Abstract
To investigate the efficacy and safety of recombinant human epidermal growth factor (rhEGF) in advanced diabetic foot ulcers (DFU) A double‐blind trial was carried out to test two rhEGF dose levels in type 1 or 2 diabetes patients with Wagner’s grade 3 or 4 ulcers, with high risk of amputation. Subjects were randomised to receive 75 (group I) or 25 μg (group II) rhEGF through intralesional injections, three times per week for 5–8 weeks together with standardised good wound care. Endpoints were granulation tissue formation, complete healing and need of amputation. Safety was assessed by clinical adverse events (AEs) and laboratory evaluations. Forty‐one patients were included. After 5–8 weeks of treatment, 83% patients in the higher dose group and 61% in group II achieved useful granulation tissue covering more than 98% of the wound area. At long‐term assessment, 13 (56·5%) patients healed in group I and 9 (50%) in group II. The mean time to complete healing in group I was 20·6 weeks (95% CI: 17·0–24·2) and 19·5 weeks (16·3–22·7) in group II. After 1‐year follow‐up, only one patient relapsed. Amputation was not necessary in 65% and 66·7% of groups I and II, respectively. The AEs rates were similar. The most frequent were sepsis (33%), burning sensation (29%), tremors, chills and local pain (25% each). rhEGF local injection enhances advanced DFU healing and reduces the risk of major amputation. No dose dependency was observed.
Keywords: Diabetic foot ulcers, Epidermal growth factor, Wound healing
Introduction
The number of people with diabetes mellitus is expected to rise from 171 million in 2000 to 366 million in 2030 (1). Major complications include foot ulcers, gangrene and amputation, which represent leading causes of hospitalisation among those patients. Around 15% of people with diabetes mellitus develop a diabetic foot ulcer (DFU). This condition precedes 85% of major amputations in this population (2). The annual incidence of DFU is more than 2% in all diabetic patients (3) and rises largely when peripheral neuropathy is present (4). It is estimated that 7–20% of total expenditure on diabetes might be attributable to diabetes foot disease (5).
Metabolic control, wound care, debridement, pressure relief, dressings and antibiotics are among the basic interventions for DFU management. New therapies are emerging to promote wound healing and to reduce the incidence of lower‐limb amputations, including recombinant human platelet‐derived growth factor (PDGF) 6, 7 and skin equivalents obtained by tissue engineering techniques 8, 9. However, these products have only been studied in small, neuropathic origin wounds. At present, there is no specific treatment for advanced or ischaemic DFU and amputation is the foreseeable outcome.
Epidermal growth factor (EGF) is a 53‐aminoacid polypeptide, isolated by Stanley Cohen from adult mouse submaxillary glands (10) that exerts potent mitogenic activity through binding to a specific cell membrane, tyrosine kinase‐type receptor on the target cells (11). EGF topical application or subcutaneous injection produces skin keratinocytes and fibroblasts hyperplasia and hypertrophy, as well as corneous layer thickening 12, 13. Exogenous EGF can also play a significant stimulating role in peripheral nerve regeneration (14). Some clinical trials have been conducted to evaluate the efficacy and safety of topical application of EGF in different indications such as DFU 15, 16, radiogenic ulcers (17), venous ulcers (18), burns 19, 20 and graft donor sites (21).
The availability of the growth factors on the wound's deeper layers is an important issue to obtain an adequate efficacy. This can be a limitation with topical formulations because active agent diffusion is affected by necrotic tissue, sepsis, inflammation and wound proteases (22). Growth factor intralesional injection could take it to the desired region.
A preliminary clinical study, where recombinant human EGF (rhEGF) (25 μg thrice weekly for 5 weeks) was injected intralesionally, in advanced DFU yielded encouraging positive results in terms of useful granulation tissue formation and major amputations prevention in more than 50% of the 29 patients treated (23). The present study evaluated the efficacy and safety of this treatment to promote healing of advanced DFU and prevention of limb amputation at two dose levels in a randomised, double‐blinded design.
Patients and methods
Trial design and patients
A randomised, double‐blinded trial was carried out at five centres. Diabetic patients (type 1 or 2), both genders, older than 18 years were included if they had a grade 3 or 4 foot ulcer according to Wagner’s classification (2), with high risk of amputation, and gave their written, informed consent to participate. Exclusion criteria were foot ulcer area ≤1 cm2, haemoglobin <100 g/l, uncontrolled chronic diseases (coronary or heart disease, diabetic coma or ketoacidosis, renal failure defined as a serum creatinine >200 μmol/l and oligoanuria), malignancies, psychiatric or neurological diseases that could impair proper reasoning for consent, pregnancy and nursing. The trial protocol was approved by the Ethics Committee at each investigation site and by the Cuban Regulatory Authority.
