such, further validation of this relationship with larger sample preferably from a different site by independent investigators is needed. The suggested conversion table between BS and PPS levels also requires independent validation of its accuracy. In addition, this study only considered BS and PPS values at baseline without tracking the relation over time.
Conclusions
Pressure ulcers are highly prevalent in the setting of patients with advanced illness. They are associated with increased morbidity and mortality, reduced quality of life and escalating health care expenditures. BS has been shown to be an effective screening tool for risk assessment for the development of pressure ulcers in a number of clinical scenarios. This study shows a strong linear correlation between BS and PPS in the setting of patients with advanced illness referred for supportive and palliative care. Therefore, PPS should be considered as a proxy measure for BS in the pursuit of screening for pressure ulcer risk in the setting of supportive and palliative care.
Acknowledgements
We wish to thank Shiraz Irani and Linda Trozzolo for their work in data collection and Darren Hamilton for his editorial assistance. Funding support for the study has been provided by the Canadian Institutes for Health Research New Emerging Team grant in Palliative and End‐of‐life Care.
References
- 1. Maida V, Corbo M, Dolzhykov M, Ennis M, Irani S, Trozzolo L. Wounds in advanced illness: a prevalence and incidence study based on a prospective case series. Int Wound J 2007;5:305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braden BJ, Bergstrom N. Clinical utility of the Braden Scale for predicting pressure sore risk. Decubitus 1989;2:44–6, 50–1. [PubMed] [Google Scholar]
- 3. Braden BJ, Maklebust J. Preventing pressure ulcers with the Braden Scale. Am J Nurs 2005;105:70–4. [DOI] [PubMed] [Google Scholar]
- 4. Pancorbo‐Hidalgo PL, Garcia‐Fernandez FP, Lopez‐Medina IM, Alvarez‐Nieto C. Risk assessment scales for pressure ulcer prevention: a systematic review. J Adv Nurs 2006;54:94–110. [DOI] [PubMed] [Google Scholar]
- 5. Halfens RJG, Van Achterberg T, Bal RM. Validity and reliability of the Braden Scale and the influence of other risk factors: a multi‐centre prospective study. Int J Nurs Stud 2000;37:313–9. [DOI] [PubMed] [Google Scholar]
- 6. Anderson F, Downing MG, Hill J, Casorso L, Lerch N. Palliative Performance Scale (PPS): a new tool. J Palliat Care 1996;12:5–11. [PubMed] [Google Scholar]
- 7. Fainsinger RL, Demoissac D, Cole J, Mead‐Wood K, Lee E. Home versus hospice inpatient care: discharge characteristics of palliative care patients in an acute care hospital. J Palliat Care 2000;16:29–34. [PubMed] [Google Scholar]
- 8. Downing M, Lau F, Lesperance M, Karlson N, Shaw J, Kuziemsky C, Bernard S, Hanson L, Olajide L, Head B, Ritchie C, Harrold J, Casarett D. Meta‐analysis of survival prediction with Palliative Performance Scale. J Palliat Care 2007;23:245–54. [PubMed] [Google Scholar]
- 9. Doberman D, Yasar S, Durso S. Would you refer this patient to hospice? An evaluation of tools for determining life expectancy in end‐stage dementia. J Palliat Med 2007;10:1410–9. [DOI] [PubMed] [Google Scholar]
- 10. Lobato LS, Beirão I, Silva M, Fonseca I, Queirõs J, Rocha G, Sarmento AM, Sousa A, Sequeiros I. End‐stage renal disease and dialysis in hereditary amyloidosis TTR V30M: presentation, survival and prognostic factors. Amyloid 2004;11:27–37. [DOI] [PubMed] [Google Scholar]
- 11. Binder EF, Kruse RL, Sherman AK, Madsen R, Zweig SC, D’Agostino R, Mehr DR. Predictors of short‐term functional decline in survivors of nursing home‐acquired lower respiratory tract infection. J Gerontol A Biol Sci Med Sci 2003;58:60–7. [DOI] [PubMed] [Google Scholar]
- 12. Bennett RG, Bellantoni MF, Ouslander JG. Air‐fluidized bed treatment of nursing home patients with pressure sores. J Am Geriatr Soc 1989;37:235–42. [DOI] [PubMed] [Google Scholar]


