Abstract
Previous research has shown −125 mmHg to be the optimal negative pressure for creating an environment that promotes wound healing, and this has therefore been adopted as a standard pressure for patients with deep sternal wound infection. However, it has not yet been clearly shown that −125 mmHg is the optimal pressure from a haemodynamic point of view. Furthermore, there have been reports of cardiac rupture during −125 mmHg negative pressure therapy. We therefore studied the effects of a lower pressure: −75 mmHg. Twelve pigs were used. After median sternotomy, sealed negative pressure therapy of −75 mmHg was applied. Baseline measurements were made and continuous recording of the cardiac output, end‐tidal CO2 production, mean arterial pressure, mean pulmonary pressure (pulmonary artery pressure), systemic vascular resistance, pulmonary vascular resistance, left atrial pressure and central venous pressure was started. Six pigs served as controls. No statistically significant difference was observed in any of the haemodynamic parameters studied, compared with the controls. The present study shows that, with a suitable foam application technique, −75 mmHg can be applied without compromising the central haemodynamics.
Keywords: Animal model, Experimental surgery, Haemodynamics, Mediastinum, Wound closure
Introduction
Deep sternal wound infection (DSWI) following cardiac surgery is a rare (0·5–3·2%) but devastating complication with a reported mortality rate between 7% and 36% 1, 2, 3, 4. Conventional forms of treatments involve surgical revision with open dressing, closed irrigation and reconstruction with vascularised soft tissue flaps such as omentum or pectoral muscle flaps. Several studies have reported promising results with the use of topical negative pressure wound therapy [vacuum‐assisted closure (VAC)] in patients with DSWI after cardiac surgery 5, 6, 7, 8, 9. Negative pressure wound therapy combines improved wound healing and stabilisation of the open sternum. Previous basic research in pig models has shown −125 mmHg to be the optimal negative pressure for creating an environment that promotes wound healing, and this pressure has therefore been adopted as a standard pressure for patients with postoperative DSWI (10). However, it has not yet been clearly shown that −125 mmHg is the optimal pressure from a haemodynamic point of view or whether negative pressure wound therapy causes changes in the haemodynamics. Furthermore, there have been reports of life‐threatening complications, such as cardiac rupture, during high‐pressure (−125 mmHg) suction drainage 11, 12, 13. The organs in the mediastinum are susceptible to mechanical forces; too great a negative pressure on the cardiac wall might impair the right ventricle function or compromise the function of coronary bypass grafts.
Two previous animal studies suggested that −125 mmHg negative pressure therapy applied to the heart through a median sternotomy incision (open sternal wounds) had negative haemodynamic effects resulting in a decrease in cardiac output (CO) 14, 15. In an earlier study, we showed that negative pressures of −50 to −175 mmHg did not impair the central haemodynamics in a porcine sternotomy wound model over a 30‐minute period (16).
The aim of the present study was to evaluate changes in haemodynamics during application of negative pressure therapy at −75 mmHg over a 5‐hour period in a realistic large animal sternotomy wound model compared with a sham‐operated control group.
Methods
Animals
Twelve Swedish domestic pigs with a mean weight of 70 ± 2 kg (range 68–72 kg) were used. All the animals received care in compliance with the European Convention on Animal Care. Ethics Committee for Animal Research at Lund University, Sweden, approved the experimental protocol for this study. All experiments were performed on the same group of pigs during a 1‐week period. The 12 pigs used in this study were selected 1–2 weeks before the planned start of the study. At the breeding farm, they were allowed to move freely between indoor/outdoor environments. All animals arrived at the research facility 5 days before the start of the study. The animals were fastened overnight the day before the experiment, but had free access to water.
