Abstract
Diabetic foot ulcers (DFUs) are a leading cause of morbidity and hospitalisation among patients with diabetes. We analysed claims data for Medicare part B diabetic foot ulcer patients treated with Negative Pressure Wound Therapy at home (N = 1135) and diabetic foot ulcer patients from a published meta‐analysis of randomised controlled wet‐to‐moist therapy. The expected costs of care for the two treatments were also compared. A significantly greater proportion of wounds treated with NPWT achieved a successful treatment endpoint compared with wet‐to‐moist therapy at both 12 weeks (39·5% versus 23·9%; P < 0·001) and 20 weeks (46·3% versus 32·8%; P < 0·001). NPWT‐treated patients reached a successful wound treatment endpoint more rapidly, and the benefit was apparent in all wound sizes. Expected 20‐week treatment costs for NPWT were similar to those for wet‐to‐moist therapy if one nursing visit per day for the latter is assumed but 42% less if two nursing visits per day are made. Thus, NPWT may improve the proportion of DFUs that attain a successful wound treatment endpoint and decrease resource utilisation by a given health care system compared with standard wet‐to‐moist therapy.
Keywords: Diabetic foot ulcer, Negative pressure wound therapy, Treatment costs, Vacuum‐assisted closure, Wound treatment
Introduction
Foot ulcers resulting from peripheral neuropathy, peripheral vascular disease and mechanical trauma are common, occurring in nearly 2% of individuals with diabetes per year (1). The lifetime risk of diabetic foot ulcers (DFUs) may be as high as 25% (2); they are a major public health problem, as they often progress to infections of surrounding tissue and bone and lead to lower extremity amputations. In fact, a DFU precedes the vast majority (at least 85%) of non traumatic amputations in patients with diabetes 3, 4, 5, 6, about 82 000 of which are performed each year in the USA (7). Given the significant reduction in the quality of life as a result of amputations 8, 9 and the consequent increase in mortality 10, 11, 12, 13, it is of significant value to avoid the need for such surgery by reducing the number of DFUs and effectively managing those that develop. However, standard wound therapy (i.e. with debridement, offloading/protective measures, etc.) fails to heal some DFUs, and amputation rates varying between 16% and 29% have been reported in the literature 1, 14, 15.
As new therapies are introduced, their success is often influenced by reimbursement and coverage guidelines by insurance companies. Although randomised controlled trials (RCTs) investigating the efficacy and safety of new therapies are important for regulatory processes governing their introduction into the market, the availability of and access to new products by health care providers are often affected by the coverage guidelines and the limits of such guidelines. A new treatment modality for DFUs is negative pressure wound therapy (NPWT) using the Vacuum Assisted Closure® (V.A.C.®) system (Kinetic Concepts Inc., San Antonio, TX). NPWT has become an increasingly popular treatment for healing chronic and difficult‐to‐manage wounds. It is thought to promote healing through a number of mechanisms, including facilitating physical wound closure through the effects of negative pressure, removing infectious materials and interstitial fluid and maintaining a closed, moist wound‐healing environment (16).
For several wound types, NPWT using the V.A.C.® system has been found to be an effective treatment modality that assists tissue granulation formation and helps decrease wound size 17, 18, 19, 20. At least one RCT providing evidence of its effectiveness in the treatment of diabetic foot amputation wounds has been completed (21). Additional evidence has been provided by retrospective studies of patients with DFU20, 22, 23, 24 and smaller prospectively designed investigations 18, 19. The present study was aimed at assessing the proportion of patients who reached a successful wound treatment endpoint with V.A.C.® therapy by retrospectively analysing a claims database and comparing these patients with data from an historical control group who received standard wound therapy in controlled clinical trial environments 25, 26. As earlier work has shown that initial wound size and wound duration may be predictors of healing success 27, 28, the influence of these variables on the treatment outcome with NPWT was also investigated. In addition, an economic analysis was modelled based on the outcomes of NPWT compared with those of standard wound therapy in the home care setting.
Materials and methods
Treatment groups
This retrospective study of patient outcomes used data from two distinct data sets. For the NPWT group, data were routinely collected by Kinetic Concepts Inc. (KCI, San Antonio, TX) from patients who treated for wound care with NPWT between 1996 and 2004. KCI maintains clinical data pertaining to Medicare Part B patients who receive NPWT with the V.A.C.® system in an outpatient setting as a condition of Medicare reimbursement. Therefore, this proprietary database is an excellent one to investigate treatment outcomes associated with use of the V.A.C.® system in real‐world clinical practice. It does not include data or tracking for comparator wound care products. As the database is established to capture V.A.C.® system use in actual practice, it is subject to the usual clinical decisions and federal policies related to prescribing decisions. An example of the latter is the Medicare durable medical equipment (DME) coverage guidelines for NPWT using the V.A.C.® system, which require that specific criteria be met for reimbursement. These criteria include the requirement that a complete wound therapy programme ‘should have been tried or considered and ruled out prior to application of NPWT’(29).
