Abstract
Silver is a broad‐spectrum antimicrobial agent that is gaining more and more importance in the wound‐management industry. By incorporating silver ions into alginate fibres, highly absorbent alginate wound dressings with antimicrobial properties can be made. Laboratory studies have shown that by incorporating fine particles of silver sodium hydrogen zirconium phosphate, the silver‐containing alginate fibres can maintain the white physical appearances while providing a sustained release of silver ions when in contact with wound exudates. Test results have proven that these fibres are highly effective against bacteria.
Keywords: Alginate fibre, Antimicrobial, Silver ions
Silver as an Antimicrobial Agent
Silver has a long history as an antimicrobial agent (1, 2), especially in the treatment of burns. While metallic silver is relatively inactive, silver ions are effective against a wide range of bacteria. When low concentrations of silver ions accumulate inside cells, they can bind to negatively charged components in proteins and nucleic acids, thereby effecting structural changes in bacterial cell walls, membranes and nucleic acids that affect viability. Interestingly, although silver is a highly effective antimicrobial agent, it has a limited toxicity to mammalian cells (3). It was found that in clean wounds in pigs, the use of silver‐containing dressings can increase the rate of epithelialisation by 28%, indicating a beneficial effect of silver ions in wounds, in addition to its antimicrobial activity (4).
In recent years, silver has been gaining importance in the wound‐management industry as an effective antimicrobial agent, and a number of silver‐containing wound dressings have been developed. These function by the sustained release of low concentrations of silver ions over time and generally appear to stimulate healing, as well as inhibiting microorganisms. A number of laboratory studies have shown the excellent antimicrobial performances of the silver‐containing wound dressings 5, 6, 7, 8, 9). Human clinical studies have also shown encouraging clinical benefits 10, 11, 12, 13).
Alginate wound dressings are an important type of modern wound‐management materials. Since they are highly absorbent, these dressings are mainly used on highly exuding wounds where microbial infection is common. By incorporating silver ions into alginate fibres, it is expected that a highly absorbent wound dressing with good antimicrobial properties can be obtained.
The Addition of Silver into Alginate Fibres Through Chemical Reaction
In the wound‐management industry, a number of silver compounds are used for antimicrobial purposes. Aqueous silver nitrate solutions have been used as a simple wound‐cleansing agent for over a long time. Since its introduction in 1968 (14), silver sulphadiazine (SSD) has also become widely used in burn treatment. Although it was first recommended as a topical treatment for the prevention of pseudomonad infections in burns, SSD has since been demonstrated to possess broad‐spectrum antibacterial, antifungal and antiviral activity 15, 16, 17).
Since alginate is a polymeric acid, it can form salt with silver ions. However, unlike calcium alginate, which is highly insoluble in water, when sodium alginate solution is extruded into a silver nitrate solution, it is difficult to form silver alginate fibre, mainly because silver is a monovalent ion and solidification of the newly spun filament is slow and difficult. A mixed solution of calcium chloride and silver nitrate can be used to produce fibres that are a mixture of calcium alginate and silver alginate. Table 1 summarises the calcium and silver contents of alginate fibres made with a mixed coagulation bath containing calcium and silver ions (18).
Table 1.
Preparation conditions and properties of the calcium silver alginate fibre (18)
| Sample | Number 1 | Number 2 |
|---|---|---|
| Alginate content of the spinning solution (%) | 6 | 6 |
| Coagulation duration (seconds) | 30 | 600 |
| Fibre silver content (%) | 5·12 | 7·30 |
| Fibre calcium content (%) | 4·98 | 6·18 |
| Ratio between silver/calcium | 1:03 | 1:18 |
| Fibre strength (cN/dtex) | 1·09 | 1·15 |
| Extension at break (%) | 10·5 | 8·9 |
In order to attach silver ions onto the alginate fibres, calcium alginate fibres can be treated with aqueous solutions of silver nitrate. The silver ions in the solution exchange with the calcium ions in the fibre, resulting in the formation of calcium alginate fibre containing silver ions. These fibres are highly antimicrobial; however, due to the oxidative power of the silver ions, they are sensitive to light exposure and can become dark‐to‐black in appearance.
