Abstract
Mast cells are predominantly found in the vicinity of connective tissue vessels of skin and mucosa. The main immunological functions of mast cells are in IgE‐mediated reactions and in helminth infestations. Mast cells respond to tissue injury by releasing inflammatory mediators and have been implicated in diseases of excessive fibrosis of the dermis such as scleroderma. Current evidence suggests that mast cells exert its role during inflammation and cellular proliferation. Animal models have shown that by stabilising mast cells at the early stages of wound healing, wound contraction is reduced. Mast cells are an ideal candidate to play a pivotal role in wound healing due to its location, substances released and clinical associations.
Keywords: mast cell, wound healing, inflammation, cellular proliferation
INTRODUCTION
Inflammation plays an integral part in the process of wound healing. Immediately following an injury inflicted upon human tissue, platelet aggregation and haemostasis begin. The scene is then set for a complex interplay of inflammatory cells and mediators with the ultimate aim of restoring function to the traumatised tissue. This is initiated by neutrophils and swiftly followed by macrophages, ultimately orchestrated by a host of chemical signals, namely cytokines and growth factors (1).
The immune system operates alongside the much‐studied inflammatory process and is seen as the less illustrious counterpart during wound healing. The innate and acquired immune system is relatively well understood in disease prevention and ill health, for example, the development of vaccines and disease modifying drugs in rheumatoid arthritis. The immune system plays an essential role in protecting the tissues against pathogenic invasion when the first line of defence, usually a barrier‐like skin, has been breached. Cells of the acquired immune system, namely B and T cells, may play a role as their levels at wound edges correspond with various stages of wound healing 2, 3. There is emerging evidence that another immune cell, that is mast cells, may play a larger role in wound healing than previously thought. This study will focus on the role of mast cells in the wound healing process and its clinical applications.
MAST CELLS
Mast cells (mästen, from German to feed or fatten) were first stained by Paul Erhlich as part of his thesis in 1878 (4). The functions of mast cells were previously shrouded in much mystery as disease rarely results from the absence of mast cells and the cells were not readily cultured (5). Other blood‐borne immune cells, such as lymphocytes, are circulated in the blood and therefore easily cultured from blood samples. Mast cells, however, only mature once they arrive at the tissues (6) and so were previously not readily cultured. Mast cells are derived from bone marrow and originate from pluripotent stem cells, which are less differentiated than precursors of neutrophils and monocytes (7). Mast cells are predominantly found in the vicinity of connective tissue vessels of skin and mucosa (8). Thus, mast cells are akin to sentries of the body, residing in the junction between the body and the environment, namely skin and mucosa.
Two main functions of mast cells are in IgE‐mediated reactions and in helminth infestations (6). Vasodilatation and vessel permeability is predominantly controlled by the autonomic nervous response following injury. Mast cells perform a vital role in the neural‐independent pathway of the vascular response. The release of histamine, prostglandins and leukotrienes by the mast cell causes early vasodilatation and venule permeability, even in the absence of neural input. In IgE‐mediated inflammatory reactions, antigens bind with mast cell surface IgE upon subsequent exposures. This results in degranulation of mast cells and the release of it contents, namely histamine and inflammatory mediators, causing angioedema and subsequently anaphylactic shock. In helminth infestations, the by‐products of helminth metabolism are antigens that trigger IgE‐mediated immune response. This in turn activates eosinophils and mast cells, causing inflammation, granuloma formation and rarely anaphylaxis, in an effort to expel the organisms.
Mast cells have an armamentarium of inflammatory mediators (for example, TNF‐α, IL‐1), growth factors [for example, TGF‐β1 , platelet‐derived growth factor (PDGF)] and proteases (mainly serine proteases, such as chymase and tryptase) that are released during tissue injury from physical injuries, such as trauma, heat and irradiation, and when activated by chemical agents such as IgE, bacterial antigen, venom, lysosomes and complement 8, 9, 10. Figure 1 illustrates this process. Mast cells have also been implicated in disease processes where there is excessive fibrosis of the dermis where it resides, such as scleroderma (11) and hypertrophic scars (12). The mast cell is an ideal candidate to play a pivotal role during wound healing, due to its location, substances released and clinical associations.