Beyond this federal guideline, patients were eligible for inclusion into the NPWT group of the present analysis based on the presence of the following: (1) wound categorised as diabetic ulcer/neuropathic ulcer, (2) wound treated with NPWT, (3) wound of chronic nature, (4) debridement of necrotic tissue performed, (5) comprehensive diabetes management included with the case plan, (6) reduction in pressure of affected ulcer, as needed and (7) description of the wound size and duration prior to NPWT. To determine wound size, its length and width were measured by the treating clinician and the area of a corresponding ellipse was used. The wound duration was recorded and categorised as having been present for more or less than 1 month.
Wounds were excluded from the analysis if either untreated osteomyelitis or cancer was present within the wound, if there was no record of treatment termination or no reason was given for treatment termination, or if multiple treatment termination entries were present (this excluded patients with multiple wounds). Similar criteria were used to classify wounds in the control group, as noted below.
The control group for the present study was selected by an extensive literature review aimed at identifying an appropriate comparator DFU population that had been treated with standard wound care regimens. PubMed and Cochrane databases were searched to identify studies reporting pertinent data. Possible comparators were prioritised based on the primary study metrics, study population, study design/rigor, sample size and treatment endpoint. More than 130 articles reported in the literature were reviewed. After prioritisation, a meta‐analysis by Margolis et al. (26) was selected as the most appropriate study based on the number of subjects and the availability of sufficient patient and treatment information to allow meaningful comparisons. Specifically, data were reported for wound area and duration, patient age and gender, and treatment assessments were made at 12 and 20 weeks. This study also provided the largest and best‐controlled description of DFU treatment endpoints for standard wet‐to‐moist wound therapy in the published literature (henceforth, the terms ‘standard’ and ‘wet‐to‐moist’ are used interchangeably). The authors pooled data on 586 patients with neuropathic DFUs who had participated in the control groups of five different RCTs published between 1992 and 1998. Inclusion criteria for the control groups included chronic wounds categorised as diabetic/neuropathic ulcers, appropriate offloading, as needed, the presence of adequate perfusion, infection control (if present) and debridement of necrotic tissue.
Patients in the control group received well‐monitored care typical of that provided in a controlled clinical trial environment; however, intermittent dressing changes were provided in an outpatient or home care setting. Thus, some variability was inevitable because the study treatments were applied and wound dressings were changed by patients or caregivers outside of the study visits.
Treatment endpoints
Treatment endpoints were defined for each group. For the NPWT group, a wound was deemed to have reached a successful treatment endpoint if closure through secondary intention or through a surgical intervention or if adequate granulation for closure by these methods was documented. Surgical interventions included flaps, grafts and primary closure. For the control group, which included patients enrolled in different trials, a wound was deemed to have reached a successful treatment endpoint when either of the following was documented: wound completely healed, i.e. wound closure (no drainage) or full epithelialisation with no drainage. Outcomes in both the NPWT and the control groups were evaluated from the perspective of no longer requiring wound care services and thus represent the attainment of successful treatment endpoints and expected discharge from wound care clinical services.
The proportions of wounds attaining a successful treatment endpoint in the two groups were compared after 12 and 20 weeks of treatment. For consistency with patients in the control group, initial wound sizes in the NPWT group were stratified into three categories: small wounds with a starting area of less than 2 cm2, medium‐sized wounds with an area of 2–4 cm2 and large wounds with an area larger than 4 cm2. Similarly, wound durations were stratified into three categories: short duration (less than 6 months), medium duration (6–12 months) and long duration (more than 12 months).
We also investigated whether variables such as initial wound size and duration predict the outcome of treatment with NPWT using logistic regression.
Economic analysis
To assess the differential cost of care in the outpatient setting between the NPWT and the control (wet‐to‐moist therapy) groups, we calculated the 20‐week expected cost of care for a patient in each group. The 20‐week treatment endpoint has been used in other cost‐effectiveness analyses and represents a relatively standardised measure of effectiveness (30). The expected cost is defined as follows:
where E(C) is the expected cost, c is the weekly cost and p t is the probability of being successfully treated in week t. p t is obtained from linear interpolation of the 12 and 20 weeks’ successful treatment rates. The weekly cost consists of nursing visits, supplies and physician costs. For nursing visits, we assumed either one or two home visits per day at $112 per visit for the wet‐to‐moist therapy group, based on data reported by Ovington 31, 32, with costs adjusted for inflation at ∼4% per annum for 3 years. For each scenario, the cost of wet‐to‐moist therapy supplies is based on three daily dressing changes, and we have assumed that the additional dressing changes each day that are not performed by a nurse are performed by another caregiver, such as a family member.