Adding particles of water‐insoluble silver compounds to the alginate fibre is one way to avoid oxidation and maintain the white physical appearance that is highly desirable for a biomedical material. Le et al. (18) developed a method to incorporate SSD into alginate fibres by mixing the water‐soluble sodium sulphadiazine with sodium alginate to form a spinning solution, which was then extruded into a 2% calcium chloride solution containing silver nitrate. During the fibre forming process, sodium alginate reacts with calcium ions to form the filament, whilst sodium sulphadiazine reacts with silver ions to form SSD, which is deposited inside the fibre structure. Alternatively, after sodium sulphadiazine and sodium alginate are dissolved to form a spinning solution, silver nitrate is added to this solution before extrusion. In this process, the SSD particles formed through the reaction between sodium sulphadiazine and silver nitrate is dispersed in the spinning solution. When extruded to form fibre, the SSD particles are embedded in the fibres.
The Addition of Silver to Alginate Fibres Through Blending
As has been mentioned before, although silver is a highly effective broad‐spectrum antimicrobial agent, it is also highly oxidative to organic materials. Skin discoloration and irritation associated with the use of silver nitrate is well known. In order to protect the host material from oxidation and discoloration, some novel silver‐containing compounds have been developed in recent years, and these have been made into fine particles that can be blended with fibre forming polymers during extrusion.
Alphasan RC5000 is a silver sodium hydrogen zirconium phosphate. This microbiologically active ingredient is a synthetic inorganic polymer. Under a scanning electron microscope, it resembles cube‐shaped crystals, with an average particle size of about one micron (about the size of an average bacterium). It consists of a three‐dimensional, repeating framework of sodium hydrogen zirconium phosphate, with many equally spaced cavities containing silver. Silver (at 3·8% by weight) provides the main antimicrobial properties, while the framework matrix acts to distribute silver evenly (without clumping or pooling) throughout the individual fibres where the Alphasan particles are added.
When Alphasan RC5000 is mixed with sodium alginate solution, the fine particles can be evenly distributed in the spinning solution under a high rate of shearing. Because the particles are very fine, they can be suspended uniformly while the solution is extruded to form fibres. Since the sodium hydrogen zirconium phosphate framework prevents the silver ions from oxidising the alginate, this type of silver‐containing alginate fibre remains white even after sterilisation through irradiation (19). Figure 1 shows the photomicrograph of alginate fibre with the Alphasan RC5000 particles uniformly distributed inside the fibre structure.
Figure 1.

Photomicrograph of alginate fibre with the Alphasan RC5000 particles uniformly distributed inside the fibre structure. Magnification: ×200.
Release of Silver Ions From Silver‐Containing Alginate Fibres
When alginate fibres containing Alphasan RC5000 particles are in contact with wound exudates, the silver ions can be released into the wound exudate by three mechanisms. First, there is an ion exchange between the silver ions in the fibre and the sodium and calcium ions in the wound fluid. Second, silver ions can be chelated by protein molecules in the wound fluid. Third, Alphasan particles attached to the surface of the fibres can also be detached from the fibres and get into the wound exudate.
Table 2 summarises the results of the silver ion concentration when alginate fibre containing 1% Alphasan RC5000 is placed in contact with normal saline or human serum. It can be seen that the silver ions are slowly released into the solution, acting as an antimicrobial agent. More silver ions can be seen to release into human serum, suggesting the high silver‐binding abilities of the protein components in the wound exudates.
Table 2.
Silver concentrations in extract solutions (19)
| Sample | Duration of contact | Silver content of extract (µg/ml) |
|---|---|---|
| Normal saline | ||
| 1 | 30 minutes | 0·50 |
| 2 | 48 hours | 0·40 |
| 3 | 7 days | 1·32 |
| Human serum | ||
| A | 30 minutes | 2·18 |
| B | 48 hours | 2·74 |
| C | 7 days | 3·74 |
In the test as summarised in Table 2, 1 g of silver‐containing fibres were placed in contact with 40 ml of either solution A (aqueous solution containing 142 mmol of NaCl and 2·5 mmol of CaCl2) or human serum. Analysis by atomic absorption spectrum showed that 1 g fibre contained 0·17 mg of silver ions. After 7 days, up to 3·74 µg/ml of silver was present in the human serum extract. The mass of silver ions released from the dressing = mass of solution × concentration of silver = 40 ml × 3·74 µg/ml = 150 µg = 0·150 mg. This means that 0·15/0·17 = 88% of the available silver is released into the human serum.
