Abstract
Chitin, a unique biopolymer based on the N‐acetyl‐glucosamine monomer is envisioned to promote rapid dermal regeneration and accelerate wound healing. It has many useful and advantageous biological properties for its application as a wound dressing. Chitin membranes were prepared using lithium chloride/dimethylacetamide solvent system and evaluated for use as a wound dressing. Swelling behaviour, moisture vapour transmission rate, microbial impermeability and antimicrobial efficacy of the dressings was evaluated. The chitin dressing provided an effective barrier to microbial penetration and exerted a broad bacteriostatic action against Gram‐positive and Gram‐negative organisms. Gamma irradiation at 25 kGy was found suitable for sterilisation of the dressings. The thermal decomposition of unirradiated and irradiated chitin membranes was investigated. No significant change in the thermal behaviour because of irradiation at 25 kGy was observed. In vitro biodegradation of unirradiated and irradiated chitin membranes showed the susceptibility of the chitin dressing to lysozyme. Irritant effect of the chitin membrane dressings on skin was tested. Subcutaneous and scarification test in guinea pigs showed no signs of inflammation. This was further supported by the Finkelstein’s test performed in rabbits. The chitin membranes were found to have optimal performance characteristics of a wound dressing and showed no toxicity or possible adverse reactions. The study shows the chitin dressings as useful adjunct in wound care.
Keywords: Chitin membrane, Properties, Toxicity, Wound dressing
Introduction
Wound dressings are indispensable for the effective healing of skin wounds such as burns and ulcers. The general functions of wound dressing are protection of the wound, prevention of infection and promoting healing by providing an optimum microenvironment for healing. To retain these functions until a regenerating skin forms, ideal wound dressings must satisfy various requirements such as high strength, good adhesion to the lesion, high water adsorption, high absorbability of exudates, high moisture permeability, biocompatibility, the absence of antigenicity, and the ability to promote skin regeneration. Different types of materials, including traditional absorbent or impregnated dressings, synthetic dressings such as semipermeable films, foam dressings, hydrogels, hydrocolloids and biological dressings are currently applied to lesions characterised by skin loss 1, 2. Various natural and synthetic polymers with good biocompatibility have been used to develop wound dressings. However, none of the wound dressing proposed so far is completely satisfactory for the treatment of skin wounds.
Chitin is a versatile and promising biomaterial based on the N‐acetyl‐glucosamine monomer (N‐acetyl‐2‐amino‐2‐deoxy‐d‐glucopyranose). It is a highly insoluble material resembling cellulose in its solubility and low chemical reactivity. It has a broad range of applications in various biomedical fields 3, 4. Chitin has been shown to be useful as a wound dressing material, drug delivery vehicle and increasingly a candidate for tissue engineering. It has been found to have an accelerating effect on the wound‐healing process 5, 6. The repeat unit N‐acetyl‐glucosamine occurs in hyaluronic acid (a GAG), that is responsible for the formation of fibrous networks for protein attachment during wound healing. Chitin is a natural polymer that possesses excellent properties that are advantageous for wound dressing namely biocompatibility, biodegradability (7), haemostatic activity and anti‐infection properties 8, 9. Chitin is reported to promote the ordered healing of tissues, activation of macrophages and works as a bacteriostatic and immunoadjuvant agent (10). It has been shown that chitin and its derivatives provide pain relief (11), induce faster wound healing and stimulate the formation of connective tissue 12, 13.
Studies related to film formation of chitin have not been popular as those of its deacetylated derivatives. Chitosan, the deacetylated derivative of chitin, has been the better researched version of the biopolymer 14, 15. This is because chitin is insoluble in most common organic solvents, a direct result of the strong intra‐ and intermolecular hydrogen bonding, while chitosan can even be dissolved in dilute organic acids. However, chitin is more favourable than chitosan in certain applications, especially in the biomedical fields. This is because of the fact that the acetamide group present in chitin is similar to the amide linkage of protein in living tissues (16) making chitin more biocompatible than chitosan. However, insolubility of chitin in common organic solvents makes its direct application difficult and expensive. Chitin‐based wound‐healing products are still at the early stages of research. The aim of the present study was to prepare and evaluate chitin membranes intended for wound dressing application. The physical properties, thermal properties, antimicrobial properties and toxicology of the chitin membranes were investigated for use as a wound dressing.