NPWT requires dressing changes every 48 hours, based on standard usage guidelines (33). Supply costs of $3·50 per dressing for wet‐to‐moist dressings [inflation‐adjusted figures based on Sebern (34) and Alterescu 35, 36] and $107 per day rental for NPWT were applied (37). We assumed physician costs to be based on one visit every 2 weeks at $66 per visit. The numbers of weekly physician visits were assumed to be equivalent in the two treatment groups.
Statistical analyses
Statistical analyses were performed using mathlab (Mathworks, Natick, MA). All univariate comparisons by treatment (wet‐to‐moist versus NPWT) were performed using Fisher’s exact test for categorical variables and t‐test for continuous variables (38). Logistic regression analysis was also used for multivariable analysis using a generalised linear model with an underlying binomial distribution and a logistic link function (39). Statistical significance was defined as P < 0·05 with a two‐tailed test.
Results
The KCI Medicare database contained data from 2209 patients with DFUs who had undergone NPWT. Of these, data on 2091 wounds (from 2091 patients) satisfied the inclusion criteria, and this group comprised the ‘unmatched’ NPWT data set. As shown in Table 1, the demographic characteristics of this group differed from those of the control group (n = 586) in several respects: the NPWT population consisted of older patients, and the wounds were of shorter duration and larger. To more closely match the demographic characteristics of the control group, a matched data set was therefore constructed from the KCI Medicare database by excluding patients older than 70 years and those with wounds of less than 1 month in duration. This resulted in a ‘matched’ NPWT data set (n = 1135) whose demographics approximated more closely those of the control group (Table 1).
Table 1.
Demographic features of patients in the control (wet‐to‐moist therapy) group (26) and the NPWT groups with and without matching
| Characteristic | Control group | NPWT‐unmatched group | NPWT‐matched group |
|---|---|---|---|
| No. of patients | 586 | 2091 | 1135 |
| Age, years (mean) | 58 | 65·2 ± 12·7 | 58·5 ± 9·4 |
| Gender (% male) | 73·2 | 64·5* | 64·5* |
| Initial wound size, cm2 (mean) | 1·61 | 13·5 ± 16·0 | 13·8 ± 15·8 |
| Wound duration, weeks (mean) | 30 | 22·9 ± 24·0 | 26·5 ± 24·7 |
NPWT, negative pressure wound therapy.
P < 0·01 using a Fisher’s exact test for comparing the observed proportion with that seen in the control group.
Even after matching, the NPWT group differed from the control group, notably with regard to the initial wound size. This is indicative of the differences in the enrolled population of a controlled clinical trial environment versus a real‐world setting. To enable a comparison and better account for these differences, which could potentially have affected the results, the data were stratified by initial wound size and wound duration, in the same manner as the analysis undertaken by Margolis et al. (26). Stratification of the data showed a greater prevalence of shorter duration and larger wounds in the NPWT group, whereas the wet‐to‐moist therapy group showed a greater prevalence of long duration and smaller wounds. This reflects the influence that a reimbursement coverage policy may have on the treatment selection criteria for patients with DFU, as the Medicare NPWT policy clearly states that other therapies must be utilised or considered prior to use of NPWT.
Successful treatment endpoints
Overall, 39·5% of wounds in the matched NPWT group achieved a successful treatment endpoint with NPWT after 12 weeks compared with 23·9% of wounds that attained a successful treatment endpoint with standard wet‐to‐moist therapy (P < 0·001) (Figure 1). Similarly, after 20 weeks, 46·3% of wounds treated with NPWT achieved a successful treatment endpoint compared with only 32·8% of wounds treated with wet‐to‐moist therapy (P < 0·001).
Figure 1.
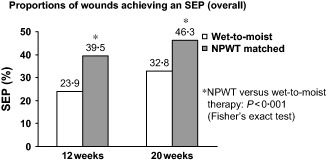
Proportion of wounds achieving a successful treatment endpoint (SEP) after 12 and 20 weeks for the wet‐to‐moist therapy (control) group from Margolis et al. (26) and the matched NPWT group.