Study medication and interventions
Recombinant human EGF was obtained from a transformed Saccharomyces cerevisiae strain at the Centre for Genetic Engineering and Biotechnology, Havana (24). For the trial, it was produced as lyophilised powder containing 75 or 25 μg per vial (Citoprot‐P®; Heber Biotec, Havana, Cuba). Both vials were indistinguishable in order to guarantee blindness during preparation and manipulation.
Patients were randomised to receive intralesional injections of rhEGF at 75 μg (group I) or 25 μg (group II), three times per week on alternate days. Simple randomisation was performed according to a computer‐generated random list, stratified by investigation site. The product was dissolved in 5 ml of physiological saline. This volume was distributed throughout the lesion at each administration. The treatment lasted until complete response or 5 weeks. If a partial response (see below for response definitions) was observed at this point, treatment continued for three additional weeks. Patients were hospitalised during the treatment.
The study medication was administered together with a standardised good wound care regimen. Ulcers were sharply debrided, gangrenous and necrotic tissue removed whenever necessary, saline‐moistened gauze dressing was used and the affected area was pressure off‐loaded. Broad‐spectrum antibiotics were used to manage infections and metabolic control was strictly followed.
Evaluation and follow‐up
Evaluation consisted in baseline and weekly wound clinical examination. Ankle/brachial index, digital plethysmography and transcutaneous oxygen pressure (TcpO2) were measured at baseline, end of treatment and 12‐month follow‐up to evaluate vascular haemodynamics. Ulcers were classified regarding their aetiopathogeny and in grades according to Wagner. Laboratory tests (at baseline, 3 weeks, end of treatment, and 3, 6 and 12 months after the end of treatment) included blood cell counts, haemoglobin, haematocrit, globular sedimentation rate, glycohaemoglobin (HbA1C), creatinine and aspartate aminotransferase, which were performed by usual clinical laboratory methods. Blood glucose was measured more frequently for the patients’ metabolic control. Wound cultures were performed before and during therapy to monitor bacterial infections.
The main efficacy endpoint was the per cent of the ulcer surface covered by granulation tissue. The protocol previewed to classify it as covering ≤25% (no response), 26–50% (minimal response), 51–75% (partial response) or >75% (complete response) of the ulcer surface. Lesion areas and the fraction with granulation were calculated as the product of the longest and shortest diameters. A corresponding diagram was drawn in the case report forms and a standardised photograph was taken to permit further audit of the result. However, as all the complete responses obtained consisted in more than 98% of the wound area covered by granulation tissue, this was the actual variable evaluated. Secondary efficacy endpoints were time to obtain complete response, complete healing (no exudates or need of dressing), time to get it and need for amputation. Recurrence up to 1‐year follow‐up was also assessed. All evaluations were performed blindly because the code was only opened after the end of the trial.
Biopsy samples were collected before, after 1 week, 3 weeks and at the end of treatment, preferably from a wound zone, macroscopically suggesting granulation tissue. Samples were fixed in 10% buffered formalin, paraffin embedded and stained with haematoxylin/eosin and Masson’s trichrome staining for collagen.
Safety was monitored by daily adverse‐event (AEs) evaluation during treatment. The severity of the AEs was classified as (i) mild, if no therapy was necessary; (ii) moderate, if specific treatment was needed and (iii) severe, when hospitalisation or its prolongation was required, if the event was life‐threatening or contributed to the patient’s death.
Statistics and data management
Sample size was estimated for each group considering that the treatment would represent a clinically significant benefit if it provides a 30% advantage in granulation tissue formation rate, considering that, at so advanced stage, the probability of granulation and healing is unlikely. Under this hypothesis, 20 individuals per group was estimated that could provide this result with a 0·05 alpha error and a 0·2 CI precision.
Data were double entered and validated on Microsoft Visual FoxPro version 5·0 and then imported to SPSS version 11.5 for further analysis. Quantitative variables were expressed as mean ± SD or median ± interquartile range and minimum and maximum values. Qualitative variables were given as absolute values and percentages. The probabilities of response were estimated using a Bayesian logistic model for fixed effects in WinBUGS14 package. The influence of baseline and demographic variables on response was tested using univariate analyses by the chi‐squared or Fisher’s exact tests as well as the odds ratio between the conditions compared and their 95% CI. Then, the variables more likely to be significant were tested in a multivariate analysis with a logistic regression model.
Despite that the objective of the trial was not to compare the efficacy between dose levels, it was carried out to look if there was evidence of any dose‐dependent effect. Normality (Shapiro–Wilks test) and homogeneity of variance (Levene’s test) assumptions were tested and depending on whether they were verified or not, differences between groups were assessed by the Student’s t‐test or Mann–Whitney U‐test, respectively. Qualitative variables comparison between groups was assessed by the chi‐squared or Fisher’s exact tests. The level of significance chosen was P < 0·05. All analyses were carried out on intention‐to‐treat basis.