Materials and methods
Chitin in 5% lithium chloride and dimethylacetamide solvent system was used. 0·25%, 0·5% and 1·0% chitin was suspended in solvent and shaken overnight. The viscous clear solution was filtered through glass wool. Dressing films were caste from this solution on flat glass plates. Membrane formation following gradual coagulation or using different solvents – butanol, propanol, acetone was attempted. The chitin gel dressings formed were washed repeatedly with water to ensure complete removal of the solvent. The chitin membranes were subjected to air drying and oven drying. The swelling behaviour of the chitin membranes (0·25% and 0·5% chitin) was determined by measuring the weight of the membranes after immersion in distilled water at periodic intervals for 5 days in comparison with the dry weight of the membranes prior to the immersion. The degree of swelling was determined according to the following relationship:
where W s and W d represent the weight of the membranes in swollen and dry state (17). Moisture vapour transmission rate (MVTR) of 0·25% and 0·5% chitin membranes was measured as per the standard test methods for water vapour transmission of materials, ASTM E96 (18). A water‐filled container was covered with the membrane. The containers were weighed and placed in an incubator at 37°C for a period of 96 hours. The containers were reweighed periodically. The loss in weight because of passage of moisture vapour through the dressing was determined by the difference. The MVTR (g/m2/24 hours) was calculated as decrease in weight of the container per squaremeter area of chitin dressing covering the container on 24‐hour basis as follows:
where G is weight loss of the samples (g), t is test time (hours) and A is effective membrane area (m2).
Thermogravimetric analysis (TGA) was used to investigate thermal behaviour of the films (19). Thermogravimetric analyzer TGA Q500 by TA Instruments Inc. (Newcastle, DE) was used. Thermal stability of the air‐dried and oven‐dried membranes on irradiation at 25 kGy was evaluated. Analysis was carried out under nitrogen atmosphere and at a heating rate of 20°C/min from 30 to 800°C. In vitro biodegradation of unirradiated and irradiated chitin membranes was evaluated by immersing in 1 mg/ml lysozyme in phosphate buffer saline (PBS) solution. The samples were incubated at 37°C and removed at periodic intervals. The membranes were repeatedly washed with ethanol, dried at 40°C and weighed.
Microbiological studies
Impermeability of unirradiated and irradiated chitin dressings to various Gram‐positive and Gram‐negative bacteria –Bacillus, Escherichia coli, Flavimonas, Micrococcus, Proteus, Pseudomonas, Staphylococcus and Streptococcus was tested. Pieces of chitin membranes were placed on soyabean caesin digest agar plates. Suspensions of bacteria were prepared in sterile water and inoculated on top of the membranes and incubated (20). Plates were checked for the growth after 48 hours of incubation. The antimicrobial efficacy of the chitin dressing was also evaluated against various microbial organisms. Agar plates were evenly inoculated with the test organism. Chitin dressings were placed on the inoculated agar plates. A negative control plate (containing no dressing) was also prepared for each challenge organism to determine viability. All agar plates were then incubated at 35 ± 2°C for 48 hours. Each dressing was then removed to allow examination of the effect of chitin dressing on microbial activity (i.e. growth or no growth) on the agar beneath the test dressing. Microbicidal efficacy of the unirradiated and irradiated chitin dressings was tested using two clinically relevant organisms –Pseudomonas aeruginosa and Staphylococcus aureus. Each organism was inoculated in tryptone soya broth and saline. The unirradiated and irradiated chitin dressings were immersed in tryptone soya broth and saline inoculated with the test organisms and incubated. The samples were withdrawn at different intervals and plated for viable counts to determine the number of surviving organisms. Microbial load of chitin dressings was determined by the membrane filtration technique. Soyabean casein digest agar and thioglycollate medium incubated at 32–35°C for bacterial counts and 20–24°C for fungal counts was used. The plates were observed for growth up to 5 days. Counts were calculated per 100 cm2 and per g of chitin dressing. The effect of different doses of gamma radiation viz. 5, 10, 15, 20 and 25 kGy was examined on the microbial load of the chitin dressings. The samples after irradiation at different doses were tested for microbial counts.