Figure 1.
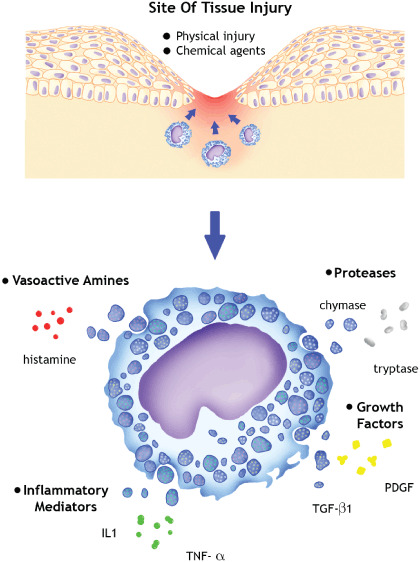
Triggers of activation and key inflammatory mediators produced by mast cells. IL, interleukin; TNF, tumour necrosis factor; TGF, transforming growth factor; PDGF, platelet‐derived growth factor.
INFLAMMATION
In the early phases of inflammation, the migration of neutrophils and macrophages to the wound site is essential. Mast cells accumulate within 24 hours of the injury, as was seen in pancreatic cancer (13), and exert its effect by releasing histamine, a vasoactive amine, readily. The role of histamine in IgE‐mediated angioedema has been outlined above. Interleukins, such as IL‐6 and IL‐8, and growth factors, such as vascular endothelial growth factor (VEGF), are released by mast cells. It is postulated that they alter the permeability of the vessels through activating the protease‐activated receptor‐2 (PAR2) (9).
Serine proteases are released from mast cells in the early stages of inflammation. Chymase and tryptase both readily breakdown the extracellular matrix to prepare for the next stage of wound healing, which is the proliferation of fibroblasts and endothelial cells. Chymase degrades the basement membrane of the epidermis (5). Tryptase binds to PAR2 and also stimulates the release of kinnins that serves to augment the changes in vascular permeability (9). Vasodilatation and increased vascular permeability ensues, allowing the infiltration of neutrophils and other inflammatory mediators.
There is growing body of evidence in recent clinical research, indicating that mast cells influence inflammation in various tissues. Conjunctival scarring is associated with the degree of inflammation. Chang et al. (14) found the presence of significantly increased mast cell in patients with previous surgery for glaucoma compared with their control group. The degree of pulmonary inflammation following lung injury is associated with the number of mast cell activated (15). Higuchi et al. (16) found mice had better outcomes following viral myocarditis if mast cell activity was dampened. This was postulated to be due to the reduced mast cell‐induced inflammatory reaction.
CELLULAR PROLIFERATION
Two key features of the cellular proliferation phase are the laying down of collagen by fibroblasts and angiogenesis by endothelial cells. Previous studies have shown that TGF‐β1 is a potent stimulator of fibroblasts and wound contraction, as proven by upregulating α‐smooth muscle actin (αSMA) (17), and clinically when adult scars mimic foetal wound healing following the application of anti‐TGF‐β1 antibodies (18). Mast cells are not the main producers of TGF‐β1 but perhaps other factors secreted by mast cells, such as VEGF along with TGF‐β1 account for its effect on fibroblast proliferation and angiogenesis. A similar argument could be made for the role of mast cells in angiogenesis.