When the wounds were stratified by initial size (Figure 2), wounds of all sizes treated with NPWT were more likely to reach a successful treatment endpoint after both 12 weeks (P < 0·05) and 20 weeks (P < 0·05) than wounds treated with wet‐to‐moist therapy. The trend was more apparent after 12 weeks of treatment than after 20 weeks but consistently more wounds achieved a successful treatment endpoint after 12 weeks of NPWT than after 20 weeks of wet‐to‐moist therapy. Thus, use of NPWT increased the overall proportion of DFUs that reached a successful treatment endpoint for all wound sizes. The higher proportion of wounds of all sizes successfully treated with NPWT at 12 weeks in comparison with those successfully treated with standard therapy at 20 weeks (Figure 2) indicates that NPWT increases the speed with which a successful outcome is reached.
Figure 2.
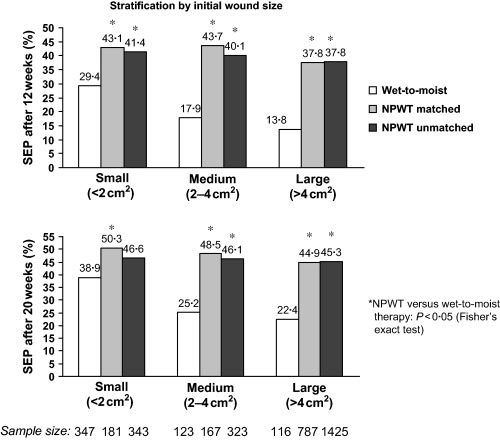
Proportion of wounds achieving a successful treatment endpoint (SEP) after 12 weeks (top) and 20 weeks (bottom). The data are stratified by initial wound size and are shown separately for the wet‐to‐moist therapy (control) group from Margolis et al. (26) and for the unmatched and matched NPWT groups.
When the wounds were stratified by duration (Figure 3), a higher proportion of NPWT‐treated wounds achieved a successful treatment endpoint than wounds treated with wet‐to‐moist therapy. After 12 weeks of treatment, the higher successful treatment rate with NPWT was statistically significant for all wounds except those of between 6 and 12 months duration, for which the sample size in the control group was considerably smaller. After 20 weeks of treatment, the higher successful treatment rate with NPWT reached significance only for wounds of more than 1 year in duration (P < 0·05) (Figure 3).
Figure 3.
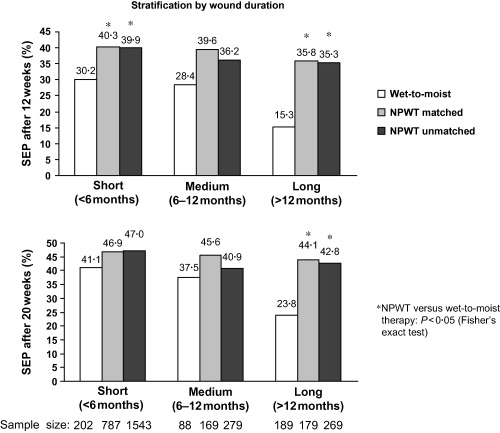
Proportion of wounds achieving a successful treatment endpoint (SEP) after 12 weeks (top) and 20 weeks (bottom). The data are stratified by wound duration and are shown separately for the wet‐to‐moist therapy (control) group from Margolis et al. (26) and for the unmatched and matched NPWT groups.
Logistic regression analysis, performed to determine the dependence of DFU healing with NPWT on wound variables, showed that as with a study by Margolis et al. (27), the initial wound size predicted treatment success with NPWT (P < 0·05), such that smaller wounds tended to heal with greater success than larger wounds. In contrast, the wound duration was not associated with treatment success (P > 0·3).
Expected 20‐week costs of NPWT and wet‐to‐moist therapy
Table 2 depicts the assumptions underlying the weekly treatment cost estimates and indicates the expected 20‐week costs for NPWT and standard wet‐to‐moist therapy. For the latter group, the 20‐week expected costs were evaluated for two scenarios: one nursing visit per day and two nursing visits per day.
Table 2.
Comparison of 20‐week costs for NPWT and two scenarios of wet‐to‐moist therapy with differing numbers of nursing visits per day
| Cost | NPWT group | Control group (wet‐to‐moist therapy) | |
|---|---|---|---|
| One nursing visit/day | Two nursing visits/day | ||
| Weekly nursing cost ($) | 336 | 784 | 1568 |
| Number of visits/week | 3 | 7 | 14 |
| Cost/visit* ($) | 112 | 112 | 112 |
| Weekly physician cost ($) | 33 | 33 | 33 |
| Number of visits/week | 0·5 | 0·5 | 0·5 |
| Cost/visit ($) | 66 | 66 | 66 |
| Weekly treatment cost ($) | 749 | 74 | 74 |
| Applications/week | 7 | 21 | 21 |
| Cost/application ($) | 107 | 3·50 | 3·50 |
| Total weekly cost ($) | 1118 | 891 | 1675 |
| 20‐week expected cost† ($) | 16 733 | 15 258 | 28 691 |
NPWT, negative pressure wound therapy.