Results
Course of the trial
From September 2003 to December 2004, 41 patients with DFU were recruited (23 in the high dose group I and 18 in group II). A flow chart of the trial course and the reasons for treatment discontinuation are shown in Figure 1. Of the 41 patients included, 13 (46%) did not complete the treatment schedule: 5 (22%) in group I and 8 (44%) in group II. The interruptions in group I were because of AEs (sepsis) in three patients (13·0%) and wound progression in two (8·7%). In group II, there was wound progression in four patients (22%), voluntary abandon in two (11%), and sepsis in two patients (11%). Four patients (two in each group) prolonged the treatment because of partial response at week 5. One from each group withdrew before week 8 because of adverse reactions (sepsis in the group I case and exclusion criteria in the group II subject). However, these four patients achieved total response at the end. The 30 patients who achieved total response were followed for at least one additional year.
Figure 1.
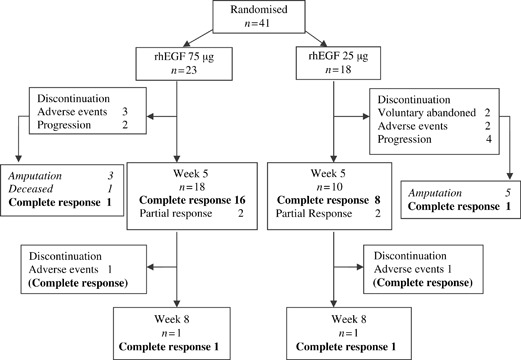
Study flowchart.
Demographic and baseline characteristics of the patients
Table 1 shows the baseline characteristics of the treatment groups. Patients in both groups were predominantly males and white. They suffered diabetes, mostly type 2, for a long time. Wound size medians were 22·5 and 25·0 cm2 in groups I and II, respectively; three patients had >100 cm2 ulcers. Lesions were located on all foot regions, more frequently on the toes. Ulcer aetiopathogeny was predominantly ischaemic. Although all Wagner’s grade 4 cases were in group I, this difference did not reach statistical significance (P = 0·056). There were no significant differences between groups in any of the other baseline characteristics.
Table 1.
Baseline characteristics of the study population
| Characteristic | Group I (n = 23) | Group II (n = 18) |
|---|---|---|
| Age in years, median ± QR (min; max) | 63·0 ± 12·0 (21; 76) | 67·5 ± 19·5 (46; 82) |
| Gender, n (%) | ||
| Males | 12 (52·2) | 10 (55·6) |
| Females | 11 (47·8) | 8 (44·4) |
| Race, n (%) | ||
| White | 15 (65·2) | 13 (72·2) |
| Non white | 8 (34·8) | 5 (27·8) |
| History of heart disease | 6 (26·1) | 3 (16·7) |
| Diabetes mellitus, n (%) | ||
| Type 1 | 2 (8·7) | 2 (11·1) |
| Type 2 | 21 (91·3) | 16 (88·9) |
| Time with diabetes in years | 20·1 ± 8·5 | 17·5 ± 10·1 |
| Ulcer duration in months, median ± QR (min; max) | 1·0 ± 1·5 (0·3; 120) | 1·0 ± 1·5(0·4; 12) |
| ABI > 0·8 | 7 (30·4%) | 4 (22·2%) |
| ABI < 0·8 | ||
| Femoral–popliteal | 7 (30·4%) | 1 (5·6%) |
| Distal | 9 (39·1%) | 13 (72·2%) |
| Ulcer size (cm2), median ± QR (min; max) | 22·5 ± 35·0 (6; 300) | 25·0 ± 10·9 (10; 110·5) |
| Predominant aetiopathogenic feature, n (%) | ||
| Neuropathic | 6 (26·1) | 8 (44·4) |
| Ischaemic | 17 (74·9) | 10 (55·6) |
| Wagner’s classification, n (%) | ||
| Grade 3 | 18 (78·3) | 18 (100·0) |
| Grade 4 | 5 (21·7) | 0 (0) |
| Ulcer location, n (%) | ||
| Toes | 15 (65·2) | 12 (66·7) |
| Internal edge | 1 (4·3) | – |
| External edge | 3 (13·0) | 4 (22·2) |
| Dorsum | 4 (17·4) | 2 (11·1) |
| Sole | 5 (21·7) | 4 (22·2) |
| Transmetatarsal | 3 (13·0) | 2 (11·1) |
| Ankle | 3 (13·0) | 2 (11·1) |
ABI, Ankle‐brachial index; QR, interquartile range.
Efficacy
More than 30% granulation tissue‐covered wound area was obtained since the first week of treatment in most patients of both groups. The granulation response rate is shown in Table 2. By the fifth week, complete response was achieved in 73·9% of group I patients and 50·0% in group II. The four partial responders who were evaluated at 8 weeks attained complete response, so the final complete response rates were 83% in group I and 61% in group II. The probability to attain 50% of the wound area covered by granulation tissue (total and partial response) was higher for group I and complete response was attained 1 week earlier. None of these variables were statistically different between both groups.