Anaesthesia and surgical preparation
All the animals received premedication consisting of intramuscular ketamine (100 mg/ml; Ketaminol vet, Farmaceutici Gellini S.p.A, Aprilia, Italy) at 15 mg/kg body weight, combined with midazolam (1 mg/ml; Dormicum, Roche, Stockholm, Sweden) and xylazine (20 mg/ml; Rompun vet, Bayer AG, Leverkusen, Germany) at 2 mg/kg, in the animal box before being transferred to the laboratory. Anaesthesia was induced by continuous intravenous infusion of propofol (20 mg/ml; Diprivan, AstraZeneca, Södertälje, Sweden) at a dosage of 0·1–0·2 ml/kg/min in combination with intermittent fentanyl (Leptanal; Jenssen‐Cilag, Sollentuna, Sweden) and atracurium besylate (Tracrium; Glaxo, Täby, Sweden) at doses of 0·02 μg/kg and 0·2–0·5 mg/kg, respectively. Before surgery a tracheotomy was performed (Portex tracheal tube, 7·5 mm internal diameter; SIMS Portex, Keene, NH). A ventilator (Servo‐Ventilator 900; Elema‐Schönander, Sweden) was used for mechanical ventilation. The same settings were used for all animals: volume controlled, 8·5 l/min, 15 breaths/min, positive end‐expiratory pressure 5 cm H2O and an inhaled oxygen fraction of 35%. The arterial pressure was monitored via a catheter in the left carotid artery. A double‐lumen central venous catheter was inserted into the left external jugular vein. A sternotomy was performed, the heart was exposed and the pulmonary trunk was dissected free; a flow transducer (CardioMed TraCe System; Medi‐stim, Oslo, Norway) was connected to the pulmonary artery to enable continuous measurement of CO of the animal. The left atrial pressure (LAP) was measured with a catheter through the left atrial appendage. The pressure in the pulmonary artery was measured with a line into the vessel. The haemodynamic data were collected with a data acquisition system (PowerLab; AD Instruments Ltd, Castle Hill, Australia).
A lower midline abdominal incision was made over the urinary bladder. The urinary bladder was exposed and a urinary catheter (Silicone Foley Catheter; Tyco Healthcare, Tullamore, Ireland) was inserted, sutured and connected to a urine‐collection bag (Unomedical a/s, Haarlev, Denmark). The abdominal incision was continuously sutured with Dermalon 2.0 (Davis‐Geck, Hampshire, UK). A midline sternotomy was performed and the pleurae were routinely opened. A layer of polyurethane foam (KCI, Copenhagen, Denmark) was placed between the sternal edges. A second layer of foam was placed over the first layer and sutured to the skin. Two evacuation tubes (KCI) were inserted into the layers of foam. The wound was sealed with a transparent adhesive drape (KCI) and the two tubes were connected to a continuous suction source supplying a pressure of −75 mmHg (V.A.C. pump unit; KCI). The procedure was performed in the same way as that on the clinical patients with the exception of the paraffin gauze coverage of the heart. The control pigs, termed ‘sham therapy’, underwent the same surgical procedure, except that the pump unit was not turned on.
Experimental protocol
After 1 hour of stabilisation, baseline measurements were made and continuous recording of the he mean arterial pressure (MAP), central venous pressure, heart rate (HR), CO, systemic vascular resistance (SVR), pulmonary vascular resistance (PVR), pulmonary artery pressure (PAP) and LAP was started. The 1‐hour stabilisation period (baseline) was followed by the application of continuous negative pressure (VAC system) of −75 mmHg to the sternotomy wound in six pigs. The remaining six pigs, ‘sham therapy’ served as controls.
Data collection and analysis
MAP, central venous pressure, CO, PAP, HR, SVR, PVR, left atrium pressure and ventilatory parameters were sampled continuously (mean values of single values every 5 seconds) throughout the experiments using a computer equipped with data acquisition system (PowerLab; AD Instruments Ltd, Castle Hill, Australia).
The experiments were performed on 12 pigs. Six served as control and six were treated with topical negative pressure. First, the pigs were allowed to stabilise without treatment for 30 minutes. At the end of this period, a baseline value was recorded (baseline). Every hour after the onset of therapy (negative pressure therapy or control), a new value was recorded and statistically compared with baseline. Statistical analysis was performed using Kruskal–Wallis test with Dunn’s test for multiple comparisons. Significance was defined as P < 0·05. Values are presented as means ± SEM.