Abe et al. (19) determined that mast cell leukotriene D4 and prostaglandin D2 have a complex relationship with type I collagen production by fibroblasts. The study also confirmed that tryptase had a pronounced effect on collagen synthesis. Garbuzenko et al. (20) found tryptase to be central in mast cell‐induced deposition of collagen in the extracellular matrix. They utilised human mast cell line (HMC‐1) to study the effect of mast cell and its secretions on fibroblast proliferation and collagen synthesis. The effects of the mast cells were measured on monolayer cultures and collagen lattices, whereas Abe et al. (19) utilised monolayer cultures and calculated the amount of fibroblast and collagen. Despite the differences in the methodology, both studies reached a similar conclusion, that mast cell tryptase plays a large role in collagen synthesis and deposition. The findings of Garbuzenko et al. (20) were similar to another earlier study carried out by Lohi et al. (21), that is histamine and tryptase stimulated fibroblast proliferation and collagen synthesis, whereas heparin and TNF‐α had no effect. Although Lohi et al. (21) concluded that tryptase activated fibroblast matrix metalloproteinase‐2 (MMP‐2), Garbuzenko et al. (20) did not identify any fibroblast MMP or tissue inhibitor of metalloproteinase (TIMP) modulation. Rat mast cells were used in the former experiment, and thus the different species phenotypes may explain the discrepancy in results.
The vast majority of studies on mast cells have been carried out on rats and mice. Mast cell phenotypes varied between species and even among different parts of the body within a species 22, 23. This results in minor variations in the substances secreted, such as the chymase family as described by Solivan et al. (24). Therefore, whether animal models will predict mast cell functions in humans with accuracy is difficult to interpret.
Following collagen deposition, fibroblasts differentiate into myofibroblasts at the latter stages of the proliferative phase. The production of αSMA is indicative of this process. Gailit et al. (25) developed a skin equivalent culture system to study the expression of αSMA and wound contraction. HMC‐1 induced αSMA expression and contraction. It was noted that the number of cells did not correlate to degree of contraction. This remains an observation, as no statistical calculation was made to confirm this. Tryptase alone had a similar effect to HMC‐1, indicating that tryptase not only produces fibroplasia, but also induces actinic contraction. Histamine stimulated the production of αSMA but did not affect wound contraction. TNF‐α had no effect. Therefore, it can be inferred from this study that wound contraction is dependent on the presence of products released by mast cells, namely tryptase and histamine.
Garbuzenko et al. (20) identified that wound contraction required the presence of both mast cells and fibroblasts. Prior to this study, wound contraction in vitro was found to have been impaired when there was no cell to cell adhesion between human mast cells and fibroblasts (26). Stem cell factor (SCF) and c‐kit induce cell adhesion between the two cells. When antibodies to SCF and c‐kit were applied, wound contraction was inhibited by up to 70%. Another theory was put forth as the results of Yamamoto et al. (26) were not consistently replicable. Moyer et al. (27) proposed that there were gap junctions that allow intercellular communication and anchorage as the basis of wound contraction. The chemical cell adhesion theory was disproved by showing that increased mast cell secretion or addition of mast cell secretions to mast cells and fibroblasts in a three‐dimensional lattice did not increase the contractile force. In addition, Moyer et al. (27) had shown that dye was transferred from mast cell to fibroblasts via gap junctions. Although homogenous cells commonly develop gap junctions, it is less common between different cells. Both studies utilised the same cell line; therefore, the difference in observations are not due to mast cell phenotype. Further studies into the effect of SCF and c‐kit on gap junctions would be required to understand the mechanism of cell adhesion between mast cells and fibroblasts, which seems integral to wound contraction. It would not be surprising if the answer lay somewhere between the two opposing theories.
Angiogenesis is essential for the provision of oxygen and nutrients to the repairing cells and rapidly dividing tumour cells which have a high metabolic demand. Angiogenesis is stimulated by growth factors (of which VEGF is the most potent), such as hypoxia and shear stress (28). Mast cells have been observed to be closely associated with tumour, usually amassing at the periphery of the neoplasm. The number of mast cells seemed to be related to the degree of angiogenesis (29). This led to the hypothesis that mast cells could be involved in angiogenesis. Incidentally, mast cells were first studied on mast cell tumour, which remains a rare tumour.
