Abstract
Reports of spider bites appear throughout North America. Bites associated with the brown recluse spider (Loxosceles recluse) cause serious medical complications because the venom of this spider contains a powerful necrotising agent with the potential to cause severe cutaneous necrosis. Although not much is known regarding the application of negative pressure wound therapy (NPWT) to spider bites, this therapy has considerable literature support for its efficacy, cost‐effectiveness and ease of use in chronic, difficult‐to‐heal wounds. A case study using NPWT to successfully treat a non healing upper arm wound presumed due to a venomous spider bite is presented here. The patient was successfully treated with a new, less costly NPWT product called the Versatile 1® and a new combination drain plus dressing called the Miller DermiVex® drain, both manufactured by Blue Sky Medical (Carlsbad, CA).
Keywords: NPWT, Negative pressure wound therapy, Spider bite
Introduction
Envenomations by the brown recluse spider (Loxosceles recluse) have been reported throughout North America (1). The brown recluse spider is most prevalent and has the most potent venom (2). The venom contains a powerful necrotising agent with the potential to cause severe cutaneous necrosis (3). Complications from spider bites include mild itching, pain and erythema surrounding the bite site (4), which have been known to cause death 5, 6. These bites pose several challenges to the clinician in that diagnosis can be difficult, systemic manifestations can occur and healing can be resistant to conventional measures (5). Because no laboratory test is available to identify the cause of symptoms in these cases, the diagnosis is made clinically. Early intervention can make a significant difference in cosmetic outcome, so a high index of suspicion is warranted (5).
Treatment for spider bites typically includes debriding devitalised tissue, applying non cytotoxic topical antimicrobials if a bioload is suspected and following routine principles for management of exudates and moist wound healing (6). Cold packs seem to slow the activity of sphyngomyelinase‐D 7, 8.
Because some bites cause systemic loxoscelism, drug therapy with dapsone may limit the severity of the bite and prevent complications 5, 9. Hyperbaric oxygen therapy has been used successfully to bring adverse response to the bite under control 10, 11, 12, 13, but not in all cases (14).
The use of negative pressure wound therapy (NPWT) is not well documented for spider bites, but it has considerable literature support for use in a variety of chronic, non healing wounds 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32. NPWT turns an open wound into a controlled, closed wound while removing the excess fluid from the wound bed, enhancing circulation and disposing of cellular waste from the lymphatic system (16). The effects of NPWT are thought to promote wound healing through multiple actions including the removal of exudates from the wounds to help establish fluid balance (17), provision of a moist wound environment (18), removal of slough (18), a potential decrease in bacterial burden in the wound (19), a reduction in oedema and third‐space fluids, an increase in the blood flow to the wound 18, 19, 20, 21, an increase in growth factors and the promotion of white cells and fibroblasts within the wound (22). Based on these documented effects of NPWT, it was theorised that this therapy could be beneficial in a severe spider bite case.
Case review
The patient is a 53‐year‐old male with Multiple Sclerosis, who receives daily injections of CopaxoneTM (glatiramer acetate; Teva Pharmaceutical Industries Ltd, Kansas City, MO), administered by his wife. He noticed unusual tenderness approximately 10 cm above an injection site, which progressively worsened over the next few days. Patient’s visit to the emergency room resulted in a diagnosis that this may have been related to the injection; however, the patient did not agree (Figure 1).
Figure 1.
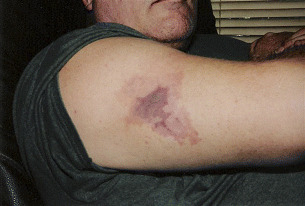
20 April 2005. Initial presentation of the wound.
His neurologist confirmed that the patient was not experiencing a reaction to his injections and started the patient on an oral antibiotic. The patient and his wife had previously identified several spiders in their house and believed that the wound may be due to a venomous insect bite. After returning home, the patient found a brown recluse spider in the bedroom under the mattress. As the wound continued to worsen with an increase in swelling, necrosis and pain, the patient referred to a nearby wound care centre. They recommended topical gentamicin and Collagenase Santyl® (Abbott Laboratories Inc, Columbus, OH) twice a day. The wound continued to worsen, and the patient was referred to our facility, the Wound Healing Center in Bedford, Indiana, approximately 4 weeks later. On examination on 17 June 2005, the wound was found to be necrotic, with deep tissue induration, limited to the immediate wounded area. The wound itself was covered with eschar and had minimal serous drainage. The wound was debrided to healthy, grossly uninvolved margins (Figure 2).