Toxicological studies
Dermal irritancy tests were conducted using guinea pigs and New Zealand white strain of rabbits supplied by Laboid, Meerut (Uttar Pradesh, India) as the experimental animals. The study was conducted in accordance with the Standards for the Care and Use of Laboratory Animals. Subcutaneous injection test was performed in guinea pigs using extracts of chitin membranes in normal saline. Animals were divided into three groups consisting of five guinea pigs. The shaved area was prepared on either side of the back. On one side, 0·5 ml of extracting medium as blank was injected and on the other side 0·5 ml of test solution was injected. Each solution was injected in five guinea pigs. Animals were kept under observation for any sign of inflammation, sloughing or necrosis for 72 hours. The extracts of the chitin membranes were evaluated using the scarification test in guinea pigs. Animals were divided into three groups, each group consisting of five guinea pigs. Either side of the back was shaved (2·5 cm). Both the sides of the shaved areas were abraded using a sterile scalpel. On one side, a known irritant substance (turpentine oil) and on the other side, solution under study was applied to see the reaction of the animals. The chitin membranes were assessed for skin irritation using the Finkelstein’s test (21). Rabbits (of either sex, average weight 1·5 kg) were used and the irritant effect of test substance and a primary irritant (Finkelstein 63) was tested. Animals were anaesthetised with phenobarbitone sodium (5 mg/kg body weight) and secured to animal board. Circular areas of 2·5 cm diameter were drawn on the animal’s abdomen. The test material under study was put on the areas. On one area, a flannel pad (2·5 cm) soaked in known irritant (turpentine + camphor) was applied. The pieces of dressing materials and flannel cloth containing irritant substance were allowed to remain firmly in contact with skin area. About 0·5 ml of trypan blue solution (0·5%) was injected in the axilla of the animal. After 16 hours, the pads were removed and the degree of irritancy of the substance was estimated by the accumulation of trypan blue at the treated site. The most intense area of blueness is ranked as 100% and no colour as 0%.
Results
Membrane formation with 0·25% and 0·5% chitin and following gradual evaporation of the solvent was found to be the most suitable. About 0·5% chitin films were found to be advantageous compared with the 0·25% chitin films with respect to the ease of handling. The films also had flexibility and pliability to permit conformation to irregular wound surface. The swelling behaviour of the chitin films was determined to evaluate the fluid handling capacity. The degree of swelling of 0·25% and 0·5% chitin membranes as a function of immersion time in distilled water is presented in Table 1. The degree of swelling of the 0·25% films was higher compared with 0·5% chitin films. The films containing 0·5% chitin exhibited lower water uptake despite the higher concentration of chitin. These results are expected because chitin is not a hydrophilic polymer. Therefore, having a higher amount of chitin in the films did not improve their ability to absorb water. The higher ability of 0·25% chitin to absorb water may therefore be because of the higher porosity of 0·25% chitin matrix than the membrane with 0·5% chitin. The dressings initially absorbed water at a faster rate and the rate reduced drastically with time. No significant change in the swelling (%) of the chitin films was observed after 24 hours. MVTR (g/m2/24 hours) of chitin membranes is presented in Table 2. MVTR of 0·5% chitin membranes was significantly higher compared with the 0·25% chitin films. Vapour transmission rate for both the 0·25% and 0·5% chitin films was found to decrease with time. At 24 hours, the MVTR was 1389·29 ± 296·25 g/m2/24 hours for 0·25% chitin and 2027·04 ± 760·96 g/m2/24 hours for 0·5% chitin films.
Table 1.
Swelling behaviour of chitin dressings
| Time (h) | Degree of swelling (%) | |
|---|---|---|
| 0·25% Chitin | 0·5% Chitin | |
| 1 | 30·75 ± 7·47 | 21·13 ± 8·31 |
| 2 | 51·29 ± 17·74 | 25·49 ± 6·98 |
| 3 | 65·18 ± 19·93 | 29·31 ± 10·69 |
| 4 | 73·08 ± 16·35 | 32·32 ± 13·37 |
| 5 | 79·99 ± 18·38 | 36·38 ± 12·36 |
| 6 | 87·35 ± 15·25 | 47·51 ± 9·52 |
| 24 | 100·35 ± 15·44 | 50·70 ± 11·91 |
| 30 | 107·59 ± 10·90 | 56·71 ± 11·00 |
| 120 | 109·43 ± 10·47 | 57·93 ± 12·11 |
Table 2.