The Antimicrobial Effect of Silver‐Containing Alginate Fibres
For alginate fibres, it is well known that when they are in contact with wound exudate, the calcium ions in the fibres exchange with sodium ions in the fluid, and the fibres are transformed from water‐insoluble calcium alginate into water‐soluble sodium alginate, resulting in the absorption of a large amount of water by the fibres. In a wound dressing, typically with a non woven structure, as the fibres absorb water and swell, the spaces between the fibres are closed and any bacteria that is carried in the wound exudate is trapped in the wound dressing. This can help reduce the spreading of bacteria, giving the alginate wound dressings bacteria static properties.
The silver ions in the alginate fibre can enhance its antimicrobial activities by the sustained release of the broad‐spectrum antimicrobial silver ions. As Table 2 summarises, silver ions can be released over a 7‐day period. These ions can kill the bacteria that are trapped in the alginate wound dressing, thus making the dressings antimicrobial. Figure 2 shows the antimicrobial mechanism for the silver‐containing alginate wound dressings.
Figure 2.
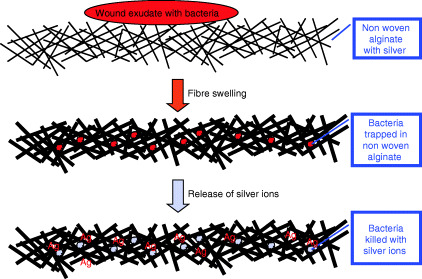
The antimicrobial mechanism of the silver‐containing alginate wound dressings.
3, 4 show the antimicrobial action of silver‐containing alginate fibres against Pseudomonas aeruginosa and Escherichia coli. In both cases, there was a 100% reduction in bacterial count within 5 hours when the fibres were placed in contact with solutions containing the two types of bacteria. Interestingly, although the Sorbsan™ alginate fibres showed some antimicrobial activity against E. coli, in the solution containing the other alginate fibre and Aquacel™ (made of carboxymethyl cellulose), antimicrobial effect was not noted and there was increased rate of bacteria growth in some cases.
Figure 3.
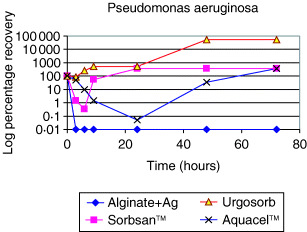
The antimicrobial action of silver‐containing alginate fibres against Pseudomonas aeruginosa (19).
Figure 4.
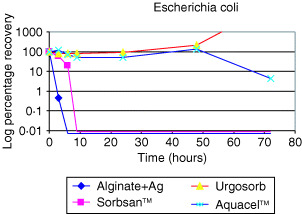
The antimicrobial action of silver‐containing alginate fibres against Escherichia coli (19).
Clinical Safety of the Silver‐Containing Alginate Fibres
In a review on the use of silver sulfadiazine in burn wound treatment, Fuller (20) mentioned that silver is inert when deposited in human tissues. It is normally present in mammalian tissue in concentrations <10 µg/dl. Serum silver concentrations of up to 20 µg/dl are regarded as non toxic from the standard point of the laboratory analysis for trace metals (21). In a study by Boosalis, it was found that the silver concentration in the urine samples of those undergoing silver sulfadiazine treatment is consistently higher during treatment, indicating the fact that there is a metabolic mechanism whereby excess silver ions in the body can be excreted. Other studies have also shown the systemic distribution and excretion of silver in urine (3). Exposure of wounds to low levels of silver ions can therefore be considered as safe.
Bacterial Resistance to Silver in Wound Care
Since ionic silver exhibits antimicrobial activity against a broad range of microorganisms, silver is now included in many commercially available health care products. The use of silver is increasing rapidly in the field of wound care, and a wide variety of silver‐containing dressings are now available. However, concerns associated with the overuse of silver and the consequent emergence of bacterial resistance are being raised. The current understanding of the biochemical and molecular basis behind silver resistance has been documented in a detailed review by Percival et al. (22).