Figure 2.
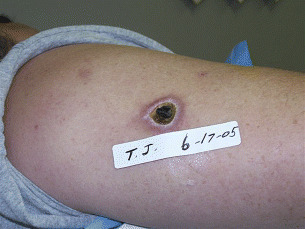
17 June 2005. The debrided wound.
Based on the severity of the wound and the failure of traditional treatments, NPWT was selected because we found that it often provides fast, painless healing. The new Versatile 1® (V1®; Blue Sky Medical, Carlsbad, CA) was chosen to administer the NPWT because of its unique potential to deliver active therapy only at night while the patient is sleeping (Figure 3).
Figure 3.

The Versatile 1 device by BlueSky Medical Group for Negative Pressure Wound Therapy.
A new concept in NPWT called the Miller DermiVex® (Blue Sky Medical) drain was commenced. This allows negative pressure to be applied to minimal depth wounds that are not amenable to use of drains or foam placed into their depths. This particular drain acts as a combination dressing and drain. It is made of soft silicone and is funnel shaped (Figure 4). It rests over the wound on healthy skin and provides a moist wound environment. The DermiVex drain protects the wound during active therapy and provides NPWT to the wound as needed.
Figure 4.
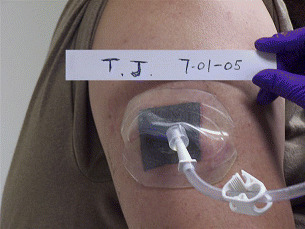
The Miller DermiVex drain acts as a combination dressing and drain for NPWT.
Due to the unknown origin of the wound, there were concerns over bioburden, so a silver dressing (SilverlonTM; Argentum Medical, LLC, Chicago, IL, USA) was applied to the surface of the wound. The Miller DermiVex drain was cut to a shape to allow 2‐ to 3‐cm borders around the wound so that it would rest on the healthy skin. The Miller DermiVex drain was secured to the wound with a few small pieces of a clear semi‐permeable membrane (TegadermTM; 3M, St. Paul, MN, USA) to prevent shifting during therapy. Tubing was connected to the Miller DermiVex drain and then to the V1 NPWT device.
The V1 was set at 100 mmHg of negative pressure based on the more dense muscular tissue identified at the base of the wound. For fatty or less dense tissues, the pressure settings of 60–80 mmHg are typically used 32, 33. The patient was instructed to change dressings three times a week. At home, the patient used NPWT for a period of 6–8 hours in every 24‐hour period. Therapy was usually administered during night hours when the patient was sleeping. This facilitates normal activities of daily living, which is a unique feature of the V1. In the morning, after pressure therapy ended, the Miller DermiVex drain was removed by the patient and a piece of Tegaderm was applied over the Silverlon and the wound.
Under this treatment plan, NPWT was administered by the patient for approximately 2·5 months until healing occurred. The therapy was continued even after a healthy bed of granulation was identified, based on the unique potential to take a wound to epithelialisation under the influence of NPWT with the Miller DermiVex drain (Figure 5).
Figure 5.
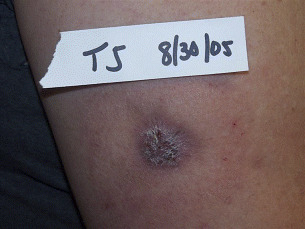
30 August 2005. After 2.5 months of NPWT; final healing.
Discussion
The V1 Wound Vacuum System allows negative pressure to be maintained within the wound dressing. In the morning, when pressure therapy ended, the patient clamped off the tubing to the pump and taped it to his arm. This unique feature of the V1 creates the potential for providing active treatment primarily at night. Treating this patient with NPWT using the parameters associated with the V1 and Miller DermiVex drain (100 mmHg applied for 6–8 hours) allowed excellent healing and yet allowed free mobility during the day.
Because of the location and depth of the wound, we selected the Miller DermiVex drain. This soft, concave silicone cover provided protection to the arm wound when the patient was moving about during the day. The Miller DermiVex drains rest on healthy skin around the wound. We theorise that by not resting on the wound, the Miller DermiVex drain promotes epithelialisation since it allows the unimpeded proliferation and progression of epithelial cells across the wound surface. In addition, the Miler DermiVex drain was easily sealed over the wound with Tegaderm and avoided having to prep the wound by cutting saline‐moistened gauze to fit. Last, the Miller DermiVex drain was easily resized to fit the wound as it healed. The patient’s wife cut the drain to size as the wound grew smaller.