Human mast cells release VEGF and fibroblast growth factor‐2 amongst other angiogenic growth factors. Serine proteases such as chymase degrade the extracellular matrix, and thus prepare the surrounding area for angiogenesis (30). Furthermore, mast cell metalloproteinases can stimulate the release of angiogenic factors found in the extracellular matrix. When mast cell metalloproteinases break down the matrix, fragments of hyaluronic acid is released, which are angiogenic fragments (31).
Mast cell proteinase‐5 (MCP‐5) is a chymase released by mast cells. Its levels peak on the 5th day after the injury. The appearance of neovasculature around the same time lends credence to the theory of mast cell‐induced angiogenesis (10). Russo et al. (32) used ketotifen, a mast cell stabiliser, and compared it with chlorphenamine (also known as chlorpheniramine), an anti‐H1 antagonist. Ketotifen, as predicted, significantly inhibited granuloma formation and there was no difference with the anti‐histamine. Haemoglobin content and TNF‐α levels of the granulomatous tissue were reduced in the ketotifen group, suggesting that mast cells positively influence angiogenesis. This was a statistically significant finding. Polymerase chain reaction analysis showed a reduction in mRNA of MCP‐5 in the ketotifen group, indicating that MCP‐5 may play a role in angiogenesis.
Most of our understanding of the role played by mast cells in angiogenesis comes from work on tumours. Soucek et al. (13) investigated pancreatic cancer cells and found mast cell accumulation at the periphery of tumour cells within the first 24 hours. In contrast, macrophages and neutrophils appeared a week after initial seeding of the tumour. Although interleukin‐1β induces angiogenesis, mast cells seem to be required for maintenance of angiogenesis, rather than initiation. Tumours with established blood supply, but with the mast cell population inactivated, reduced in size or disappeared, indicating that mast cells are integral to tumour maintenance as well. Earlier studies have shown varying tumour response to histamine modulation. Lawson et al. (33) reported a decrease in colonic cancer volume with the use of cimetidine but not with ranitidine, both H2 receptor antagonists. Bowrey et al. (34) conducted a small clinical trial to investigate the effect of histamine on breast cancer. Although the expression of histamine receptors was higher in tumour cells compared with mammary cells, cimetidine did not significantly influence the proliferation of tumour cells. Perhaps, the effect of mast cells on tumour angiogenesis is more complex than previously hypothesised.
Mast cells play an important role in fibroblast proliferation and wound contraction. Its secretion of tryptase and histamine are keys to fibroblast production of collagen and αSMA contraction. They maintain angiogenesis to supply vital nutrients to repairing cells. Without the functioning of mast cells, wound healing is impaired in animal models (35). Figure 2 summarises the role of mast cells in the different phases of wound healing.
Figure 2.
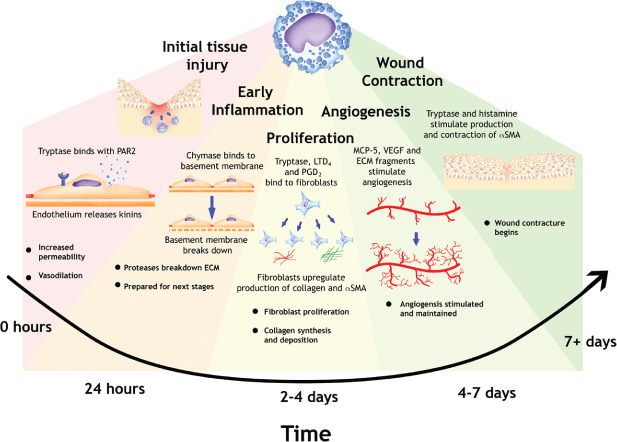
Role of mast cells in the different stages of wound healing. PAR, protease activated receptor; ECM, extracellular matrix; LT, leukotriene; PG, prostaglandin; MCP, mast cell proteinase; VEGF, vascular endothelial growth factor; SMA, smooth muscle actin.