Results
All animals subjected to the application of −75 mmHg negative pressure to the sternotomy wound survived the experiment. The total duration of the experiment was almost 6 hours per animal. No statistically significant difference was observed in CO (Figure 1), end‐tidal CO2 concentration (Figure 2), MAP (Figure 3) or mean pulmonary pressure (Figure 4) between the pigs treated with −75 mmHg negative pressure and the control pigs. Neither were there any statistically significant differences in SVR (Figure 5), PVR (Figure 6), LAP or central venous pressure between the pigs treated with −75 mmHg negative pressure and the control pigs.
Figure 1.
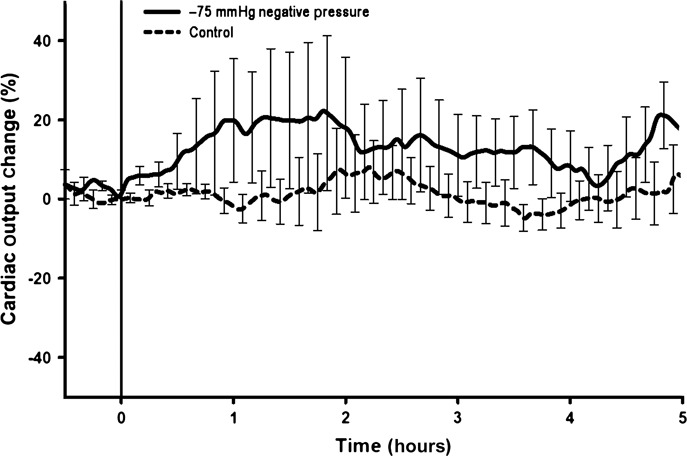
Cardiac output (CO) change (%) during negative pressure application at −75 mmHg over a 5‐hour period. No significant change in CO change was seen compared with the control group.
Figure 2.
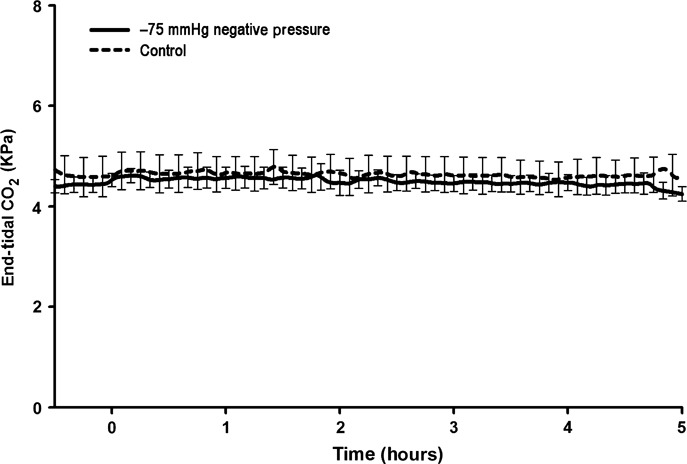
End‐tidal CO2 concentration (KPa) during negative pressure application at −75 mmHg over a 5‐hour period. No significant change in end‐tidal CO2 was found compared with the control group.
Figure 3.
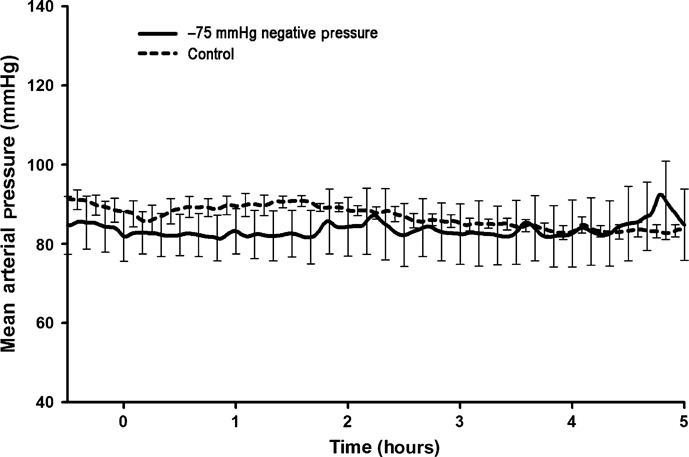
Mean arterial pressure (MAP) (mmHg) during negative pressure application at −75 mmHg over a 5‐hour period. No significant change in MAP was seen compared with the control group.