MVTR of chitin dressings
| Time (h) | MVTR (g/m2/24 hours) | |
|---|---|---|
| 0·25% Chitin | 0·5% Chitin | |
| 1 | 3249·12 ± 1203·51 | 6747·37 ± 883·51 |
| 2 | 1624·56 ± 601·75 | 5329·29 ± 1284·38 |
| 3 | 1523·62 ± 369·70 | 3827·25 ± 944·44 |
| 4 | 1462·45 ± 174·21 | 3753·50 ± 1153·33 |
| 5 | 1432·07 ± 62·81 | 3635·49 ± 1177·82 |
| 6 | 1254·44 ± 50·23 | 3014·21 ± 1002·16 |
| 24 | 1389·29 ± 296·25 | 2027·04 ± 760·96 |
| 30 | 1370·64 ± 342·92 | 1925·53 ± 738·63 |
| 120 | 485·92 ± 3·53 | 636·46 ± 91·92 |
MVTR, moisture vapour transmission rate.
Thermal stability of the unirradiated and irradiated chitin dressings was observed by TGA technique. Figure 1 shows the TGA curves for air‐dried and oven‐dried chitin films. The decreasing line depicts the % weight loss of the samples and the line with the peaks (degradation peaks) is the derivative curve illustrating the rate of weight loss (%/min). Both air‐dried and oven‐dried samples tested showed initial weight loss at approximately 50°C, likely a result of moisture evaporation upon heating. No significant change in the thermal behaviour because of irradiation at 25 kGy was observed. Table 3 lists the degradation peak values (denoted T d) observed for the unirradiated and irradiated chitin films. The degradation behaviour of the irradiated films was found to be comparable with those of unirradiated films.
Figure 1.
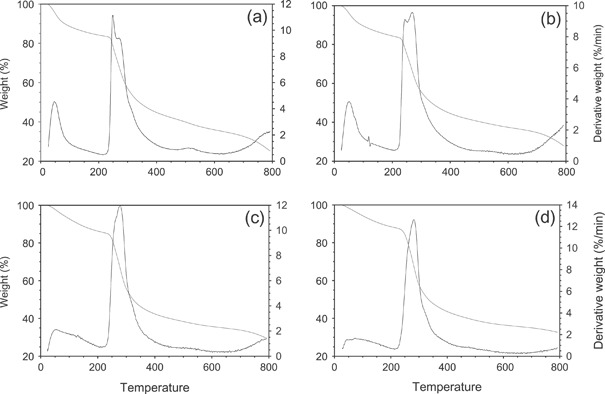
TGA curves for (a) air‐dried unirradiated; (b) air‐dried irradiated; (c) oven‐dried unirradiated and (d) oven‐dried irradiated chitin membranes.
Table 3.
Degradation temperature(s) of unirradiated and irradiated chitin dressings
| Type of membrane | First T d (°C) | Second T d (°C) |
|---|---|---|
| Air‐dried unirradiated | 255 | 280 |
| Air‐dried irradiated | 245 | 275 |
| Oven‐dried unirradiated | 270 | 285 |
| Oven‐dried irradiated | 278 | 288 |
Figure 2 illustrates the biodegradation profile of chitin membranes. Weight loss for both unirradiated and irradiated chitin dressings increased with time in the PBS lysozyme solution. Irradiated chitin membranes were found to be more susceptible to the enzyme. No statistically significant difference was obtained in the biodegradability of the unirradiated and irradiated chitin membranes. Both the unirradiated and irradiated chitin membranes degraded within 1 week demonstrating susceptibility to the lysozyme.
Figure 2.