CLINICAL PRACTICE
Mast cell modulation by pharmaceutical means is an established medical practice and our knowledge comes mainly from IgE‐mediated reactions. For example, anti‐histamines are routinely used for type I hypersensitivity reactions and mast cell stabilisers are utilised for asthma. Animal models have been used by Gallant‐Behm et al. (36) to evaluate the efficacy of ketotifen on wound scarring. The results and implications of this study are discussed.
Yorkshire pig skin heals histologically like humans (37). Red Duroc pigs, unfortunately heal with the equivalent of human hypertrophic scars (38). Gallant‐Behm et al. (36) inflicted wounds on four pigs and studied the wound healing with and without ketotifen and followed up the wounds for a minimum of 10 weeks. Wound contraction was expressed as the percentage of scar area at any given time over the area of scar from the original wounding. In the Yorkshire pigs, there was no difference in wound healing with or without ketotifen. In the Red Duroc pig with ketotifen, the wounds healed with contraction similar to the Yorkshire pigs, and less contraction than that of the Red Duroc pig without ketotifen. Application of ketotifen after wound healing had no effect. The histological findings and the number of cells including myofibroblasts and mast cells were in keeping with the clinical findings. The treatment was given over a period of 10 weeks and it remains to be seen if a shorter period of treatment would produce the same results. Delayed treatment, started 28 days after wounding did not reduce the amount of contraction in the Red Duroc pigs. Mast cell stabilisation proved beneficial in the early stages of wound healing in this study, specifically during inflammation and the proliferative phase.
There is scope for the development of mast cell stabilisers to produce finer scars that are less prone to contraction and altered pigmentation in humans. This may be of clinical importance for those who are predisposed to developing hypertrophic scars. There needs to be a balance between achieving good scars and compromising the immune function around the area of healing.
The main difficulty is the use of animal models for studying the effects of mast cells on wound healing. As previously mentioned, mast cell phenotypes and the secretory products are different from site to site and from species to species 22, 23. What may hold true for a mouse or pig, may not necessarily hold true for a human being. The HMC‐1 that is currently used is the closest approximation to in vivo conditions. Human skin mast cells will differ to a certain degree from human mast cells from different tissues. Therefore, the interpretation of results must be treated with caution.
There is the possibility of utilising drugs that can modulate mast cell function to aid wound healing, such as mast cell stabilisers and histamine antagonists. Human clinical trials would be invaluable to evaluate the effect of readily available, low‐cost agents such as ketotifen, sodium cromoglicate, chlorphenamine and cimetidine on the outcome of wound healing and the eventual scarring.
In conclusion, mast cells are poorly understood compared to other immune cells. They play a pivotal role in the early stages of wound healing, especially in the proliferative phase. Although mast cells may not be major players in wound healing, animal models suggest that the absence of mast cells function impairs wound healing, and the converse is true for the presence of too many mast cells.
ACKNOWLEDGEMENTS
This article was originally written as a requirement as part of the MSc course in Wound Healing and Tissue Repair at Cardiff University. Illustrations by Miss Lucy Hyde, University of Dundee.
REFERENCES
- 1. Hart J. Inflammation 1: its role in the healing of acute wounds. J Wound Care 2002;11:205–9. [DOI] [PubMed] [Google Scholar]
- 2. Martin CW, Muir IFK. The role of lymphocytes in wound healing. BrJ Plast Surg 1990;43:655–62. [DOI] [PubMed] [Google Scholar]
- 3. Boyce DE, Jones WD, Ruge F, Harding KG, Moore K. The role of lymphocytes in human dermal wound healing. Br J Dermatol 2000;143:59–65. [DOI] [PubMed] [Google Scholar]
- 4. Ehrlich P. Beiträge zur theorie und praxis der hitologischen Färbung. Thesis, Leipzig University, 1878.