Cost/visit is based on $112/visit, as reported by Ovington 31, 32, and adjusted by ∼4% per annum for 3 years to account for inflation. Such a fee‐for‐service reimbursement is only true for some private payors, but payment reflects cost‐to‐system for a nursing visit and is therefore relevant to a Medicare setting as well.
Incorporates therapy outcomes as well as direct equipment/medical personnel costs (see text for method of calculation).
The expected 20‐week costs associated with NPWT are similar to those of wet‐to‐moist therapy for one nursing visit per day, and 42% less than those of wet‐to‐moist therapy for two nursing visits per day. For NPWT, the 20‐week expected costs of therapy were $16 733. For wet‐to‐moist therapy, they were $15 258 and $28 691, respectively, for the one or two nursing visits per day scenarios.
Discussion
NPWT is a common treatment modality for DFUs, but few studies have compared NPWT with standard wound therapy. The present study is the first large‐scale, retrospective analysis of the efficacy of NPWT versus wet‐to‐moist therapy for DFUs. The results suggest that NPWT increases the proportion of wounds that reach a successful treatment endpoint at 12 and 20 weeks of treatment. These findings are consistent with the results of smaller, prospective studies showing that NPWT helps promote wound healing and helps decrease wound surface area (19) or volume and depth (18) compared with conventional dressings. Additionally, the results are consistent with those of a large RCT that reported healing of diabetic foot amputation wounds with NPWT compared with a control group that received standard moist wound care (21).
To evaluate outcomes with NPWT, the present study relied on a database used to process DME claims for reimbursement of treatment costs. The data evaluated in this retrospective analysis were for claims by patients with neuropathic diabetic ulcers. As such, it has the associated limitations of a retrospective comparison. Additionally, the available data points are limited as they are based solely on the criteria outlined in Medicare’s DME coverage policy. On the contrary, an advantage of the database is the consistency of clinical information provided because complete records are required for reimbursement.
The control (wet‐to‐moist therapy) group relied on a robust meta‐analysis of wound healing from several RCTs of non infected, non ischemic diabetic foot wounds (26), and differences between this group and the NPWT group in some patient characteristics and inclusion criteria may reflect inherent differences between a RCT setting and a real‐world treatment setting. While the control group was an historical one, we believe it provided an interesting benchmark from which to compare outcomes and represented the most viable and comprehensive comparison group available. The risks and assumptions of using an historical control group are acknowledged. As patients in the NPWT group were derived from a real‐world clinical practice setting, they were not randomised to treatment. Thus, while the two populations in this study appeared to be similar in terms of wound characteristics, definitive conclusions on their comparability cannot be drawn.
The present study did not allow the opportunity for patient screening to detect comorbid conditions in the NPWT group that may have influenced treatment outcomes. It is assumed that because V.A.C.® therapy requires a physician’s prescription order for reimbursement and that the coverage policy requires documentation of wound‐healing progress, ‘good wound care’ practices were being followed. Part of good wound care includes ensuring adequate circulation to the wound site and ruling out peripheral vascular disease prior to wound treatment with NPWT. However, it cannot be assured that all patients with DFU who received NPWT were non ischemic or non infected. In contrast, for the control group, adequate perfusion is an established criterion for enrolment in RCTs, and it would be expected that this criterion was adhered to. An additional potential limitation is the consistency of wound location, as this is not a criterion for reimbursement purposes. Thus, treatment locations were not controlled for in the present study, and the patient populations differed for several variables such as patient age, gender, initial wound size and wound duration. Although a matching procedure was carried out to equalise mean patient ages and wound durations, some differences in the treatment groups may have remained.
While these between‐group differences could have had some influence on the findings of the study, we believe they were adequately addressed and did not have a significant impact on the results or bias them in favour of NPWT. In fact, any differences between the two groups based on wound severity, size and the lack of any documented comorbidities or vascular assessments would tend to bias the results against the V.A.C.® therapy group. If peripheral vascular disease or significant comorbid conditions were present in the NPWT patient group, these factors would tend to create a greater clinical challenge and the possibility of a poorer treatment outcome in comparison with the control group. In contrast, these comorbid or complicating factors are ruled out or minimised in controlled clinical trials, which could result in a greater likelihood of treatment success for wet‐to‐moist therapy.