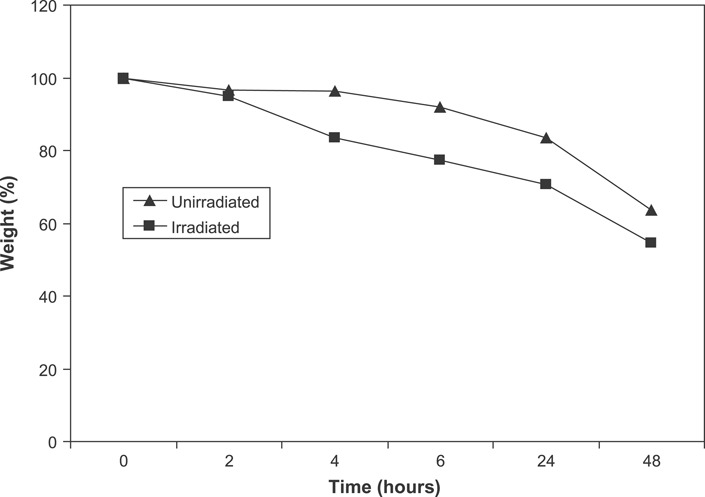
Biodegradation profile of chitin membranes in lysozyme solution.
A wound dressing must constitute a barrier against the external contaminating agents. Impermeability of the chitin dressing to different bacterial strains –Bacillus, Escherichia coli, Flavimonas, Micrococcus, Proteus, Pseudomonas, Staphylococcus and Streptococcus was tested. Chitin dressings were found to be impermeable to various bacilli and cocci strains. No effect of gamma irradiation on the impermeability of the chitin membranes to bacteria was observed. The chitin dressings exhibited strong bacteriostatic effect on the eight bacterial strains tested –Bacillus, Escherichia coli, Flavimonas, Micrococcus, Proteus, Pseudomonas, Staphylococcus and Streptococcus. Complete inhibition of bacterial growth was observed in contact with the dressings. The antimicrobial efficacy of the unirradiated and irradiated chitin membranes on Pseudomonas aeruginosa in broth and saline is presented in Figure 3. The counts in broth after 24 hours in the absence of chitin were 1·36 × 104/ml. However, the counts were 9·36 × 102/ml in the presence of unirradiated chitin dressing and 4·24 × 102/ml in the presence of irradiated chitin dressing. About 2‐log reduction in the counts were observed in the presence of chitin. Reduction in the viable counts was also observed in saline in the presence of chitin dressings. Irradiated chitin dressings were found to be more effective in controlling the bacterial growth. Similar antimicrobial effect of chitin dressings was also observed against Staphylococcus aureus (Figure 4). Microbial profile of chitin dressings at different doses of gamma radiation (0, 5, 10, 15, 20 and 25 kGy) is presented in Figure 5. Initial microbial contamination of the chitin films was estimated to be 5·72 × 103 CFU/100 cm2. Reduction in counts was observed on irradiation. No viable counts were detected at 25 kGy. Radiation dose of 25 kGy was found suitable for sterilisation of the chitin dressings.
Figure 3.
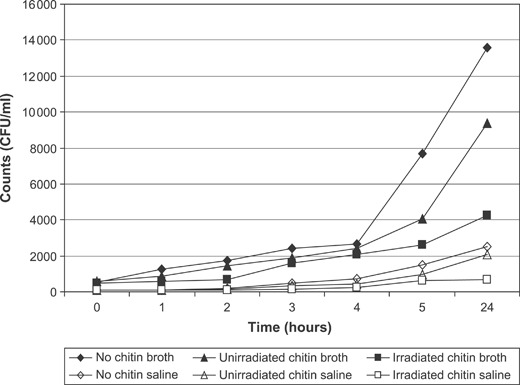
Antimicrobial effect of chitin on Pseudomonas aeruginosa.
Figure 4.
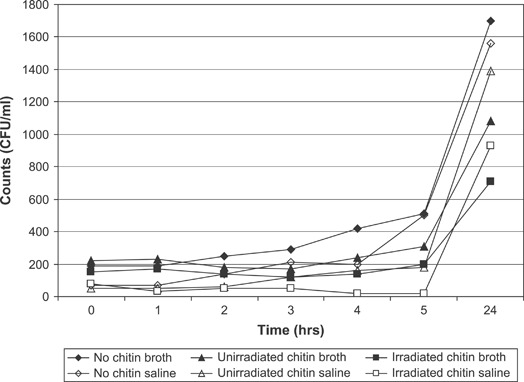
Antimicrobial effect of chitin on Staphylococcus aureus.
