Abstract
Necrotising fasciitis is a rare infection of the subcutaneous tissues. If untreated, it is invariably fatal, and thus a high index of suspicion for the diagnosis is required. The disease's manifestation can range from a fulminant presentation to a subtle and insidious development. The priority in every case is to proceed to radical surgical debridement. On review of the literature and based on our clinical experience, we propose a new classification based on clinical presentation and suggest an algorithm to facilitate the management of this devastating condition. Increasing awareness should be given to the management of the large wounds resulting from the surgical debridement of necrotising fasciitis.
Keywords: Management, Necrotising fasciitis
Introduction
Soft tissue infections encompass a diverse spectrum of disease (1). These include simple pyodermas like erysipelas, furunculosis, impetigo and folliculitis, which are confined to the epidermis and dermis. Cellulitis involves infection along the superficial fascia. Necrotising fasciitis is an uncommon, devastating and progressively fatal infection that will involve the subcutaneous fat and deep fascial layers (2).
This review will focus on necrotising fasciitis, first by placing the disease within a historical context, then addressing its epidemiology, risk factors, aetiology, clinical presentation, investigations, management and prognosis. We propose a new management algorithm (Figure 1) based upon the clinical disease types and address the aspects of wound care and reconstruction for these patients if they survive.
Figure 1.
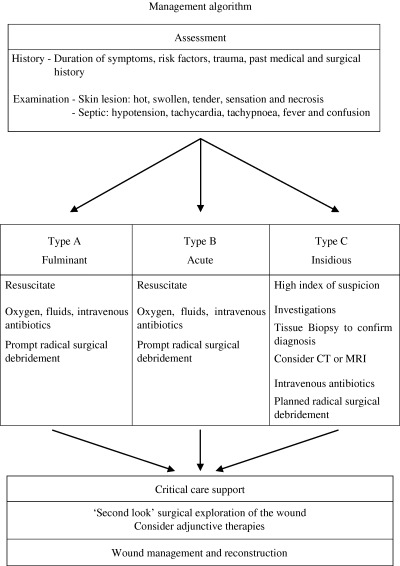
Management algorithm.
Historical background
Hippocrates in the 5th century BC was the first to describe the disease necrotising fasci‐ itis (3). In the 19th century, Joseph Jones, a Confederate army surgeon, described 2642 cases of ‘hospital gangrene’, with an almost 50% mortality (4). Fournier in 1883 described the pathology necrotising fasciitis affecting the perineum and external genitalia (Fournier's gangrene) (5). Meleney in 1924 reported on 20 cases with a 20% mortality of ‘necrotising erysipelas’ in China and was the first to establish the haemolytic streptococcus as the aetiological agent (6). Wilson in 1952 was the first to coin the term ‘necrotising fasciitis’ as he described the dominant feature of the disease, namely inflammation and necrosis of the subcutaneous fat and deep fascia, with sparing of the muscle (7).
Epidemiology and risk factors
Necrotising fasciitis is rare. Population‐based surveillance for group A streptococcal necrotising infection in Canada showed an incidence range from 0·15 to 0·55 cases per 100 000 from those younger than 45 to those older than 65 years (8). Increasing age is a consistent risk factor across several case series, although the condition can affect any age group (8, 9). A sex predilection to the disease has not been consistently demonstrated 8, 9, 10). The inoculation of the bacteria into the subcutaneous space can occur with any damage to the overlying skin or via haematogenous spread from a distant site. Reported mechanisms of injury have included cuts, burns, blunt or penetrating trauma, chronic skin conditions, animal or insect bites, childbirth, intravenous injection of illegal drugs, postoperative infections, perirectal or other abscesses, incarcerated hernias and even secondary to an acute pharyngitis (11). Unfortunately, in up to 36% cases, no preceding skin lesion or antecedent injury can be found (8).
The majority of adults that acquire necrotising fasciitis have at least one other underlying disease that will increase their susceptibility to infection (8, 11). Diabetes, chronic renal failure, peripheral vascular disease, alcohol abuse, malignancy, immunosuppressive therapy, HIV infection, and chronic cardiac and pulmonary disease have all been implicated as significant risk factors (Table 1) (11, 12). Non steroidal anti‐inflammatory drugs (NSAIDs) have also been implicated as a risk factor, but the strength of the association is still to be established (13). It is possible that these drugs can either weaken the host response to infection or minimise the symptoms and signs of infection. This may lead to a delay in diagnosis and thus a delay in definitive management.
Table 1.