References
- 1. Klasen HJ. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns 2000;26(2):117–30. [DOI] [PubMed] [Google Scholar]
- 2. Klasen HJ. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 2000;26(2):131–8. [DOI] [PubMed] [Google Scholar]
- 3. Lansdown AB . Silver. 2: Toxicity in mammals and how its products aid wound repair. J Wound Care 2002;11(5):173–7. [DOI] [PubMed] [Google Scholar]
- 4. Geronemus RG, Mertz PM, Eaglstein WH. Wound healing. The effects of topical antimicrobial agents. Arch Dermatol 1979;. 115(11):1311–4. [DOI] [PubMed] [Google Scholar]
- 5. Furr JR, Russell AD, Turner TD, Andrews A. Antibacterial activity of Actisorb Plus, Actisorb and silver nitrate. J Hosp Infect 1994;27(3);201–8. [DOI] [PubMed] [Google Scholar]
- 6. Lansdown AB, Jensen K, Jensen MQ. Contreet Foam and Contreet Hydrocolloid: an insight into two new silver‐containing dressings. J Wound Care 2003;12(6):205–10. [DOI] [PubMed] [Google Scholar]
- 7. Wright JB, Hansen DL, Burrell RE. The comparative efficacy of two antimicrobial barrier dressings: in vivo examination of two controlled release silver dressings. Wounds 1998;10(6):179–88. [Google Scholar]
- 8. Thomas S, McCubbin P. A comparison of the antimicrobial effects of four silver‐containing dressings on three organisms. J Wound Care 2003;12(3): 101–7. [DOI] [PubMed] [Google Scholar]
- 9. Thomas S, McCubbin P. An in vitro analysis of the antimicrobial properties of 10 silver containing dressings. J Wound Care 2003;12(8): 305–8. [DOI] [PubMed] [Google Scholar]
- 10. Wunderlich U, Orfanos CE. Treatment of venous ulcera cruris with dry wound dressings. Phase overlapping use of silver impregnated activated charcoal xerodressing. Hautarzt 1991;42(7): 446–50. [PubMed] [Google Scholar]
- 11. Tebbe B, Orfanos CE. Therapy of leg ulcers and decubitus ulcers with a xero‐dressing: modern wound dressings with antibacterial activity. H+G Brand (Special Edition) 1996;71(9):11–13. [Google Scholar]
- 12. Bornier C, Jeannin C. Clinical trials with ACTISORB–carried out on 20 cases of complex wounds. Soins Chir 1989;99: 39–41. [PubMed] [Google Scholar]
- 13. Cassino R, Ricci E, Carousone A. Management of infected wounds: a review of antibiotic and antiseptic treatments (poster presentation). In ‘10th European Wound Management Association Conference’, Dublin 2001.
- 14. Fox C. Topical therapy and the development of silver sulphadia zinc. Surg Gynecol Obstet 1983;157: 82–8. [PubMed] [Google Scholar]
- 15. Hamilton‐Miller JM, Shah S, Smith C. Silver sulphadiazine: a comprehensive in vitro reassessment. Chemotherapy 1996;39(6):405–9. [DOI] [PubMed] [Google Scholar]
- 16. Wlodkowski TJ, Rosenkranz HS. Antifungal activity of silver sulphadiazine. Lancet 1973;2(7831):739–40. [DOI] [PubMed] [Google Scholar]
- 17. Rahn RO, Setlow JK, Landry LC. Ultraviolet irradiation of nucleic acids complexed with heavy atoms. 3. Influence of Ag+ and Hg2+ on the sensitivity of phage and of transforming DNA to ultraviolet radiation. Photochem Photobiol 1973;18: 39–41. [DOI] [PubMed] [Google Scholar]
- 18. Le Y, Anand SC, Horrocks AR. Medical Textiles ‘96. Cambridge, UK: Woodhead Publishing, 1997. [Google Scholar]
- 19. Qin Y, Groocock MR. PCT WO/02/36866A1 May 2002.
- 20. Fuller J. J Burn Care Rehabil 1994;15: 213–6. [DOI] [PubMed] [Google Scholar]
- 21. Boosalis MG. Serum and urinary silver levels in thermal injury patients. Surgery 1987;101: 40–43. [PubMed] [Google Scholar]
- 22. Percival SL, Bowler PG, Russell D. Bacterial resistance to silver in wound care. J Hosp Infect 2005;60(1):1–7. [DOI] [PubMed] [Google Scholar]