Some variability existed in the endpoint definitions used in the present study; however, we believe that defining the study endpoint as ‘treatment success’ no longer requiring wound care services is reasonable. Each data source accurately measured the percentage of wounds that was treated successfully. The endpoint used for the NPWT group was a discharge record generated after successful treatment. Reasons for discontinuation of treatment included surgical closure, secondary closure and adequate granulation. Because NPWT is typically used to create a healthy layer of granulation tissue, wounds in the NPWT database were not typically tracked to the point of full epithelialisation. For the control wet‐to‐moist therapy group, which comprised pooled data from several clinical trial studies (26), the endpoints were ‘healed’ or ‘completely healed’. Consequently, it is likely that some wounds in the wet‐to‐moist therapy group could have been tracked to full epithelialisation. The present study was based on the best available comparative data source at the time of analysis. Further studies are encouraged to confirm its findings.
Some clinicians may reserve NPWT for large or more severe wounds (16). The present study showed that NPWT was disproportionately used for larger wounds, and the results showed substantial benefit with NPWT in such cases. However, the results also suggest that NPWT may provide clinical benefit for small wounds, which tended to heal faster than larger wounds. Although not surprising, this finding strongly supports the use of NPWT on small wounds. Future studies should evaluate potential differences in the efficacy of this treatment based on wound size.
In contrast, wound duration did not appear to influence treatment success. It is possible that this finding was a result of the poor temporal resolution for wound duration. However, Figure 3 shows that this is unlikely because the treatment success rate for wounds treated with NPWT did not differ between the various wound durations, whereas it did for wet‐to‐moist therapy. This implies that the treatment success rate with NPWT is similar regardless of wound duration, which contrasts with the findings of Margolis et al. (26), who reported that wound duration was one of the key predictors for healing success with wet‐to‐moist therapy. This reinforces the assertion that NPWT can be successful in instances in which other treatments have failed to generate the desired outcome 16, 24, 40.
The economic analysis of NPWT versus wet‐to‐moist therapy showed that NPWT can result in enhanced treatment outcomes for DFUs at an equivalent or potentially lower overall cost in the home care setting. If nursing visits on a once‐daily basis for those receiving wet‐to‐moist therapy are assumed, the 20‐week expected costs of NPWT are similar to those of wet‐to‐moist therapy (Table 2). If two nursing visits per day are assumed, NPWT costs 42% less than wet‐to‐moist therapy over a 20‐week period. The economic differential associated with NPWT incorporated therapy outcomes as well as the direct costs of equipment and medical personnel time. This illustrates the importance of evaluating cost implications in relation to therapy outcomes rather than simply evaluating unit costs of dressings or equipment. The potential cost savings associated with NPWT are realised because of the requirement for fewer weekly nursing visits (with substantial weekly cost savings) and the achievement of higher successful treatment rates in a shorter time. It is important to emphasise that if treatment decision‐makers focus only on the larger weekly supplies/rental costs for NPWT ($749) versus for those for saline gauze ($74), the potential cost savings would be grossly overlooked. Instead, by taking a comprehensive approach to the treatment of patients with DFU, a robust representation of the overall cost‐efficiency of NPWT can be seen. In fact, the present study is rather conservative in that other potential clinical and economic benefits of NPWT, such as a reduction in amputation rates and total treatment costs and improvement of quality of life, were not considered. Preliminary evidence suggests that patients receiving NPWT tend to have a lower incidence of amputations in comparison with standard therapies (21). With estimated costs of more than $73 000 per patient for amputations [direct costs plus subsequent costs arising from prosthesis fabrication, fitting and rehabilitation, and maintaining bipedal ambulation (41)], the potential economic savings to the health care system associated with a modality such as NPWT versus standard therapy could be very substantial when taking into account reductions in amputations alone.
This retrospective study of NPWT in patients with DFU is useful in that it provides additional information related to effective treatment of this increasingly prevalent disease condition. However, more research in real‐world settings is needed to further confirm the findings of the present study and the cost‐effectiveness of NPWT.
Conclusions
Foot ulcers are a leading cause of hospitalisation for patients with diabetes and are a major source of morbidity and health care resource usage (1). This study provides further support for NPWT, improving successful wound treatment outcomes for DFUs in comparison with standard wound therapy and reducing the treatment time until a successful outcome is achieved. It also provides data to suggest that use of NPWT for treating DFUs in the home care setting may have favourable real‐world economic advantages that can potentially benefit the health care system. As such, the study provides important data to facilitate the design and conduct of further large‐scale RCTs to examine the utility of NPWT in the treatment of DFUs and its potential to avoid amputations.