Risk factors associated with necrotising fasciitis
| Risk factor |
|---|
| Diabetes |
| Peripheral vascular disease |
| Human Immunodeficiency Virus infection |
| Chronic renal failure |
| Malignancy |
| Chronic cardiac and pulmonary disease |
| Non steroidal anti‐inflammatory drugs (NSAIDs) |
| Immunosuppresive chemotherapy |
It must also be appreciated that necrotising fasciitis can and does affect the young and previously healthy individuals (14). In children, varicella infection is a common risk factor for developing necrotising fasciitis (8).
Microbiology
Microbiological classification
Giuliano et al. classified the bacteriological culture results commonly seen in necrotising fasciitis into two distinct subtypes: type 1 and type 2 (15) (Table 2). Type 1 infections are typically polymicrobial and will include non group A streptococci and at least one or more facultative anaerobes (Bacteroides, Peptostreptococcus or Clostridium) and/or members of the Enterobacteriaceae (Escherichia coli, Klebsiella and Proteus) (15). Type 2 infections typically involve group A β‐haemolytic streptococcus or group A streptococcus (GAS) (Streptococcus pyogenes) solely or in combination with a staphylococcus (15). Type 1 infections tend to predominate where necrotising fasciitis occurs secondary to some form of penetrating trauma, or previous surgery in the abdomen, perineum and external genitalia (16). Type 2 infections are the most prevalent and tend to occur on the extremities (11).
Table 2.
Microbiology of necrotising fasciitis
| Organisms | |
|---|---|
| Type 1 infections | |
| Group B streptococci | |
| Anaerobes: | Bacteroides sp. |
| Peptostreptococcus sp. | |
| Clostridium sp. | |
| Enterococci | |
| Gram‐negative bacteria: | Escherichia coli |
| Proteus sp. | |
| Klebsiella | |
| Pseudomonas | |
| Serratia marcescens | |
| Pasteurella sp. | |
| Type 2 infections | |
| Group A haemolytic streptococci | |
| Coagulase negative and positive staphylococci | |
| Other | |
| Marine vibrios: | Vibrio vulnificus |
| Vibrio damsela | |
| Vibrio parahaemolyticus | |
| Fungi: | Candida sp. |
Streptococcus pyogenes and the streptococcal toxic shock syndrome
Invasive GAS species are isolated in necrotising fasciitis in anywhere from 62% (17) to 97% cases (8). GAS may also cause the serious life‐threatening streptococcal toxic shock‐like syndrome (STSS), first described in 1983 (18). It is characterised by fever, myalgia, vomiting and diarrhoea, and rarely a scarlatina‐like rash, progressing to multiorgan failure, hypotension, confusion, coma and death (19). Approximately, half the cases of STSS will have necrotising fasciitis as a feature (1). However, this syndrome can insidiously present secondary to apparently mild infections at various mucosal sites in the paranasal sinuses and pharynx (20).
GAS posse's different M proteins on their cell membranes, which allow them to adhere, colonise and subsequently invade the host (21). Various population surveillance studies have implicated that a clone of streptococci with serotypes M1 and M3 are responsible for sporadic outbreaks of STSS (22). The clinical effects of STSS are mediated by pryrogenic exotoxins A, B and C (23). These may directly damage host tissues or indirectly act as superantigens to stimulate T cells and macrophages to release pro‐inflammatory cytokines such as tumour necrosis factor‐α, interleukin (IL)‐1β, and IL‐6 which will mediate septic shock (23).
Clinical presentation
Pathogenesis, signs and symptoms
Classically, necrotising fasciitis will present with severe pain at a localised site with or without cutaneous inflammatory changes (rubor, calor, dolour and tumour) and may deceptively appear as a mild cellulitis (24) (Figure 2). The bacteria will release enzymes such as hyaluronidase and lipases which will degrade connective tissue and fat to allow spread along fascial planes. Thrombosis of dermal vessels can occur due to local toxin‐induced ischaemia as the subcutaneous necrosis progresses (17). The sequence of cutaneous changes may manifest as erythema, then bronzing and induration of the skin, followed by breakdown with purple bullae formation within 3–5 days and finally the dull blue‐grey hue of frank skin necrosis. Lymphangitis and lymphadenitis are rarely reported (11). The overlying cutaneous sensation can vary from exquisite tenderness early in the disease process to anaesthesia, as the superficial nerves are destroyed. If the infection contains a gas forming organism, localised crepitus may be detectable clinically (usually type 1 infections in diabetics) (20). The problem is that the late cutaneous changes mentioned are those that are most specific for necrotising fasciitis, and thus the diagnosis is difficult to make until the disease is locally or systemically advanced.