Figure 4.
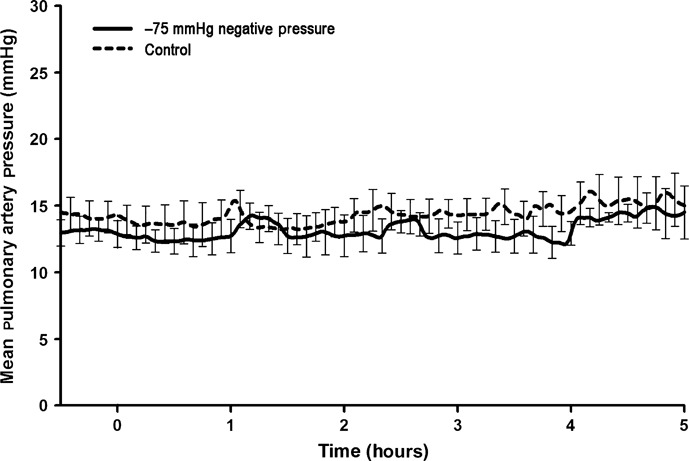
Mean pulmonary artery pressure (PAP) (mmHg) during negative pressure application at −75 mmHg over a 5‐hour period. No significant change in mean PAP was found compared with the control group.
Figure 5.
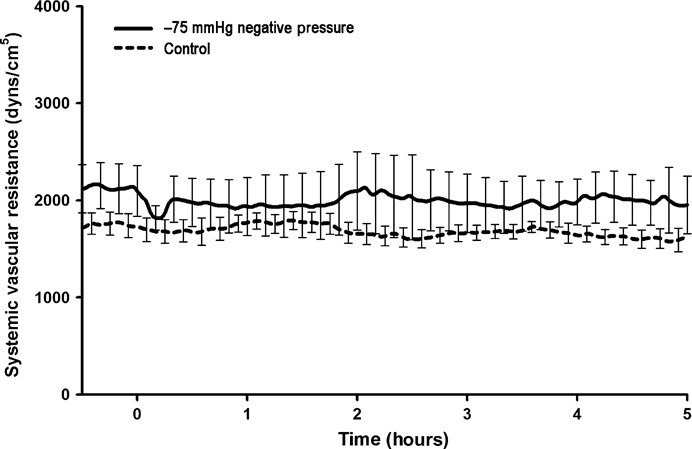
Systemic vascular resistance (SVR) (dyn s/cm5) during negative pressure application at −75 mmHg over a 5‐hour period. No significant change in SVR was observed compared with the control group.
Figure 6.
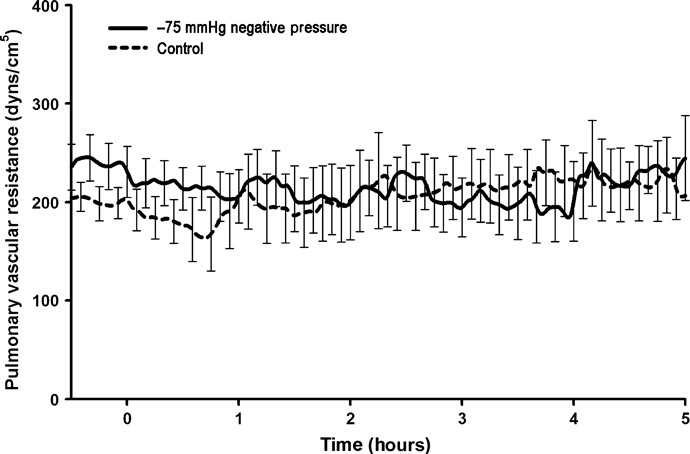
Pulmonary vascular resistance (PVR) (dyn s/cm5) during negative pressure application at −75 mmHg over a 5‐hour period. No significant change in PVR was found compared with the control group.