- 5. Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev 1997;77:1033–79. [DOI] [PubMed] [Google Scholar]
- 6. Parslow TG, Stites DP, Terr AI, Imboden JB, editors. Medical immunology. 10th ed. London, McGraw‐Hill, 2001. [Google Scholar]
- 7. Sonoda T, Kitamura Y, Haku Y, Hara H, Mori KJ. Mast cell precursors in various hematopoietic colonies of mice produced in vivo and in vitro. Br J Haematol 1983;53:611–20. [DOI] [PubMed] [Google Scholar]
- 8. Woolf, N. Acute inflammation II: cellular events and chemical mediators. In: Woolf N, editor. Pathology: basic and systemic, 1st ed. London: W B Saunders, 1998:41–62. [Google Scholar]
- 9. Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev 2007;217:65–78. [DOI] [PubMed] [Google Scholar]
- 10. Kagawa S, Matsuo A, Yagi Y, Ikematsu K, Tsuda R, Nakasono I. The time‐course analysis of gene expression during wound healing in mouse skin. Legal Med 2008;11(2):70–5. [DOI] [PubMed] [Google Scholar]
- 11. Nishioka K, Kobayashi Y, Katayama I, Takijiri C. Mast cell numbers in diffuse scleroderma. Arch Dermatol 1987;123(22):205–8. [PubMed] [Google Scholar]
- 12. Kischer CW, Bunce H, Shetlar MR. Mast cell analysis in hypertrophic scars, hypertrophic scars treated with pressure and mature scars. J Invest Dermatol 1978;70:355–7. [DOI] [PubMed] [Google Scholar]
- 13. Soucek L, Lawlor ER, Soto D, Shchors K, Brown Swugart L, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc‐induced pancreatic islet tumours. Nat Med 2007;13:1211–8. [DOI] [PubMed] [Google Scholar]
- 14. Chang L, Wong T, Ohbayashi M, Bunce C, Barton K, Ono S, Khaw PT. Increased mast cell numbers in the conjunctiva of glaucoma patients: a possible indicator of preoperative glaucoma surgery inflammation. Eye 2009;23:1859–65. [DOI] [PubMed] [Google Scholar]
- 15. Kalin TV, Meliton L, Meliton AY, Zhu X, Whitsett JA, Kalinichenko VV. Pulmonary mastocytosis and enhanced lung inflammation in mice heterozygous null for the Foxf1 gene. Am J Respir Cell Mol Biol 2008;39:390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higuchi H, Hara M, Yamamoto K, Miyamoto T, Kinoshita M, Yamada T, Uchiyama K, Matsumori A. Mast cells play a critical role in the pathogenesis of viral myocarditis. Circulation 2008;118:363–72. [DOI] [PubMed] [Google Scholar]
- 17. Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor‐β1 induces α‐smooth muscle actin expression in granulation tissue myofibrobalsts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993;122:103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shah M, Foreman DM, Ferguson MW. Neutralising antibody to TGF‐beta 1,2 reduces cutaneous scarring in adult rodents. J Cell Sci 1994;107: 1137–57. [DOI] [PubMed] [Google Scholar]
- 19. Abe M, Kurosawa M, Ishikawa O, Miyachi Y. Effect of mast cell‐derived mediators and mast cell‐related neutral proteases on human dermal fibroblast proliferation and type I collagen production. J Allergy Clin Immunol 2002;106: S78–84 [DOI] [PubMed] [Google Scholar]
- 20. Garbuzenko E, Nagler A, Pickholtz D, Gillery P, Reich R, Maquart F‐X, Levi‐Schaffer F. Human mast cells stimulate fibroblast proliferation, collagen synthesis and lattice contraction: a direct role for mast cells in skin fibrosis. Clin Exp Allergy 2002;32:237–46. [DOI] [PubMed] [Google Scholar]
- 21. Lohi J, Narvima I, Keski‐Oja J. Pericellular substrates of human mast cell tryptase: 72000 dalton gelatinase and fibronectin. J Cell Biochem 1992;106:337–49. [DOI] [PubMed] [Google Scholar]