Figure 2.
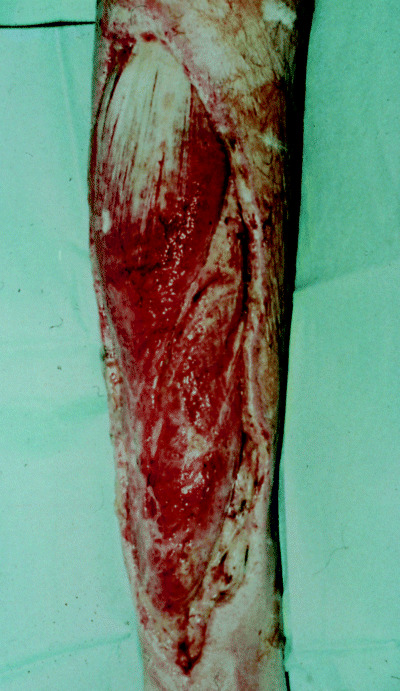
Necrotising fasciitis affecting the calf.
As the bacteria and toxins spread into the bloodstream, the characteristics of sepsis (fever, tachycardia, tachypnoea, hypotension, confusion and multiorgan failure) will manifest. At this point, no prizes can be given for recognising the seriousness of the situation, as the patient will most certainly die without intervention. However, the difficulty is the variability of the speed and severity of manifestation in which this progression can occur. We believe that this distinction allows a system of classification of the presentation of necrotising fasciitis that can be used to guide management decisions.
Clinical classification
A strong case has been made for viewing necrotising fasciitis as a clinical spectrum (10). We propose classifying necrotising fasciitis into three types: A, B and C. Type A is the most severe, whereby these patients present with extensive tissue necrosis, progressing rapidly over a matter of hours and are systemically septic. These patients are frequently moribund and usually have a poor prognosis. In contrast, the symptoms and signs of type B cases tend to develop over a time span of days. There is usually an identifiable skin lesion, or history of trauma, with a wound that is painful out of proportion to the clinical picture. The patient may be well or unwell, but their condition can deteriorate over hours to days to exhibit frank tissue necrosis with systemic upset.
Type C cases are more insidious in onset, with non specific or variable symptoms. Localised pain at the site of a skin lesion, which is usually the most consistent feature in necrotising fasciitis, may be mild or absent. This is especially relevant in diabetic patients, as they can have concomitant peripheral neuropathy that may mask painful stimuli. Interestingly, the host immune/bacterial interaction is such that systemic manifestations of the disease are also minimal. Thus there is a potential for extensive local tissue invasion and destruction, to the ignorance of the patient and clinician. With such a paucity of objective findings, these patients may be seen frequently in the community over several days and be treated as a case of simple cellulitis. There is a lack of data published on this group, but we have found that they have a significant morbidity unless intervention occurs early.
Anatomical location
Various retrospective studies have reported with consistent findings on the common sites of infection of necrotising fascitis (8, 9, 17). Childers and colleagues found that the lower limb was most commonly involved (32%), followed by the upper limb (24%), the perineum (16%), the trunk (16%), head and neck (10%) and buttock (>1%) (17).
There are also numerous case reports of the disease occurring in rarer locations. These include necrotising fasciitis of the retroperitoneum masquerading as an acute appendicitis (25). Also, necrotising fasciitis affecting the chest has been described as a complication of the placement of a thoracostomy tube (26) and secondary to cardiothoracic surgery (27).
Investigations
The diagnosis of necrotising fasciitis, especially in type A infections, is essentially clinical and requires immediate and radical debridement. However, in type B and type C infections, there may be a role for various investigations to heighten the index of suspicion of the disease's presence or to determine the extent of the disease.
The blood results may show an anaemia, a raised white cell count and elevated inflammatory markers. Hyperglycaemia may be present, and hypocalcaemia can occur due to saponification of free fatty acids with ionic calcium in areas of fat necrosis (28). The creatinine kinase may be raised, indicating muscle involvement. A confirmed bacteraemia has been reported in 46% of blood cultures obtained (8). Arterial blood gases should be performed to determine the degree of metabolic derangement, especially if signs of sepsis are present.
Plain radiographs may be used to detect gas within the soft tissue, and it is more sensitive than clinical examination for crepitus (29, 30). CT scanning has been demonstrated to show that asymmetric fascial thickening, fat stranding and soft‐tissue gas are valuable clues in assessing a patient with suspected necrotising fasciitis (31). It can also provide information about coexistent deep collections, and it is helpful in determining the extent of spread of infection (31).