In our recent review article (34), we identified several areas of confounding results between current commonly accepted NPWT practices and the literature. One such area is the optimal pressure level. While experience‐based protocols with NPWT suggest pressures typically around −125 mmHg to be beneficial (20), early Russian literature (32) and a recent article suggest that lower pressures around −80 mmHg’ may be beneficial, especially in soft tissue, to minimise possible ischaemic effects’(33). These lower pressures were used successfully in this case report.
To date, there are unanswered questions with NPWT needed to define the optimal parameters for pressure intensity, duration of treatment, interval between treatments and mode and timing of application. There are also many unanswered questions needed to define treatment for non responsive spider bites. Further research is needed to explore the optimal practice protocols to deliver the most effective and efficient treatment. For now, the Miller DermiVex drain and the V1 may be considered as an option to provide NPWT to non responsive spider bite wounds.
Acknowledgement
This study was supported by a grant from Blue Sky Medical (Carlsbad, CA).
References
- 1. Vetter RS, Bush SP. Reports of presumptive brown recluse spider bites reinforce improbable diagnosis in regions of North America where the spider is not endemic. Clin Infect Dis 2002;35(4):442–5. [DOI] [PubMed] [Google Scholar]
- 2. Stibich AS, Schwartz RA. Brown recluse spider bite. eMedicine J 2001;2(6): sections 1–11. [Google Scholar]
- 3. Majeski J. Necrotizing fasciitis developing from a brown recluse spider bite. Am Surg 2001;67(2):188–90. [PubMed] [Google Scholar]
- 4. Fagan SP, Berger DH, Rahwan K, Awad SS. Spider bites presenting with methicillin‐resistant Staphylococcus aureus soft tissue infection require early aggressive treatment. Surg Infect (Larchmt) 2003;4(4):311–5. [DOI] [PubMed] [Google Scholar]
- 5. Leach J, Bassichis B, Itani K. Brown recluse spider bites to the head: three cases and a review [review]. Ear Nose Throat J 2004;83(7):465–70. [PubMed] [Google Scholar]
- 6. Wilson JR, Hagood CO, Prather ID. Brown recluse spider bites: a complex problem wound. A brief review and case study. Ostomy Wound Manage 2005;51(3):59–66. [PubMed] [Google Scholar]
- 7. Russell FE. Arachnid envenomations. Emerg Med Serv 1991;20(5):16–47. [Google Scholar]
- 8. Wilson DC, King LE Jr. Spiders and spider bites. Dermatol Clin 1990;8:277–86. [PubMed] [Google Scholar]
- 9. King L, Rees RS. Dapsone treatment of brown recluse bite. JAMA 1983;250:648. [PubMed] [Google Scholar]
- 10. Svendsen FJ. Treatment of clinically diagnosed brown recluse spider bites with hyperbaric oxygen: a clinical observation. J Ark Med Soc 1986;83:199–204. [PubMed] [Google Scholar]
- 11. Strain GM, Snider TG, Tedford BL, Cohn GH. Hyperbaric oxygen effects on brown recluse spider (Loxosceles reclusa) envenomation in rabbits. Toxicon 1991;29:989–96. [DOI] [PubMed] [Google Scholar]
- 12. Bangasser R. Treatment of the brown recluse spider (Loxosceles reclusa) bite with hyperbaric oxygen therapy. In: Kindwall EP, Whelan HT, editors. Hyperbaric medicine practice. Flagstaff: Best Publishing Co., 1999:869–77. [Google Scholar]
- 13. Kendall TE, Caniglia RJ. Hyperbaric oxygen with treatment of clinically diagnosed spider bites: a review of 48 cases [abstract]. Undersea Biomed Res 1989;16(Suppl):21 2929052 [Google Scholar]
- 14. Hobbs GD, Anderson AR, Greene TJ, Yealy DM. Comparison of hyperbaric oxygen and dapsone therapy for Loxosceles envenomation. Acad Emerg Med 1996;3:758–61. [DOI] [PubMed] [Google Scholar]
- 15. Chariker ME, Jeter KF, Tintle TE, Bottsford JE. Effective management of incisional and cutaneous fistulae with closed suction wound drainage. Contemp Surg 1989;34:59–63. [Google Scholar]