Acknowledgements
This research was sponsored, in part, by Kinetic Concepts Inc., San Antonio, TX. Drs LAL and JAN have received grants for educational activities from Kinetic Concepts Inc. Dr AJB has served on an Advisory Board and Dr PS on a Speaker’s Bureau for Kinetic Concepts Inc.
References
- 1. Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, Wagner EH. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999;22:382–7. [DOI] [PubMed] [Google Scholar]
- 2. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–28. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association . Consensus Development Conference on Diabetic Foot Wound Care: 7–8 April 1999, Boston Massachusetts. Diabetes Care 1999;22:1354–60. [DOI] [PubMed] [Google Scholar]
- 4. Larsson J, Agardh CD, Apelqvist J, Stenstrom A. Long‐term prognosis after healed amputation in patients with diabetes. Clin Orthop 1998;350:149–58. [PubMed] [Google Scholar]
- 5. Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care 1990;13:513–21. [DOI] [PubMed] [Google Scholar]
- 6. Moss SE, Klein R, Klein BE. The 14‐year incidence of lower‐extremity amputations in a diabetic population. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care 1999;22:951–9. [DOI] [PubMed] [Google Scholar]
- 7. Center for Disease Control and Prevention . National diabetes fact sheet, 2003. [WWW document]. URL http://www.cdc.gov/diabetes/pubs/factsheet.htm [accessed on 13 December 2005].
- 8. Peters EJ, Childs MR, Wunderlich RP, Harkless LB, Armstrong DG, Lavery LA. Functional status of persons with diabetes‐related lower‐extremity amputations. Diabetes Care 2001;24:1799–804. [DOI] [PubMed] [Google Scholar]
- 9. Coffey JT, Brandle M, Zhou H, Marriott D, Burke R, Tabaei BP, Engelgau MM, Kaplan RM, Herman WH. Valuing health‐related quality of life in diabetes. Diabetes Care 2002;25:2238–43. [DOI] [PubMed] [Google Scholar]
- 10. Sandnes DK, Sobel M, Flum DR. Survival after lower‐extremity amputation. J Am Coll Surg 2004;199:394–402. [DOI] [PubMed] [Google Scholar]
- 11. Mayfield JA, Reiber GE, Maynard C, Czerniecki JM, Caps MT, Sangeorzan BJ. Survival following lower‐limb amputation in a veteran population. J Rehabil Res Dev 2001;38:341–5. [PubMed] [Google Scholar]
- 12. Tentolouris N, Al‐Sabbagh S, Walker MG, Boulton AJ, Jude EB. Mortality in diabetic and nondiabetic patients after amputations performed from 1990 to 1995: a 5‐year follow‐up study. Diabetes Care 2004;27:1598–604. [DOI] [PubMed] [Google Scholar]
- 13. Faglia E, Favales F, Morabito A. New ulceration, new major amputation, and survival rates in diabetic subjects hospitalized for foot ulceration from 1990 to 1993: a 6.5‐year follow‐up. Diabetes Care 2001;24:78–83. [DOI] [PubMed] [Google Scholar]
- 14. Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new‐onset diabetic foot ulcers stratified by etiology. Diabetes Care 2003;26:491–4. [DOI] [PubMed] [Google Scholar]
- 15. Muller IS, De Grauw WJ, Van Gerwen WH, Bartelink ML, Van Den Hoogen HJ, Rutten GE. Foot ulceration and lower limb amputation in type 2 diabetic patients in dutch primary health care. Diabetes Care 2002;25:570–4. [DOI] [PubMed] [Google Scholar]
- 16. Armstrong DG, Attinger CE, Boulton AJ, Frykberg RG, Kirsner RS, Lavery LA, Mills JL. Guidelines regarding negative wound therapy (NPWT) in the diabetic foot. Ostomy Wound Manage 2004;50(4B Suppl):3S–27S. [PubMed] [Google Scholar]
- 17. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–77. [PubMed] [Google Scholar]
- 18. Eginton MT, Brown KR, Seabrook GR, Towne JB, Cambria RA. A prospective randomized evaluation of negative‐pressure wound dressings for diabetic foot wounds. Ann Vasc Surg 2003;17:645–9. [DOI] [PubMed] [Google Scholar]
- 19. McCallon SK, Knight CA, Valiulus JP, Cunningham MW, McCulloch JM, Farinas LP. Vacuum‐assisted closure versus saline‐moistened gauze in the healing of postoperative diabetic foot wounds. Ostomy Wound Manage 2000;46:28–32, 34. [PubMed] [Google Scholar]