T2‐weighted magnetic resonance imaging with fat suppression has been claimed to be very sensitive in demonstrating deep fascial involvement in necrotising fasciitis (32). However, this modality is not specific enough and has been found to overestimate the extent of infection and overdiagnose necrotising fasciitis (33).
Ultrasonography can be helpful in differentiating Fournier's gangrene from other causes of an ‘acute scrotum’ (11, 34). It is also good at determining the extent of the spread of the infection.
The difficulty is that the imaging characteristics of necrotising fasciitis have been gained principally from retrospective case reports. Thus the actual impact of their role in affecting outcome has yet to be determined.
Majeski and colleagues produced a case series whereby bedside biopsy, followed by frozen section evaluation, was performed on 43 patients evaluated for a suspicious inflammatory process over 15 years (35). This test was 100% sensitive and 100% specific in diagnosing the 12 patients who had necrotising fasciitis (35).
Management
The key aspects of management include early diagnosis, resuscitation of the patient, administration of broad‐spectrum antibiotics (Table 3), followed by radical surgical debridement, intensive care support and finally reconstruction of the resulting wound (Figure 1).
Table 3.
First‐line antibiotic guidelines for treatment of necrotising fasciitis
| Antibiotic (g) | Route | Dosage (g) | Frequency | Maximum dose per day (caution in renal failure) (g) |
|---|---|---|---|---|
| Clindamycin | Intravenous | 0·6–1·2 | TDS | 4·8 |
| Cefuroxime | Intravenous | 0·75–1·5 | TDS | 9 |
TDS, three times daily.
Resuscitation, antibiotic therapy and critical care
The degree of respiratory, haemodynamic, renal and metabolic compromise must be quickly assessed. If required, early resuscitation should be instituted and the intensive care unit promptly informed. The patient's tetanus status should also be determined and updated if necessary.
All patients should receive broad‐spectrum intravenous antibiotics to cover streptococci, staphylococci, Gram‐negative rods and anaerobes. We recommend a combination of clindamycin and cefuroxime, with any adjustments made once culture results have returned (Table 3). Eagle showed that penicillins failed in this type of infection because the offending bacteria reached a stationary phase of growth and stopped expressing critical penicillin‐binding proteins (36). Stephens demonstrated that the greater efficacy of clindamycin was multifactorial (19). Clindamycin was not affected by the stage of growth, it inhibited M protein synthesis thus promoting phagocytosis, and it suppressed the effects of bacterial toxins in mediating septic shock (37). Clindamycin also lasts longer than penicillins, and it has some anaerobe cover (38).
Intensive care monitoring with support of cardiac, respiratory, renal and metabolic systems is key in the postoperative period. These patients will loose considerable amounts of fluid and protein from their large wounds. Majeski and colleagues showed that early nutritional support is an important factor in improving survival and patients may need up to twice their basal caloric requirement (39).
Surgical management
Surgical exploration of the wound is the definitive treatment, and correction of the sepsis will not occur until the infection has been excised and all necrotic tissue debrided (Figure 3). The incision should be made directly over the involved skin and parallel to the neurovascular bundles and carried down to the deep fascia (20). The necrotic tissue is dull, grey and avascular, and ‘murky dishwater’ fluid may be found. The underlying deep fascia and muscle should be inspected for infective invasion and debrided if necessary. All necrotic and devitalised tissue must be removed, and the debridement must extend to where the subcutaneous tissue no longer lifts easily of the deep fascia. Tissue and fluid samples should be obtained and sent for histopathological and microbiological analysis. The wound should then be packed with saline moistened guaze.
Figure 3.
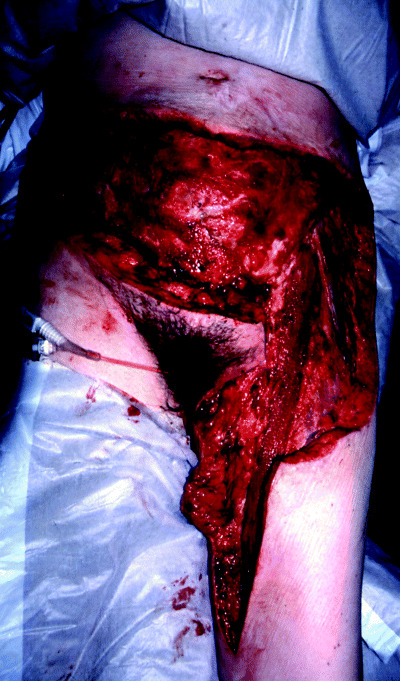
Severe necrotising fasciitis following abdomonoplasty.