Figure 5.
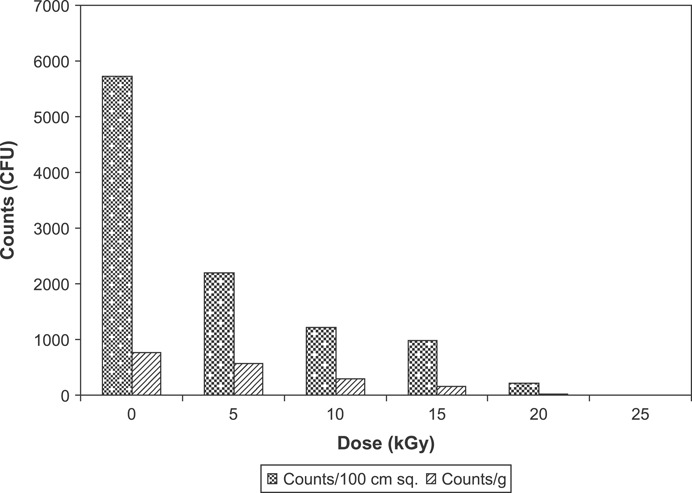
Microbial profile of chitin dressings at different doses of gamma radiation.
Subcutaneous injection test was conducted to evaluate the response of guinea pigs to the extracts of the chitin membrane. All the injection sites of the extract and blank were examined at 24, 48 and 72 hours intervals for gross evidence of tissue reactivity. No signs of inflammation at the site of injection were observed with any of the solution. Scarification test carried out in guinea pigs also suggested the absence of inflammatory activity. Animals did not respond to any of the test solution. The Finkelstein’s test showed no irritant effect of the chitin membrane. No blue coloration (0%) was detected in contact with the chitin membranes. However, in contact with the known irritant, the treated site was stained blue (100%). The toxicity tests carried out with the chitin dressings produced no irritant effect on the skin of animals viz. rabbits and guinea pigs. The results obtained indicate that chitin membranes prepared are safe and non irritant to the skin.
Discussion
A wound dressing needs to provide some basic physical and biological function during its residence in the wound bed. A variety of wound dressings are being developed for satisfying the ideal characteristics. In the present study, the chitin dressings were evaluated for the desired properties of a successful wound dressing such as fluid uptake, vapour transmission rate, microbial impermeability, antimicrobial efficacy, thermal properties, biodegradability and toxicology. Control of evaporative water loss and prevention of bacterial infection are the most important properties required for a successful wound covering. The moisture permeability of the wound dressing prevents the accumulation of fluid in heavily exudating wounds. Moisture vapour transmission thus has important implications for the ability of the dressing to cope with exudates production in vivo. Swelling behaviour, that is water absorption capacity and MVTR of the chitin dressings was determined to assess the fluid handling capacity of the dressings. Both the absorption capacity and moisture permeability of the dressings show the ability of the chitin dressing to prevent fluid accumulation in exudating wound. The chitin dressing was found to have a fluid uptake of about 50% of its weight that would prevent the wound bed from accumulation of exudates. The MVTR of the dressing was found to be 2027 g/m2/day indicating that chitin dressing can maintain a moist environment over wound bed in moderate to heavily exuding wound that would enhance epithelial cell migration during the healing process (22).
An ideal dressing should adhere closely to the wound, have a good ability to stop bleeding and act as an antibacterial barrier. It should induce no toxicity effects or immunological reaction. An ideal dressing should keep the wound wet, be permeable to air and properly stimulate the damaged tissue to regeneration. An ideal dressing should be able to be removed without inducing trauma during dressing change over healed areas in the wound bed. Resorbable materials are gaining considerable importance in all surgical disciplines and also for use as wound dressing (23). Physical and biological characterisation of the chitin membranes has shown that the membranes have ideal properties for a dressing and thus would aid in maintenance of a balanced moist wound environment to structural support for tissue regeneration. The moisture management capability of a dressing has a direct impact on the effectiveness of the dressing and helps in the healing of wounds.