- 20. Page JC, Newswander B, Schwenke DC, Hansen M, Ferguson J. Retrospective analysis of negative pressure wound therapy in open foot wounds with significant soft tissue defects. Adv Skin Wound Care 2004;17:354–64. [DOI] [PubMed] [Google Scholar]
- 21. Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–10. [DOI] [PubMed] [Google Scholar]
- 22. Clare MP, Fitzgibbons TC, McMullen ST, Stice RC, Hayes DF, Henkel L. Experience with the vacuum assisted closure negative pressure technique in the treatment of non‐healing diabetic and dysvascular wounds. Foot Ankle Int 2002;23:896–901. [DOI] [PubMed] [Google Scholar]
- 23. Armstrong DG, Lavery LA, Abu‐Rumman P, Espensen EH, Vazquez JR, Nixon BP, Boulton AJ. Outcomes of subatmospheric pressure dressing therapy on wounds of the diabetic foot. Ostomy Wound Manage 2002;48:64–8. [PubMed] [Google Scholar]
- 24. Philbeck TE Jr, Schroeder WJ, Whittington KT. Vacuum‐assisted closure therapy for diabetic foot ulcers: clinical and cost analyses. Home Health Care Consultant 2001;8:27–34. [Google Scholar]
- 25. Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta‐analysis. Diabetes Care 1999;22:692–5. [DOI] [PubMed] [Google Scholar]
- 26. Margolis DJ, Kantor J, Santanna J, Strom BL, Berlin JA. Risk factors for delayed healing of neuropathic diabetic foot ulcers: a pooled analysis. Arch Dermatol 2000;136:1531–5. [DOI] [PubMed] [Google Scholar]
- 27. Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: the association of wound size, wound duration, and wound grade on healing. Diabetes Care 2002;25:1835–9. [DOI] [PubMed] [Google Scholar]
- 28. Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: predicting which ones will not heal. Am J Med 2003;115:627–31. [DOI] [PubMed] [Google Scholar]
- 29. Palmetto DMERC Policy on NPWT . Negative pressure wound therapy pumps, 2002. [WWW document]. URL http://www.palmettogba.com [accessed on 28 May 2006].
- 30. Kantor J, Margolis DJ. Treatment options for diabetic neuropathic foot ulcers: a cost‐effectiveness analysis. Dermatol Surg 2001;27:347–51. [DOI] [PubMed] [Google Scholar]
- 31. Ovington LG. Hanging wet‐to‐dry dressings out to dry. Home Healthc Nurse 2001;19:477–83. [DOI] [PubMed] [Google Scholar]
- 32. Ovington LG. Hanging wet‐to‐dry dressings out to dry. Adv Skin Wound Care 2002;15:79–84. [Google Scholar]
- 33. Kinetic Concepts Inc . V.A.C.® therapy clinical guidelines, 2005. [WWW document]. URL http://www.kci1.com/2‐B‐128_Clin_Guidelines_Blue_Book_1‐05.pdf [accessed on 8 March 2006].
- 34. Sebern MD. Pressure ulcer management in home health care: efficacy and cost effectiveness of moisture vapor permeable dressing. Arch Phys Med Rehabil 1986;67:726–9. [DOI] [PubMed] [Google Scholar]
- 35. Alterescu V. The financial costs of inpatient pressure ulcers to an acute care facility. Decubitus 1989;2:14–23. [PubMed] [Google Scholar]
- 36. Alterescu V. Some fundamental concepts of accounting for nurses. J Enterostomal Ther 1989;16:125–8. [PubMed] [Google Scholar]
- 37. Philbeck TE Jr, Whittington KT, Millsap MH, Briones RB, Wight DG, Schroeder WJ. The clinical and cost effectiveness of externally applied negative pressure wound therapy in the treatment of wounds in home healthcare Medicare patients. Ostomy Wound Manage 1999;45:41–50. [PubMed] [Google Scholar]
- 38. Zar JH. Biostatistical analysis, 4th edn. Upper Saddle River, NJ: Prentice‐Hall International, 1999. [Google Scholar]
- 39. McCullagh P, Nelder JA. Generalized linear models. London, New York: Chapman and Hall, 1989. [Google Scholar]
- 40. Joseph E, Hamori CA, Bergman S, Roaf E, Swann NF, Anastasi GW. A prospective randomized trial of vacuum‐assisted closure versus standard therapy of chronic nonhealing wounds. Wounds 2000;12:60–7. [Google Scholar]
- 41. Niezgoda JA, Page JC, Kaplan M. The economic value of negative pressure wound therapy. Ostomy Wound Manage 2005;51(2A Suppl):44–7. [PubMed] [Google Scholar]