Table 2.
Granulation response to treatment with intralesional recombinant human epidermal growth factor
| Group I (n = 23) | Group II (n = 18) | |
|---|---|---|
| Response at 5 weeks (percentage of ulcer area covered by granulation tissue), n (%) | ||
| Complete (75–100%) | 17 (73·9) | 9 (50·0) |
| Partial (50–75%) | 2 (8·7) | 2 (11·1) |
| Minimal (25–50%) | 0 (0) | 2 (11·1) |
| No response (<25%) | 4 (17·4) | 5 (27·8) |
| Response at 8 weeks, n (%) | ||
| Complete (75–100%) | 19 (82·6) | 11 (61·1) |
| Minimal (25–50%) | 0 | 2 (11·1) |
| No response (<25%) | 4 (17·4) | 5 (27·8) |
| Probability to obtain >50% of ulcer area covered by granulation tissue (95% CI) | 0·62–0·93 | 0·38–0·79 |
| Time to obtain complete response (weeks), mean ± SD (95% CI) | 3·8 ± 2·2 (2·8–4·8) | 4·9 ± 2·2 (3·6–6·1) |
The univariate analysis of the relationship of different baseline variables with complete response rate at 5 weeks showed significantly higher probability of complete response (odds ratio; 95% CI) for ≤65 years of age (10·9; 2·3–50·0), history of cardiopathy (5·1; 1·1–25·0), neuro‐infectious ulcer (14·1; 1·6–125·0), and having received ≥15 rhEGF doses (5·2; 1·3–21·1). However, using multivariate logistic regression analysis, the only variables that had or were close to a significant effect on complete response at 5 weeks were male gender (9·5; 0·9–100), ≤65 years of age (34·1; 2·8–409), and ≥15 rhEGF doses (15·3; 1·4–165).
The final outcome is shown in Table 3. Complete wound healing was obtained in 13 (56·5%) and 9 (50·0%) patients in groups I and II, respectively, after approximately 20 weeks since the onset of treatment. Skin graft was used in one case after the complete granulation response. Ulcer relapse occurred in one of the subjects in group I. Besides, one group II patient did not reach complete healing although the lesion had improved considerably. Some examples of healed patients are shown in Figure 2. Granulation response, wound contraction and complete epithelisation can be observed.
Table 3.
Outcome of the patients (including treatment and follow‐up periods)
| Endpoint | Group I (n = 23) | Group II (n = 18) |
|---|---|---|
| Complete healing, n (%) | 13 (56·5)* | 9 (50·0) |
| Lesion persisted at the end of follow‐up, n (%) | 0 | 1 (5·1) |
| Amputations, n (%) | 8 (34·8) | 6 (33·3) |
| Withdrawals | 2 (deceased) | 2 (voluntary abandoners) |
| Probability of complete healing (95% CI) | 0·362–0·738 | 0·289–0·709 |
| Type of amputation | ||
| Above‐knee | 3 | 1 |
| Below‐knee | 2 | 4 |
| Transmetatarsian | 2 | 1 |
| Toes | 1 | 0 |
| Time to healing (weeks) | ||
| Mean ± SD | 20·6 ± 1·8 | 19·5 ± 1·6 |
| 95% CI | 17·0–24·2 | 16·3–22·7 |
| Time to amputation (months) | ||
| Mean ± SD | 15·6 ± 1·9 | 13·9 ± 2·4 |
| 95% CI | 11·9–19·3 | 9·3–18·5 |
In one case healing was reached after skin graft.
Figure 2.
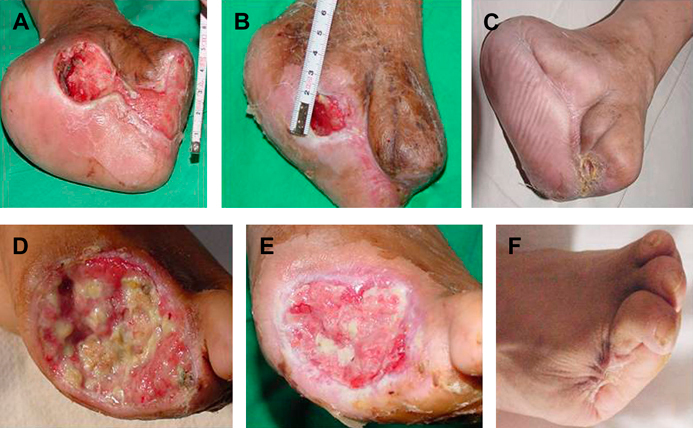
Comparative photos of some patients: (A, B, C) patient IA‐01; (D, E, F) patient IA‐11. (A, D) before treatment; (B, E) at the end of treatment; (C, F) after 1‐year follow‐up.