Surgical management of Fournier's gangrene creates special considerations. Radical debridement of all affected tissues is also carried out; however, one should try to preserve the anal sphincter if possible (20). If the anal sphincters are involved or there is bowel perforation, a diversion colostomy may be used to avoid faecal contamination of the wound. Orchidectomy is not usually necessary as the vascular supply to the testicles is different from that of the scrotum (40). If the scrotal sac has been debrided, the testicles may be placed subcutaneously in the lower abdominal wall and returned to a reconstructed scrotal sac at a later date (41, 42).
Adjunctive therapies
The use of hyperbaric oxygen (HBO) therapy in the management of necrotising fasciitis is controversial. Some studies have reported a survival benefit, with fewer debridements and thus quicker wound closure with adjunctive HBO (43, 44). However, there has been no prospective randomised trial to assess this benefit of HBO. Potential complications include claustrophobia, tympanic membrane rupture, seizures and central nervous system oxygen toxicity (45). Also, the logistics of resuscitation, if the need arose, in a confined space are difficult. However, if the facilities are on site and the patient is relatively stable, we believe that HBO does have a role to play once it does not detract from definitive management.
The use of intravenous immunoglobulin (IVIG) has been developed with the aim of modulating the immune response to streptococcal superantigens. There have been reports of a clinical benefit in terms of increased survival (46, 47). Unfortunately, the number of patients treated with IVIG has been small, and thus isolating and ascribing a benefit due to this therapy has been difficult (8). We believe that type A patients are most likely to benefit most from this therapy, as they are more likely to have a poorer outcome; further studies are required to confirm this hypothesis.
Wound management
The wound must be closely inspected and the patient brought back to theatre within 24–48 hours to assess the adequacy of the initial debridement. This should be repeated as many times as necessary until one is sure that the infection is under control, and all necrotic material has been removed. Serial photography may allow recognition of a demarcation zone of cellulitic or devitalised tissue which can be used to objectively monitor for evidence of insidious spread of infection. Nursing care is key in detecting local recurrence of infection and in protecting the integrity of the unaffected skin. The resulting wounds from necrotising fasciitis are usually extensive and pose a considerable management dilemma.
The aims of the wound dressings are to provide cover to protect against secondary infection, encourage granulation tissue formation and absorb any inflammatory exudate. Alginate (48) or Hydrogel (17) dressings have been used effectively postoperatively. If the debridement has created a significant cavity, then conforming foam dressings can be inserted to fill the dead space (48). These dressings may need to be changed often, which can be very painful for the patient. In such cases, a general anaesthetic may have to be used.
Topical negative pressure (TNP) therapy is another technique that can be used to manage these wounds 49, 50, 51). It will reduce oedema fluid and stimulate granulation tissue formation as a result of the applied tension forces (49). Krasner has also suggested that this technique may reduce wound pain and the discomfort associated with dressing changes (52). TNP has also been shown to minimise bacterial growth and has an added benefit of reverse tissue expansion (49) which may dramatically aid in the closure of the resultant defect.
Once the surgeon is sure that the infection has completely resolved and the patient has a healthy bed of granulation tissue, a reconstructive procedure may be planned. Wound closure may be facilitated by skin grafts or delayed wound closure (17). If there is exposed bone or neurovascular structures, then local transpositional or free flaps may be used to cover the wound (20).
Prognosis
The mortality rates for necrotising fasciitis vary considerably with the best centres claiming less than 10% (53) and others as high as 75% (54). The larger, more robust, retrospective case series have narrowed these rates to between 25% and 40% (8, 9, 17). With regard to location, truncal (44%) and perineal (38%) regions have a higher mortality than the extremities (22%) (55, 56). Presumably, this is because one may have the option of amputating an extremity if the infection is too extensive.
There are a host of variables associated with a higher mortality. These include delay to initial debridement, age >60, female gender, hypotension, bacteraemia, total body surface area that involved more than 250 cm3, renal failure and elevated blood lactate (8, 17, 45). Comorbid medical problems that are associated with a higher mortality include malignancy, cardiac disease, pulmonary disease, intravenous drug abuse and malnutrition (45). Diabetes mellitus, type of microbiological growth isolated and use of NSAIDs were not associated with a higher mortality (17, 45).
The most important variable that can be affected clinically is a reduction in the time from admission to initial debridement. This requires a high index of suspicion, with an awareness of atypical presentations, such as those found in type C cases.