Discussion
Postoperative DSWI is a rare but potentially lethal complication after open heart surgery, and negative pressure wound therapy of −125 mmHg has been reported to be successful in the treatment of this condition 5, 6, 7, 8, 9. The application of subatmospheric pressure to the mediastinum combines several advantageous features. Negative pressure wound therapy allows open drainage of exudates with simultaneous stabilisation of the chest and isolation of the wound (10). This stimulates granulation tissue formation in combination with an increase in blood flow in the adjacent tissue (17). Furthermore, negative pressure wound therapy approximates the wound edges and provides a mass filling effect, without establishing a new wound (as in the case of an abdominal or omental flaps surgery).
Sealed negative pressure therapy was first described in the early 1990s as a method of accelerating wound healing in patients with open fractures (18) and resulted in the development of a VAC system 19, 20. Negative pressure treatment of wounds has also been used with success in reconstructive and trauma surgery 21, 22. Basic research showed −125 mmHg to be the optimal negative pressure for creating an environment that promotes wound healing and this pressure has therefore been adapted as the standard pressure for treating patients with DSWI. Later studies reported that the application of −125 mmHg led to a large zone under the polyurethane foam with an increase in blood flow, while relative hypoperfusion was found in the zone closest to the vacuum source (0–0·5 cm). At a pressure of −75 mmHg, the increase in blood flow will not extend as deep into the tissue as −125 mmHg and the hypoperfused zone closest to the polyurethane foam will also decrease in size (17). Furthermore, there are reports that negative pressure therapy of mediastinal wounds at a pressure of −125 mmHg results in a decrease in CO 14, 15. These studies were performed with echocardiography, magnetic resonance imaging, Schwann‐Ganz catheters and flow probes. Recently, there have been several reports of life‐threatening complications, such as right ventricular rupture, during negative pressure therapy at −125 mmHg 11, 13. Application of high‐pressure suction on the cardiac wall might impair the right ventricle function or compromise the function of the coronary bypass grafts. The main reason why no recommendations have been made to decrease the negative pressure from −125 mmHg to −75 or −100 mmHg is that authorities in the field have pointed out the risk of chest wall instability and thus promote shear between the sternal edges with an increased risk of right ventricular rupture (12).
We have recently published the results of a study showing that −75 mmHg is as good as −125 mmHg regarding stabilisation of the chest wall and sternal edges. The risk of organ rupture increased during high negative pressure therapy (−150 to −175 mmHg) (23). Previous research verified that neither −75 mmHg nor −125 mmHg affected the respiratory parameters in a 70 kg pig model (24). In the present study, we investigated the haemodynamic outcome during 5 hours of negative pressure therapy and found no statistically significant change in CO, HR, MAP, mean PAP, central venous pressure, LAP, SVR, PVR or end‐tidal CO2 elimination in −75 mmHg negative‐pressure‐treated pigs compared with sham‐operated pigs without negative pressure therapy.
There is thus evidence from several studies that −75 mmHg could be used instead of −125 mmHg for the treatment of DSWI. The sternal stability is as good with −75 mmHg as with −125 mmHg and the risk of organ rupture decreases with lower negative pressures (23). The relative hypoperfusion zone closest to the vacuum source extends deeper into the tissue at a pressure of −125 mmHg than at −75 mmHg (17). Furthermore, it is well known clinically that if a patient suffers from pain at a pressure of −125 mmHg, decreasing the negative pressure to −75 or −100 mmHg often but not always decreases the level of pain.