Amputation was necessary in 8 (34·8%) and 6 (33·3%) patients in groups I and II, respectively, taking into account the whole treatment and follow‐up period. During the treatment period, amputations occurred in three and five patients in groups I and II, respectively. However, five and one patients were further amputated, despite having had complete granulation response. Mean time to amputation in group I was 15·6 months (95% CI: 11·9–19·3) and 13·9 months (95% CI: 9·3–18·5) in group II. No statistical dependence was found between the amputation and the patients’ demographic characteristics.
Histological studies confirmed that useful granulation tissue was obtained since the first week of treatment, with more than 25% of the images with neoformation vessels and a significant increase of collagen fibres and fibroblast proliferation (Figure 3).
Figure 3.
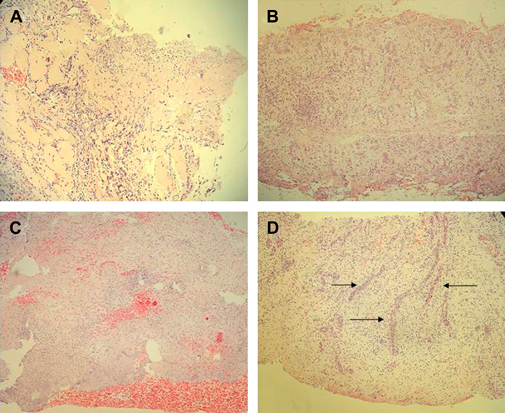
Representative histological image of neuropathic (A, B) and ischaemic (C, D) ulcers. Samples were collected just before the first Citoprot‐P infiltration (A, C) and after the fifth week of treatment. (B, D). Haematoxylin/eosin staining, magnification ×10. (A) Neuropathic lesion before treatment. At this stage an acute inflammatory infiltration is observed as the only ingredient of the granulation process. Inflammation is diffuse and not polarised. No evidences of matrix production or neovessels are detected. (B) At the fifth week, the matrix appears dense, consolidated and indurated by compact collagen material. The presence of fibroblastoid cells is largely increased. (C) Ischaemic lesion before treatment. Wound matrix appears fibrinoid, non organised and rich in cell and tissue debris. Granulation and inflammatory polarisation processes have not yet set forth. (D) At the fifth week of treatment, the wound matrix looks consolidated, organised and with well‐shaped vessels showing luminal blood (indicated by arrows).
Safety
AEs are listed in Table 4. The most frequent were sepsis, burning sensation and local pain. Most of the AEs were classified as mild or moderate. Only two patients (4·9%) had severe events. One patient had anaemia and chest pain that required blood transfusions and medication to resolve it. The other, a woman with history of cardiopathy, had interrupted the treatment because of local sepsis, and 1 week after developed an acute abdomen and a fatal arrhythmia during emergency surgery. No alterations of the clinical laboratory measurements were made.
Table 4.
Adverse events frequency
| Events | Group I (n = 23), n (%) | Group II (n = 18), n (%) | Total, n (%) |
|---|---|---|---|
| Sepsis | 5 (21·7) | 3 (16·7) | 8 (19·6) |
| Burning sensation | 5 (21·7) | 2 (11·1) | 7 (17·1) |
| Local pain | 4 (17·4) | 3 (16·7) | 7 (17·1) |
| Tremors | 5 (21·7) | 1 (5·6) | 6 (14·6) |
| Chills | 4 (17·4) | 1 (5·6) | 5 (12·2) |
| Fever | 3 (13·0) | 1 (5·6) | 4 (9·8) |
| Anaemia | 1 (4·3) | 1 (5·6) | 2 (4·9) |
| Enterocolitis | 2 (8·6) | – | 2 (4·9) |
| Chest pain | 1 (4·3) | 1 (5·6) | 2 (4·9) |
| Facial paralysis | 1 (4·3) | – | 1 (2·4) |
Discussion
This study shows that treatment with intralesional rhEGF, associated to good wound care measures, can benefit patients with advanced DFU for which otherwise there is no available specific therapy. A complete granulation response appeared after 5 weeks of treatment in more than 60% of the patients. Complete wound healing was reached in more than 50% of the patients after 20 weeks since the onset of an up to 8‐week treatment schedule. Interestingly, this result was obtained despite that the ulcers were Wagner’s grade 3 or 4, mostly larger than 20 cm2, predominantly ischaemic, and had a high risk of amputation. Only one recurrence was observed during a 1‐year follow‐up after treatment, which supports the durability of the effect. All the patients who entered the study were evaluated under ‘intention‐to‐treat’ basis.
The study, although randomised and double blind, was not placebo controlled, which limits the external validity of the results. A placebo group was not accepted by the Ethics Committee considering the high risk of amputation in these patients and the possibility to avoid it given the previous results obtained with this procedure (23). The study was not powered to determine differences between the two dose levels studied, but to compare each of them with a 30% response that was considered to be clinically significant. However, some dose effect was suggested by the tendency to an earlier response in the higher dose group. More studies are required to further elucidate this aspect.