Conclusions
Necrotising fasciitis is a rare but devastating infection of the subcutaneous tissues. The presentation of the disease is variable. Type A cases manifest with frank tissue necrosis and septicaemic shock occurring over hours. Types B cases develop over hours to days, usually with a precipitating traumatic event, and can deteriorate quickly. Type C cases have a more insidious and non specific presentation and require a high index of suspicion in order to make the diagnosis.
Radical surgical debridement is the definitive management, and it is also frequently diagnostic in sick patients with the disease, who have yet to produce the classical dermatological signs. There may be a role for special investigations, especially in type C cases in making the diagnosis and thus reducing the time to surgery. All patients should be treated with intravenous antibiotics.
Adjunctive therapies such as HBO therapy and intravenous immunotherapy require further study to determine their role in management.
BOX 1.
Table 4.
Summary of the key points
| Necrotising fasciitis is a rare, and potentially fatal subcutaneous infection. Risk factors include diabetes mellitus, peripheral vascular disease, chronic renal failure and immunosuppression. |
| Streptococcus pyogenes is the most common bacteria isolated. The disease manifests as three main clinical types: A, B and C. These range from a dramatic fulminant presentation to a more subtle and insidious development. |
| Prompt radical surgical debridement is the definitive management. All patients should receive broad‐spectrum intravenous antibiotics. Intensive care support and meticulous wound care with subsequent reconstruction are key in ensuring a good outcome. |
References
- 1. Bisno AL, Stephens DL. Streptococcal infections of skin and soft tissues. N Eng J Med 1996;334: 240. [DOI] [PubMed] [Google Scholar]
- 2. Jallali N. Necrotising fasciitis: its aetiology, diagnosis and management. J Wound Care 2003; 8: 297–300. [DOI] [PubMed] [Google Scholar]
- 3. Descamps V, Aitken J, Lee MG. Hippocrates on necrotising fasciitis. Lancet 1994;344: 556.DOI: 10.1016/S0140-6736(94)91956-9 [DOI] [PubMed] [Google Scholar]
- 4. Quirk WF, Sternbach G, Joseph J. Infection with flesh eating bacteria. J Emerg Med 1996;14(6):747–53.DOI: 10.1016/S0736-4679(96)00197-7 [DOI] [PubMed] [Google Scholar]
- 5. Stephens BJ, Lathrop JC, Rice WT et al. Fournier's gangrene: historic (1764–1978) versus contemporary (1979–1988) differences in etiology and clinical importance. Am Surg 1993;59: 149–54. [PubMed] [Google Scholar]
- 6. Meleney FL. Haemolytic streptococcal gangrene. Arch Surg 1924;9: 317–64. [Google Scholar]
- 7. Wilson B. Necrotizing fasciitis. Am Surg 1952; 18: 416–31. [PubMed] [Google Scholar]
- 8. Kaul R, McGeer A, Low DE. Population‐based surveillance for group A streptococcal necrotising fasciitis: clinical features, prognostic indicators and microbiological analysis of seventy‐seven cases. Am J Med 1997;103: 18–24.DOI: 10.1016/S0002-9343(97)00160-5 [DOI] [PubMed] [Google Scholar]
- 9. Demers B, Simor AE, Vellend P et al. Severe group A Streptococcal infections in Ontario, Canada 1987–91. Clin Infect Dis 1993;16: 792–800. [DOI] [PubMed] [Google Scholar]
- 10. Jarrett P, Rademaker M, Duffill M. The clinical spectrum of necrotising fasciitis. A review of 15 cases. Aust NZ J Med 1997;27: 29–34. [DOI] [PubMed] [Google Scholar]
- 11. Green RJ. Necrotising fasciitis. Chest 1996;110(1): 219–29. [DOI] [PubMed] [Google Scholar]
- 12. Ward RG, Walsh MS. Necrotising fasciitis: 10 years experience in a district general hospital. Br J Surg 1991;78: 488–9. [DOI] [PubMed] [Google Scholar]
- 13. Holder EP, Moore PT, Browne BA. Nonsteroidal anti‐inflammatory drugs and necrotising fasciitis: an update. Drug Saf 1997;17: 369–73. [DOI] [PubMed] [Google Scholar]