However, we must remember that there are data supporting the use of −125 mmHg. In the majority of studies showing good results with negative pressure therapy in patients with DSWI after cardiac surgery, −125 mmHg has been used 5, 6, 7, 8, 9. Furthermore, it appears that granulation tissue formation is optimal at −125 mmHg (25). These are important observations, but a clinical prospective randomised study comparing −75 mmHg and −125 mmHg should be performed before any definitive recommendations can be made regarding the optimal pressure in negative pressure wound therapy.
There are some limitations in the present study. Echocardiography was not performed during the experiments, so we were not able to study the geometry of the heart or detect possible ventricular wall stress and sudden diastolic dysfunction.
In conclusion, negative pressure wound therapy is an established alternative in the treatment of DSWI. The present study shows that, with a suitable foam application technique, –75 mmHg can be applied without compromising the central haemodynamics.
Acknowledgements
This study was supported by research grants from the Region Skåne Research Funds, the Swedish Heart‐Lung Foundation, the Swedish Medical Association, the Swedish Government Grant for Clinical Research, and the Donation Funds of Lund University Hospital.
References
- 1. Farinas MC, Gald PF, Bernal JM, Rabasa JM, Revuelta JM, Gonzalez‐Macias J. Suppurative mediastinitis after open‐heart surgery: a case‐control study covering a seven‐year period in Santander, Spain. Clin Infect Dis 1995;20:272–9. [DOI] [PubMed] [Google Scholar]
- 2. Ivert T, Lindblom D, Sahni J, Eldh J. Management of deep sternal wound infection after cardiac surgery – Hanuman syndrome. Scand J Thorac Cardiovasc Surg 1991;25:111–7. [DOI] [PubMed] [Google Scholar]
- 3. Milano CA, Kesler K, Archibald N, Sexton DJ, Jones RH. Mediastinitis after coronary artery bypass graft surgery. Risk factors and long‐term survival. Circulation 1995;92:2245–51. [DOI] [PubMed] [Google Scholar]
- 4. Ridderstolpe L, Gill H, Granfeldt H, Ahlfeldt H, Rutberg H. Superficial and deep sternal wound complications: incidence, risk factors and mortality. Eur J Cardiothorac Surg 2001;20:1168–75. [DOI] [PubMed] [Google Scholar]
- 5. Domkowski PW, Smith ML, Gonyon DL Jr, Drye C, Wooten MK, Levin LS, Wolfe WG. Evaluation of vacuum‐assisted closure in the treatment of poststernotomy mediastinitis. J Thorac Cardiovasc Surg 2003;126:386–90. [DOI] [PubMed] [Google Scholar]
- 6. Fleck TM, Fleck M, Moidl R, Czerny M, Koller R, Giovanoli P, Hiesmayer MJ, Zimpfer D, Wolner E, Grabenwoger M. The vacuum‐assisted closure system for the treatment of deep sternal wound infections after cardiac surgery. Ann Thorac Surg 2002;74:1596–600. [DOI] [PubMed] [Google Scholar]
- 7. Luckraz H, Murphy F, Bryant S, Charman SC, Ritchie AJ. Vacuum‐assisted closure as a treatment modality for infections after cardiac surgery. J Thorac Cardiovasc Surg 2003;125:301–5. [DOI] [PubMed] [Google Scholar]
- 8. Obdeijn MC, De Lange MY, Lichtendahl DH, De Boer WJ. Vacuum‐assisted closure in the treatment of poststernotomy mediastinitis. Ann Thorac Surg 1999;68:2358–60. [DOI] [PubMed] [Google Scholar]
- 9. Tang AT, Ohri SK, Haw MP. Novel application of vacuum assisted closure technique to the treatment of sternotomy wound infection. Eur J Cardiothorac Surg 2000;17:482–4. [DOI] [PubMed] [Google Scholar]