The objective of the wound dressing material employed is to accelerate wound healing. Chitin’s monomeric unit, N‐acetyl‐glucosamine, occurs in hyaluronic acid, an extracellular macromolecule that is important in wound repair. Therefore, chitin possesses the characteristics favourable for promoting rapid dermal regeneration and accelerated wound healing suitable for application extending from simple wound coverings to sophisticated artificial skin matrixes (4). Chitin and its derivatives have been reported to facilitate wound healing in clinical cases. Mizuno et al. (24) reported that chitosan was a good wound‐healing material and that the incorporation of basic fibroblast growth factor accelerated the rate of healing. Wood and coworkers reported the evaluation of chitin and chitosan on fibroblast and keratinocyte proliferation (25). The authors indicated that higher the degree of deacetylation the better the modulation of mitogenesis of cells. The concept of fluid‐absorbing chitin beads has been proposed as a wound dressing material (26). The direct use of in situ chitin with fungal mycelia from the fungus Ganoderma tsugae to produce wound‐healing sacchachitin membranes has also been shown (27). Chitosan in combination with alginate as polyelectrolyte complex films have also been prepared and evaluated as potential wound dressing materials (28). Chitin nanofibrils linked to chitosan glycolate as spray, gel and gauze preparations have been studied for wound repair (29). Good results have been reported confirming the effectiveness of chitin in healing cutaneous lesions. The chitin membranes with optimal performance characteristics of a wound dressing and no toxicity or possible adverse reactions are thus a valuable dressing material for use in the management of skin wounds of various aetiology.
References
- 1. Eisenbud D, Huang NF, Luke S, Silberklang M. Skin substitutes and wound healing: current status and challenges. Wounds 2004;16:2–17. [Google Scholar]
- 2. Singh R, Chouhan US, Purohit S, Gupta P, Kumar P, Kumar A, Chacharkar MP, Kachhawa D, Ghiya BC. Radiation processed amniotic membranes in the treatment of non‐healing ulcers of different etiology. Cell Tissue Bank 2004;5:129–34. [DOI] [PubMed] [Google Scholar]
- 3. Khor E. Chitin: a biomaterial in waiting. Curr Opin Solid State Mater Sci 2002;6:313–7. [Google Scholar]
- 4. Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials 2003;24:2339–49. [DOI] [PubMed] [Google Scholar]
- 5. Cho YW, Cho YN, Chung SH, Yoo G, Ko SW. Water soluble chitin as a wound healing accelerator. Biomaterials 1999;20:2139–45. [DOI] [PubMed] [Google Scholar]
- 6. Yusof NL, Wee A, Lim LY, Khor E. Flexible chitin films as potential wound‐dressing materials: wound model studies. J Biomed Mater Res 2003;66:224–32. [DOI] [PubMed] [Google Scholar]
- 7. Tomihata K, Ikada Y. In vitro and in vivo degradation of films of chitin and its deacetylated derivatives. Biomaterials 1997;18:567–73. [DOI] [PubMed] [Google Scholar]
- 8. Ueno H, Murakami M, Okumura M, Kadosawa T, Uede T, Fujinaga T. Chitosan accelerates the production of osteopontin from polymorphonuclear leukocytes. Biomaterials 2001;22:1667–73. [DOI] [PubMed] [Google Scholar]
- 9. Suzuki Y, Okamoto Y, Morimoto M. Influence of physicochemical properties of chitin and chitosan on compliment activation. Carbohydr Polym 2000;42:307–10. [Google Scholar]
- 10. Lloyd L, Kennedy J, Methacanon FP, Paterson M, Knill CJ. Carbohydrate polymers as wound management aid. Carbohydr Polym 1998;37:315–22. [Google Scholar]