- 14. Chelsom J, Halstensen A, Haga T et al. Necrotising fasciitis due to group A streptococci in western Norway: incidence and clinical features. Lancet 1994;344: 1111–5. [DOI] [PubMed] [Google Scholar]
- 15. Giuliano A, Lewis F, Hadley K et al. Bacteriology of necrotizing fasciitis. Am J Surg 1977;134: 52–7.DOI: 10.1016/0002-9610(77)90283-5 [DOI] [PubMed] [Google Scholar]
- 16. Gorbach SL. IDCP guideline: necrotizing skin and soft tissue infections, part 1: necrotizing fasciitis. Infect Dis Clin Pract 1996;5: 406. [Google Scholar]
- 17. Childers BJ, Potyondy LD, Nachreiner R et al. Necrotising fasciitis: a fourteen‐year retrospective study of 163 consecutive patients. Am Surg 2002; 62: 109–16. [PubMed] [Google Scholar]
- 18. Willoughby R, Greenberg RN. The toxic shock syndrome and streptococcal pyrogenis exotoxins. Ann Intern Med 1982;98: 559. [DOI] [PubMed] [Google Scholar]
- 19. Dennis LS. The Flesh eating bacterium: what's next? J Infect Dis 1999;179 (Suppl. 2):S366–74. [DOI] [PubMed] [Google Scholar]
- 20. Sutherland ME, Meyer AA. Necrotizing soft‐tissue infections. Surg Clin North Am 1994;74(3):591–607. [PubMed] [Google Scholar]
- 21. Lancefield RC. Current knowledge of type specific M antigens of group A streptococci. J Immunol 1962;89: 307–13. [PubMed] [Google Scholar]
- 22. Kishka DL, Thiede B, Caracciolo J et al. Invasive group A streptococcal infections in North Carolina: epidemiology, clinical features, and genetic and serotype analysis of causative organisms. J Infect Dis 1997;176: 992–1000. [DOI] [PubMed] [Google Scholar]
- 23. Hacket SP, Stephens DL. Streptococcal toxic shock syndrome: synthesis of tumour necrosis factor and interleukin‐1 by monocytes stimulated with pyrogenic exotoxin A and streptolysin O. J Infect Dis 1992;165: 879–85. [DOI] [PubMed] [Google Scholar]
- 24. Urschel JD. Necrotising soft tissue infections. Postgrad Med J 1999;75: 645–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Devin B, McCarthy A, Mehran R. Necrotising fasciitis of the retroperitoneum: an unusual presentation of Group A streptococcus. Can J Surg 1998; 41: 156–60. [PMC free article] [PubMed] [Google Scholar]
- 26. Chen Y‐M, Wu MF, Lee PY et al. Necrotising fasciitis: is it a fatal complication of tube thoracostomy? Report of three cases. Respir Med 1992; 86: 249–51. [DOI] [PubMed] [Google Scholar]
- 27. Frota Filho JD, Drews C, Leaes P et al. Postoperative necrotising fasciitis of the thorax in cardiac surgery. Arq Bras Cardiol 2001;76(3):245–54. [DOI] [PubMed] [Google Scholar]
- 28. Rea WJ, Walter J, Wyrick WJ Jr. Necrotising fasciitis. Ann Surg 1970;172: 957–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wall DB, Klein SR, Black S, De Virgilio C. A simple model to help distinguish necrotizing fasciitis from nonnecrotizing soft tissue infection. J Am Coll Surg 2000;191: 227–31.DOI: 10.1016/S1072-7515(00)00318-5 [DOI] [PubMed] [Google Scholar]
- 30. Fisher JR, Conway MJ, Takeshita RT et al. Necrotising fasciitis: importance of roentgenographic studies for soft‐tissue gas. JAMA 1979;241: 803–6. [DOI] [PubMed] [Google Scholar]
- 31. Wysoki MG, Santora TA, Shah RM, Friedman AC. Necrotizing fasciitis: CT characteristics. Radiology 1997;203(3):859–63. [DOI] [PubMed] [Google Scholar]
- 32. Schmid MR, Kossman T, Duewell S. Differentiation of necrotizing fasciitis and cellulitis using MR imaging. Am J Roentgenol 1998;170: 615–20. [DOI] [PubMed] [Google Scholar]
- 33. Arslan A, Pierre‐Jerome C, Borthne A. Necrotizing fasciitis: unreliable MRI findings in the preoperative diagnosis. Eur J Radiol 2000;36: 139–43.DOI: 10.1016/S0720-048X(00)00164-9 [DOI] [PubMed] [Google Scholar]
- 34. Begley MG, Shawker TH, Robertson CN et al. Fournier gangrene: diagnosis with scrotal ultrasound. Radiology 1988;169: 387–9. [DOI] [PubMed] [Google Scholar]
- 35. Majeski J, Majeski E. Necrotizing fasciitis: improved survival with early recognition by tissue biopsy and aggressive surgical treatment. South Med J 1997;90(11):1065–8. [DOI] [PubMed] [Google Scholar]