- 16. Fleck CA, Frizzell LD. When negative is positive: a review of negative pressure wound therapy. Extended Care Product News 2004;3/4:20–5. [Google Scholar]
- 17. Zagoren A. Nutritional assessment and intervention in the person with a chronic wound. In: Krasner DL, Rodeheaver GT, Sibbald RG, editors. Chronic wound care: a clinical source book for healthcare professionals, 3rd edn. Wayne: Health Management Publications Inc, 2001:117–126. [Google Scholar]
- 18. Barker DE, Kaufman HJ, Smith LA, Ciraulo DL, Richart CL, Burns RP. Vacuum pack technique of temporary abdominal closure: a 7‐year experience with 112 patients. J Trauma, Inj Infect Crit Care 2000;48(2):201–6. [DOI] [PubMed] [Google Scholar]
- 19. Kostiuchenok II, Kolker VA, Karlov VA. The vacuum effect in the surgical treatment of purulent wounds. Vestn Khir Im I I Grek 1986;9:18–21. [PubMed] [Google Scholar]
- 20. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 21. Argenta LC, Morykwas MJ. Vacuum assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–77. [PubMed] [Google Scholar]
- 22. Davydov YA, Malafeeva AP, Smirnov AP, Flegontov VB. Vacuum therapy in the treatment of purulent lactation mastitis. Vestn Khir Im I I Grek 1986;9:66–70. [PubMed] [Google Scholar]
- 23. Philbeck TE, Whittington KT, Millsap MH, Briones RB, Wight DG, Schroeder WJ. The clinical and cost effectiveness of externally applied negative pressure wound therapy in the treatment of wounds in home healthcare Medicare patients. Ostomy Wound Manage 1999;45(11):41–50. [PubMed] [Google Scholar]
- 24. Larichev AB. Vacuum therapy of wounds and wound infection (studies in clinical physiological tones). Carlsbad: BlueSky Publishing, 2005. [Google Scholar]
- 25. Karev ID, Solovyov VA, Terentyev VA. Drainage foam rubber system in treatment of purulent wounds/wounds and wound infection. All-Union Conference; 1986. 2. 87–8. [Google Scholar]
- 26. IuA Davydov, Larichev AB, Smirnov AP, Flegontov VB. Vacuum therapy of acute suppurative diseases of soft tissues and suppurative wounds. Vestn Khir Im I I Grek 1986;141:43–6. [PubMed] [Google Scholar]
- 27. IuA Davydov, Larichev AB, Men’kov KG. Bacteriologic and cytologic evaluation of vacuum therapy of suppurative wounds. Vestn Khir Im I I Grek 1988;141:48–52. [PubMed] [Google Scholar]
- 28. IuA Davydov, Larichev AB, Alu Abramov. Substantiation of using forced early secondary suture in the treatment of suppurative wounds by the method of vacuum therapy. Vestn Khir Im I I Grek 1990;144:126–8. [PubMed] [Google Scholar]
- 29. IuA Davydov, Larichev AB, Alu Abramov, Men'kov KG. Concept of clinico‐biological control of the wound process in the treatment of suppurative wounds using vacuum therapy. Vestn Khir Im I I Grek 1991;146:132–6. [PubMed] [Google Scholar]
- 30. Morykwas MJ, Argenta LC. Nonsurgical modalities to enhance healing and care of soft tissue wounds. J South Orthop Assoc 1997:6:279–88. [PubMed] [Google Scholar]
- 31. Sibbald RG, Mahoney J, VAC Therapy Canadian Consensus Group . A consensus report on the use of vacuum‐assisted closure in chronic, difficult‐to‐heal wounds. Ostomy Wound Manage 2003;49(11):52–66. [PubMed] [Google Scholar]
- 32. Usupov YN, Yepifanov MV. Active wound drainage. Vestn Khir Im I I Grek 1987:4:42–5. [PubMed] [Google Scholar]
- 33. Wackenfors A, Sjorgren J, Gustafsson R, Algotsson L, Ingemansson R, Malmsjo M. Effects of vacuum‐assisted closure therapy on inguinal wound edge mircovascular blood flow. Wound Rep Regen 2004;12(6):600–6. [DOI] [PubMed] [Google Scholar]
- 34. Miller MS, Lowery CA. Negative pressure wound therapy: “a rose by any other name”. Ostomy Wound Manage 2005;51(3):44–9. [PubMed] [Google Scholar]