Other growth factors such as recombinant human PDGF‐BB (becaplermin) have been used topically but in neuropathic and smaller lesions 6, 7. In a meta‐analysis of those studies, Smiell et al. concluded that within the setting of a comprehensive wound management programme, treatment with becaplermin gel at a dose of 100 μg/g once daily increases the incidence of complete healing (25). However, 95% of the patients included in those trials had ulcers ≤10 cm2 (median 1·4–3·5 cm2) and an adequate blood supply (defined as TcpO2 >30 mmHg) was a requisite for inclusion. On the contrary, this study treated more advanced, larger (median >20 cm2) and both neuropathic and ischaemic wounds.
Previous use of EGF topically, on DFU has been reported. A randomised, double‐blind, placebo‐controlled trial evaluated two doses of a rhEGF‐containing cream in patients with DFUs compared with a protein‐free calf blood derivative cream used as control. Healing rate was significantly enhanced by rhEGF 0·04% but not by the lower dose (0·02%). This trial also included much less severe ulcers given by Wagner’s grades 1 and 2, ≤4 cm2 size and only neuropathic (15). Another non controlled trial treated patients with grade 2–3, resistant to advanced dressing alone, neuropathic ulcers, mean size 4·8 cm2 with a topical formulation containing 0·005% rhEGF added to the dressing. In the treated patients, complete healing was noted in 76% (52/68) of patients within an average of 46 days (range from 2 to 14 weeks) (16).
A limitation to the efficacy of topical formulations can be that the growth factor cannot steadily reach the deeper layers of the wound. Diffusion of the active agent is affected by necrotic tissue, sepsis, inflammation and by the action of wound proteases (22). It has been shown that chronic wounds have elevated pro‐inflammatory cytokines, high protease activity, decreased levels of natural metalloproteinase inhibitors and diminished growth factor activity 26, 27, 28. The still active factor may be unavailable for biologic activity because of trapping or binding to molecules such as fibrinogen, macroglobulin or albumin 29, 30. Additionally, the ever‐present tissue level of bacteria in chronic wounds produce higher levels of proteases and other metalloproteinases that further degrade the growth factors and their receptors (31). These facts can contribute to explain the lack of efficacy of topical EGF and PDGF at lower doses.
Intralesional injection of the growth factor could bring the active agent into the desired region and avoid the inactivating agents. The results shown here confirm the previous proof‐of‐concept study where 86% of 29 treated patients exhibited a productive granulation with substantial wound matrix transformation, granulation tissue cell population and angiogenesis enhancement. Seventeen patients (58·6%) were rescued from amputation (23). Interestingly, more severe wounds were treated in both trials with the intralesional injection procedure, with an essentially satisfactory outcome.
Treatment was well tolerated. Besides local symptoms, most of the AEs were mild and easily manageable. The two severe AEs, including the patient who deceased, do not seem to be related to the EGF treatment. One of the major concerns of the use of exogenous EGF at concentrations much higher than physiological is that it could promote the development of malignant neoplasia. An accurate assessment of this event was included in the long‐term follow‐up in this study. It was not observed in any of the patients. Nevertheless, additional studies should be performed with a larger number of patients as long as the use of this product is extended.
Another concern with the intralesional route of administration could be the risk of inoculating or spreading bacterial infection. This was minimised by the concomitant good wound care practices, broad‐spectrum antibiotic coverage and adequate aseptic injection procedures. Around 20% of the patients developed sepsis, which accounted for most of the therapeutic failures. Infections control remains a critical problem in such advanced DFU.
In summary, intralesional administration of rhEGF improved granulation tissue formation in both neuropathic and ischaemic advanced DFU. Wound healing was thus stimulated. Future controlled studies are needed to further assess the possible impact of this promising intervention because this condition is still an unsolved medical problem and an important economic burden to medical care systems.
Acknowledgements
Authors EI‐C, CV‐S, EG‐I, JB‐A, RS‐R, BYB and PAL‐S are employees of the Centre for Biological Research, which is part of the Centre for Genetic Engineering and Biotechnology, Havana network, where rhEGF is produced and the new formulation was developed. The rest of the authors have no conflict of interests. The study was financed by Heber Biotec, Havana, Cuba (product), and the Ministry of Public Health of Cuba (hospital facilities and general medical care of the hospitalised patients. The authors also thank technicians Mariela Acevedo, and Lourdes Quesada for their participation in data processing. The authors received free drug (rhEGF) from Heber Biotec, Havana, Cuba.