- 36. Eagle H. Experimental approach to the problem of treatment failure with penicillin. 1. Group A streptococcal infection in mice. Am J Med 1952; 13: 389–99.DOI: 10.1016/0002-9343(52)90293-3 [DOI] [PubMed] [Google Scholar]
- 37. Stephens DL, Bryant AE, Yan S. Invasive group A streptococcal infection: new concepts in antibiotic treatment. Int J Antimicrob Agents 1994;4: 297–301.DOI: 10.1016/0924-8579(94)90029-9 [DOI] [PubMed] [Google Scholar]
- 38. Stephens DL, Bryant AE, Hackett SP. Antibiotic effects on bacterial viability, toxin production, and host response. Clin Infect Dis 1995;20 (Suppl.):S154–7. [DOI] [PubMed] [Google Scholar]
- 39. Majeski JA, Alexander JW. Early diagnosis, nutritional support, and immediate extensive debridement improve survival in necrotizing fasciitis. Am J Surg 1983;145: 784–7.DOI: 10.1016/0002-9610(83)90140-X [DOI] [PubMed] [Google Scholar]
- 40. Efem SEE. The features and aetiology if Fournier's gangrene. Postgrad Med 1994;70: 558–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Efem SE. Recent advances in the management of Fournier's gangrene: preliminary observations. Surgery 1993;113: 200–4. [PubMed] [Google Scholar]
- 42. Paty R, Smith AD. Gangrene and Fournier's gangrene. Urol Clin North Am 1992;19: 149–62. [PubMed] [Google Scholar]
- 43. Riseman JF, Zmboni WA, Curtis A et al. Hyperbaric oxygen therapy for necrotising fasciitis reduces mortality and the need for debridements. Surgery 1990;108: 847–50. [PubMed] [Google Scholar]
- 44. Eltorai IM, Hart GB, Strauss MB et al. The role of hyperbaric oxygen in the management of Fournier's gangrene. Int Surg 1986;71: 53–8. [PubMed] [Google Scholar]
- 45. Elliot DC, Kufera JA, Myers RAM. Necrotizing soft tissue infections. Risk factors for mortality and strategies for management. Ann Surg 1996;224(5):672–83.DOI: 10.1097/00000658-199611000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kaul R, McGeer A, Canadian Infectious Diseases Society (CIDS) Ontario Group A Streptococcal Study, Norrby‐Teglund A, Kotb M, Low DE. Intravenous immunoglobulin (IVIG) therapy in streptococcal toxic shock syndrome (STSS): results of a matched case‐control study [abstract]. Interscience Conference on Antimicrobial Agents and Chemotherapy 1995..
- 47. Norrby‐Teglund A, Kaul R, Low DE, McGeer A et al. Plasma from patients with severe group A streptococcal infections treated with normal polyspecific IgG (IVIG) inhibits streptococcal superantigen‐induced T cell proliferation and cytokine production. J Immunol 1996;156: 3057–64. [PubMed] [Google Scholar]
- 48. Holloway S, Ryder J. Management of a patient with post‐operative necrotizing fasciitis. Br J Nurs 2002; (Suppl. 11) (16):S25–S32. [DOI] [PubMed] [Google Scholar]
- 49. Banwell PE, Teot L. Topical negative pressure (TNP): the evolution of a novel wound therapy. J Wound Care 2003;12(1):122–8. [DOI] [PubMed] [Google Scholar]
- 50. Banwell PE. Topical negative pressure in wound care. J Wound Care 1999;8(2):79–84. [DOI] [PubMed] [Google Scholar]
- 51. Webb LX. New techniques in wound management: vacuum‐assisted wound closure. J Am Acad Orthop Surg 2002;10(5):303–11. [DOI] [PubMed] [Google Scholar]
- 52. Krasner DL. Managing wound pain in patients with vacuum‐assisted closure devices. Ostomy Wound Manage 2002;48(5):38–43. [PubMed] [Google Scholar]
- 53. Burge TS, Watson JD. Necrotising fasciitis. Be bloody, bold and resolute. BMJ 1994;308: 1453–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Freischlag JA, Ajalat G, Busuttil RW. Treatment of necrotizing soft tissue infections: the need for a new approach. Am J Surg 1985;149: 751. [DOI] [PubMed] [Google Scholar]
- 55. Pessa ME, Howard RJ. Necrotizing fasciitis. Surg Gynecol Obstet 1985;161: 357–61. [PubMed] [Google Scholar]
- 56. Bosshardt TL, Henderson VJ, Organ CH Jr. Necrotizing soft tissue infections. Arch Surg 1996; 131: 846–54. [DOI] [PubMed] [Google Scholar]