- 10. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 11. Bu‐Omar Y, Naik MJ, Catarino PA, Ratnatunga C. Right ventricular rupture during use of high‐pressure suction drainage in the management of poststernotomy mediastinitis. Ann Thorac Surg 2003;76:974–5. [DOI] [PubMed] [Google Scholar]
- 12. Fleck TM, Grabenwoger M. Letter to the editor (reply), (Right ventricular rupture during use of high‐pressure suction drainage in the managment of poststernotomy mediastinitis). Ann Thorac Surg 2003;76:974–5. [DOI] [PubMed] [Google Scholar]
- 13. Sartipy U, Lockowandt U, Gabel J, Jideus L, Dellgren G. Cardiac rupture during vacuum‐assisted closure therapy. Ann Thorac Surg 2006;82:1110–1. [DOI] [PubMed] [Google Scholar]
- 14. Conquest AM, Garofalo JH, Maziarz DM, Mendelson KG, Su SY, Wooden WA, Meadows WM, Nifong W, Chitwood WR. Hemodynamic effects of the vacuum‐assisted closure device on open mediastinal wounds. J Surg Res 2003;115:209–13. [DOI] [PubMed] [Google Scholar]
- 15. Petzina R, Ugander M, Gustafsson L, Engblom H, Sjogren J, Hetzer R, Ingemansson R, Arheden H, Malmsjo M. Hemodynamic effects of vacuum‐assisted closure therapy in cardiac surgery: assessment using magnetic resonance imaging. J Thorac Cardiovasc Surg 2007;133:1154–62. [DOI] [PubMed] [Google Scholar]
- 16. Sjogren J, Gustafsson R, Wackenfors A, Malmsjo M, Algotsson L, Ingemansson R. Effects of vacuum‐assisted closure on central hemodynamics in a sternotomy wound model. Interact Cardiovasc Thorac Surg 2004;3:666–71. [DOI] [PubMed] [Google Scholar]
- 17. Wackenfors A, Gustafsson R, Sjogren J, Algotsson L, Ingemansson R, Malmsjo M. Blood flow responses in the peristernal thoracic wall during vacuum‐assisted closure therapy. Ann Thorac Surg 2005;79:1724–30. [DOI] [PubMed] [Google Scholar]
- 18. Fleischmann W, Strecker W, Bombelli M, Kinzl L. [Vacuum sealing as treatment of soft tissue damage in open fractures]. Unfallchirurg 1993;96:488–92. [PubMed] [Google Scholar]
- 19. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–76. [PubMed] [Google Scholar]
- 20. Voinchet V, Magalon G. [Vacuum assisted closure. Wound healing by negative pressure]. Ann Chir Plast Esthet 1996;41:583–9. [PubMed] [Google Scholar]
- 21. Barker DE, Kaufman HJ, Smith LA, Ciraulo DL, Richart CL, Burns RP. Vacuum pack technique of temporary abdominal closure: a 7‐year experience with 112 patients. J Trauma 2000;48:201–6. [DOI] [PubMed] [Google Scholar]
- 22. Greer SE, Adelman M, Kasabian A, Galiano RD, Scott R, Longaker MT. The use of subatmospheric pressure dressing therapy to close lymphocutaneous fistulas of the groin. Br J Plast Surg 2000;53:484–7. [DOI] [PubMed] [Google Scholar]
- 23. Mokhtari A, Petzina R, Gustafsson L, Sjogren J, Malmsjo M, Ingemansson R. Sternal stability at different negative pressures during vacuum‐assisted closure therapy. Ann Thorac Surg 2006;82:1063–7. [DOI] [PubMed] [Google Scholar]
- 24. Gustafsson R, Sjogren J, Malmsjo M, Wackenfors A, Algotsson L, Ingemansson R. Vacuum‐assisted closure of the sternotomy wound: respiratory mechanics and ventilation. Plast Reconstr Surg 2006;117:1167–76. [DOI] [PubMed] [Google Scholar]
- 25. Morykwas MJ, Faler BJ, Pearce DJ, Argenta LC. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg 2001;47:547–51. [DOI] [PubMed] [Google Scholar]