Cuban citoprot‐P study group: (*Steering committee)
Patient recruitment, treatment and follow‐up (number of patients included at each site): National Institute for Angiology and Vascular Surgery (20), José I. Fernández‐Montequín*, Neobalis Franco‐Pérez, William O. Savigne‐Gutierrez, Calixto Valdés‐Pérez, Nelson Chirino‐Carreño, Héctor T. Álvarez‐Duarte, Alicia C. Ascaño‐Ortega, Maité López‐Ruiz, Gertrudis García‐Lazo, Ana J. Betancourt‐Borrell, Julia Zapata‐Vinet, Alicia Rodríguez‐Pérez, Bárbara Reyes‐Iglesias, Alfredo Aldama‐Figueroa, Rafael Suarez‐Sola, Ines P. González‐Sosa; ‘Enrique Cabrera’ Hospital (8): Heriberto Artaza‐Sanz*, Natalia Pol‐Marrón, Angela M. Blanco‐Díaz, Pedro Goicochea, Dinorah González‐Viñales; ‘Joaquín Albarrán’ Hospital (6): Lourdes Morejón‐Vega*; José A. Gonzalez‐García; ‘Hermanos Amejeiras’ University Hospital (2): Osvaldo Eliseo‐Musenden*; ‘Arnaldo Milián Castro’ Hospital (8): Cecilio González‐Benavides*, Teresita Feito‐Cantell, Felicia García‐Seco, Juan M. García‐Velázquez, Yasmín Rodríguez‐Río, Amel Alfonso‐Simón, Linet López‐Casanova. Biopsy processing and histological evaluation. ‘Hermanos Amejeiras’ University Hospital: Ernesto Arteaga‐Hernández, Agustín Chong; Centre for Biological Research: José S. Alba, Yanelda García‐Vega, Iraldo Bello‐Rivero, Isabel Guillén, Data Management and Statistical Analyses. Centre for Biological Research: Elizeth García‐Iglesias*, Carmen Valenzuela‐Silva*; Trial Protocol Design, Monitoring, Analysis of Results. Centre for Biological Research: Ena Infante‐Cristiá*, Blas Y Betancourt*, Larissa Chacón‐Corvea, Yaleysis Rosales‐Pantoja, Pedro A. López‐Saura*; Centre for Genetic Engineering and Biotechnology: Jorge Berlanga‐Acosta*, Luis Herrera‐Martínez, Ernesto López‐Mola, Ricardo Silva‐Rodríguez*.
References
- 1. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–53. [DOI] [PubMed] [Google Scholar]
- 2. Frykberg RG, Armstrong DG, Giurini J, Edwards A, Kravete M, Kravitz S, Ross C, Stavosky J, Stuck R, Vanore J. Diabetic foot disorders. A clinical practice guideline. J Foot Ankle Surg 2000;39(5 Suppl):S1–S60. [PubMed] [Google Scholar]
- 3. Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, Hann AW, Hussein A, Jackson N, Johnson KE, Ryder CH, Torkington R, Van Ross ER, Whalley AM, Widdows P, Williamson S, Boulton AJ. The North‐West diabetes foot care study: incidence of, and risk factors for, new diabetic foot ulceration in a community‐based patient cohort. Diabet Med 2002;19:377–84. [DOI] [PubMed] [Google Scholar]
- 4. Abbott CA, Vileikyte L, Williamson S, Carrington AL, Boulton AJM. Multicenter study of the incidence of and predictive risk factors for diabetic neuropathic foot ulceration. Diabetes Care 1998;21:1071–5. [DOI] [PubMed] [Google Scholar]
- 5. Boulton AJ, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–24. [DOI] [PubMed] [Google Scholar]
- 6. Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet‐derived growth factor‐BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo‐controlled double‐blind study. Diabetes Care 1998;21:822–7. [DOI] [PubMed] [Google Scholar]
- 7. Margolis DJ, Bartus C, Hoffstad O, Malay S, Berlin JA. Effectiveness of recombinant human platelet‐derived growth factor for the treatment of diabetic neuropathic foot ulcers. Wound Repair Regen 2005;13:531–6. [DOI] [PubMed] [Google Scholar]
- 8. Veves A, Falanga V, Armstrong DG, Sabolinski ML. Apligraf Diabetic Foot Ulcer Study. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001;24:290–5. [DOI] [PubMed] [Google Scholar]
- 9. Marston WA, Hanft J, Norwood P, Pollak R. Dermagraft Diabetic Foot Ulcer Study Group . The efficacy and safety of dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003;26:1701–5. [DOI] [PubMed] [Google Scholar]
- 10. Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new‐born animal. J Biol Chem 1962;237:1555–62. [PubMed] [Google Scholar]
- 11. Bazley LA, Gullick WJ. The epidermal growth factor receptor family. Endocr Relat Cancer 2005;12:S17–S27. [DOI] [PubMed] [Google Scholar]
- 12. Berlanga J, Moreira E, Perez LC, Boix E, Gonzalez T, Lopez‐Saura P. Wound healing promotion in rats treated with EGF is dose dependent. Biotecnol Apl 1996;13:181–5. [Google Scholar]