- 11. Ohshima Y, Nishino K, Yonekura Y, Kishimoto S, Wakabayashi S. Clinical application of chitin non‐woven fabric as wound dressing. Eur J Plast Surg 2004;10:66–9. [Google Scholar]
- 12. Okamoto Y, Kawakami L, Miyatake K, Morimoto M, Shigemasa I, Minami S. Analgesic effects of chitin and chitosan. Carbohydr Polym 2002;49:249–52. [Google Scholar]
- 13. Ishihara M, Nakanishi K, Ono K, Sato M, Kikuchi M, Saito Y, Yuara H, Matsul T, Hattori H, Uenoyama M, Kurita A. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials 2000;23:833–40. [DOI] [PubMed] [Google Scholar]
- 14. Mi FL, Shyu SS, Wu YB, Lee ST, Shyon JY, Huang RN. Fabrication and characterization of a sponge‐like asymmetric chitosan membrane as a wound dressing. Biomaterials 2001;22:165–73. [DOI] [PubMed] [Google Scholar]
- 15. Niekraszewicz A. Chitosan medical dressings. Fibres Textiles East Eur 2005;13:16–8. [Google Scholar]
- 16. Muzzareli RAA. Chitin In: Mark HF, Bikales NM, Overberger CG, Menges G, Kroschwitz JI, editors. Encyclopedia of polymer science and engineering, Vol. 3. New York: Wiley, 1985: 430–40. [Google Scholar]
- 17. Kim SJ, Park SJ, Lee SM, Lee YM, Kim HC, Kim SI. Electroactive characteristics of interpenetrating polymer network hydrogels composed of poly (vinyl alcohol) and poly (N‐isopropylacrylamide). J Appl Polym Sci 2003;89:890–4. [Google Scholar]
- 18. ASTM Standard E96 . Standard test methods for water vapor transmission of materials. In: Annual Book of ATSM Standards, Vol. 04.06. West Conshohocken: ASTM International, 2002;878–85. [Google Scholar]
- 19. Peesan M, Rujiravanit R, Supaphol P. Characterisation of beta‐chitin/poly (vinyl alcohol) blend films. Polym Test 2003;22:381–7. [Google Scholar]
- 20. Hilmy N, Darwis D, Hardiningsih L. Poly(n‐vinylpyrrolidone) hydrogels: 2. Hydrogel composites as wound dressing for tropical environment. Radiat Phys Chem 1993;42:911–4. [Google Scholar]
- 21. Finkelstein P, Laden K, Meichowski W. New methods for evaluating cosmetic irritancy. J Invest Dermatol 1963;40:11–4. [Google Scholar]
- 22. Balakrishnan B, Mohanty M, Umashankar PR, Jayakrishnan A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005;26:6335–42. [DOI] [PubMed] [Google Scholar]
- 23. Jurgens C, Schulz AP, Porte T, Faschingbauer M, Seide K. Biodegradable films in trauma and orthopedic surgery. Eur J Trauma 2006;32:160–71. [Google Scholar]
- 24. Mizuno K, Yamamura K, Yano K, Osada T, Sacki S, Takimoto N, Sakura T, Nimura Y. Effect of chitosan film containing basic fibroblast growth factor on wound healing in genetically diabetic mice. J Biomed Mater Res 2003;64:77–81. [DOI] [PubMed] [Google Scholar]
- 25. Howling GI, Dettmar PW, Goddard PA, Hampson FC, Dornish M, Wood EJ. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro . Biomaterials 2001;22:2959–66. [DOI] [PubMed] [Google Scholar]
- 26. Yusof NLM, Lim LY, Khor E. Preparation and characterization of chitin beads as a wound dressing precursor. J Biomed Mater Res 2001;54:59–68. [DOI] [PubMed] [Google Scholar]
- 27. Su CH, Sun CS, Juan SW, Hu CH, Ke WT, Sheu M‐T. Fungal mycelia as the source of chitin and polysaccharides and their applications as skin substitutes. Biomaterials 1997;18:1169–74. [DOI] [PubMed] [Google Scholar]
- 28. Yan X‐L, Khor E, Lim LY. Chitosan‐alginate films prepared with chitosans of different molecular weights. J Biomed Mater Res 2001;58:358–65. [DOI] [PubMed] [Google Scholar]
- 29. Mattioli‐Belmonte M, Zizzi A, Lucarini G, Giantomassi F, Biagini G, Tucci G, Orlando F, Provinciali M, Carezzi F, Morganti P. Chitin nanofibrils linked to chitosan glycolate as spray, gel, and gauze preparations for wound repair. J Bioactive Compatible Polym 2007;22:525–38. [Google Scholar]