- 22. Miller HRP, Pemberton AD. Tissue‐specific expression of mast cell granule serine proteinases and their role in inflammation in the lung and gut. Immunology 2002;105:375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henz BM. Exploring the mast cell enigma: a personal reflection of what remains to be done. Exp Dermatol 2008;17:91–9. [DOI] [PubMed] [Google Scholar]
- 24. Solivan S, Selwood T, Wang Z‐M, Schechter NM. Evidence of diversity of substrate specificity among members of the chymase family of serine proteases. FEBS 2002;512:133–8. [DOI] [PubMed] [Google Scholar]
- 25. Gailit J, Marchese MJ, Kew RR, Gruber BL. The differentiation and function of myofibroblasts is regulated by mast cell mediators. J Invest Dermatol 2001;117:1113–9. [DOI] [PubMed] [Google Scholar]
- 26. Yamamoto T, Hartmann K, Eckes B, Krieg T. Mast cells enhance contraction of three‐dimensional collagen lattices by fibroblasts by cell–cell interaction: role of stem cell factor/c‐kit. Immunology 2000;99:435–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moyer KE, Saggers GC, Ehrlich P. Mast cells promote fibroblast populated collagen lattice contraction through gap junction intercellular communication. Wound Repair Regen 2004;12:269–75. [DOI] [PubMed] [Google Scholar]
- 28. Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000;63: 389–96. [DOI] [PubMed] [Google Scholar]
- 29. Nienartowicz A, Sobaniec‐Lotowska ME, Jarocka‐Cyrta E, Lemancewicz D. Mast cells in neoangiogenesis. Med Sci Monit 2006;12:RA53–6. [PubMed] [Google Scholar]
- 30. Crivellato E, Nico B, Ribatti D. Mast cells and tumour angiogenesis: new insight from experimental carcinogenesis. Cancer Lett 2008;269: 1–6. [DOI] [PubMed] [Google Scholar]
- 31. Cox L. Present angiogenesis research and its possible future implementation in wound care. J Wound Care 2003;12:225–8. [DOI] [PubMed] [Google Scholar]
- 32. Russo A, Russo G, Peticca M, Pietropaolo C, Di Rosa M, Iuvone T. Inhibition of granuloma‐associated angiogenesis by controlling mast cell mediator release: role of mast cell protease‐5. Br J Pharmacol 2005;145:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lawson JA, Adams WJ, Morris DL. Ranitidine and cimetidine differ in their in vitro and in vivo effects on human colonic cancer growth. Br J Cancer 1996. 73:872–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bowrey PF, King J, Magarey C, Schwartz P, Marr P, Bolton E, Morris DL. Histamine, mast cells and tumour cell proliferation in breast cancer: does preoperative cimetidine administration have an effect? Br J Cancer 2000. 82:167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weller K, Foitzik K, Paus R, Syska W, Maurer M. Mast cells are required for normal healing of skin wounds in mice. FASEB 2006;20: E1628–35. [DOI] [PubMed] [Google Scholar]
- 36. Gallant‐Behm CL, Hildebrand KA, Hart DA. The mast cell stabilizer ketotifen prevents development of excessive skin wound contraction and fibrosis in red Duroc pigs. Wound Repair Regen 2008;16:226–33. [DOI] [PubMed] [Google Scholar]
- 37. Wang JF, Olson ME, Reno CR, Wright JB, Hart DA. The pig as a model for excisional skin wound healing: characterization of the molecular and cellular biology, and bacteriology of the healing process. Comp Med 2001;51:341–8. [PubMed] [Google Scholar]
- 38. Gallant CL, Olson ME, Hart DA. Molecular, histologic, and gross phenotype of skin wound healing in red Duroc pigs reveal an abnormal healing phenotype of hypercontracted, hyperpigmented scarring. Wound Repair Regen 2004;12:305–19. [DOI] [PubMed] [Google Scholar]