- 13. Berlanga J, Lodos J, Labarta V, Merino N, Gonzalez T, Hayes O, Puentes P, Mulet J, Lopez‐Saura P. The effect of the epidermal growth factor treatment schedule on the healing of full‐thickness wounds in pigs. Biotecnol Apl 1997;14:163–8. [Google Scholar]
- 14. Prats PA, Castañeda LO, Falcón V, Ortega R, De La Rosa MC, Menéndez I, Labarta V, Gómez R. Efecto del factor de crecimiento epidérmico sobre la regeneración del nervio ciático transectado en ratas. Biotecnol Apl 1998;15:237–41. [Google Scholar]
- 15. Tsang MW, Wong WK, Hung CS, Lai KM, Tang W, Cheung EY, Kam G, Leung L, Chan CW, Chu CM, Lam EK. Human epidermal growth factor enhances healing of diabetic foot ulcers. Diabetes Care 2003;26:1856–61. [DOI] [PubMed] [Google Scholar]
- 16. Hong JP, Jung HD, Kim YW. Recombinant human epidermal growth factor (EGF) to enhance healing for diabetic foot ulcers. Ann Plast Surg 2006;56:394–8. [DOI] [PubMed] [Google Scholar]
- 17. Barroso MC, Díaz C, Alsina S, Areces F, Vázquez E. Human recombinant epidermal growth factor in the treatment of radiogenic ulcers. Biotecnol Apl 1993;10:12. [Google Scholar]
- 18. Quiñones M, McCook J, Zacca E, Martínez MA. Efecto del Factor de Crecimiento Epidérmico humano recombinante sobre las úlceras de miembros inferiores causadas por insuficiencia venosa crónica. Progreso en Ciencias Médicas 1991;5:11–4. [Google Scholar]
- 19. Borges HA, Martínez A, López LD, Ung EV, Wade MM, Mella CM, Hernández F, Gonzalez T, López‐Saura P. El Factor de Crecimiento Epidérmico humano recombinante acelera la cicatrización de quemaduras en niños. Estudio a doble ciegas. Biotecnol Apl 1994;11:204–8. [Google Scholar]
- 20. Martínez A, López LD, Pérez R, Antón J, Agüero MM, Peón O, Taquechel L, De Céspedes V, Pérez L, Martínez E, Domínguez F, Ung‐Lau EV, Hernández F, González T, López‐Saura P. Uso del Factor de Crecimiento Epidérmico humano recombinante en crema de Sulfadiacina de Plata en el tratamiento de pacientes quemados. Biotecnol Apl 1994;11:209–12. [Google Scholar]
- 21. Brown GL, Nanney LB, Griffen J, Cramer AB, Yancey JM, Curtsinger LJ, Holtzin L, Schultz GS, Jurkiewicz MJ, Lynch JB. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med 1989;321:76–9. [DOI] [PubMed] [Google Scholar]
- 22. Berlanga J, Lodos J, Reyes O, Infante JF, Caballero E, López‐Saura P. Epidermal growth factor stimulated re‐epithelialization in pigs. The possible role of acute‐wound proteases. Biotecnol Apl 1998;15:83–7. [Google Scholar]
- 23. Berlanga J, Savigne W, Valdez C, Franco N, Alba JS, Del Rio A, López‐Saura P, Guillén G, Lopez E, Herrera L, Férnandez‐Montequín J. Epidermal growth factor intra‐lesional infiltrations can prevent amputation in diabetic patients with advanced foot ulcers. Int Wound J 2006;3:232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cinza AM, Quintana M, Lombardero J, Poutou R, Pérez E, Pérez LC, Mella CM, Besada V, Padrón G, Castellanos L, Estrada R, Morales‐Grillo J. A batch process for production of human epidermal growth factor in yeast. Product characterization. Biotecnol Apl 1991;8:166–74. [Google Scholar]
- 25. Smiell JM, Wieman TJ, Steed DL, Perry BH, Sampson AR, Schwab BH. Efficacy and safety of becaplermin (recombinant human platelet‐derived growth factor‐BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen 1999;7:335–46. [DOI] [PubMed] [Google Scholar]
- 26. Nwomeh BC, Yager DR, Cohen IK. Physiology of chronic wounds. Clin Plast Surg 1998;25:341–56. [PubMed] [Google Scholar]
- 27. Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen 1996;4:411–20. [DOI] [PubMed] [Google Scholar]
- 28. Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen 1996;4:321–5. [DOI] [PubMed] [Google Scholar]
- 29. Falanga V, Eaglstein WH. The trap hypothesis of venous ulceration. Lancet 1993;341:1006–8. [DOI] [PubMed] [Google Scholar]
- 30. Robson MC, Smith PD. Topical use of growth factors to enhance healing. In: Falanga V, editor. Cutaneous wound healing. London: Martin Dunitz Limited, 2001:379–98. [Google Scholar]
- 31. Robson MC, Stenberg BD, Heggers JP. Wound healing alterations caused by infection. Clin Plast Surg 1990;7:485–92. [PubMed] [Google Scholar]


