Abstract
Wound contraction is an orchestrated phenomenon that contributes to closure of wounds that heal by secondary intention. However, excessive and premature contraction results in scarring. Although the exact mechanism of contraction is unknown, the wound closure process is accompanied by and followed by changes in the physical and mechanical properties of the wound and periwound tissues during the biological transformation. Transforming growth factor‐β (TGF‐β) induces a contractile phenotype in the cellular–extracellular matrix. Meanwhile, various external and internal mechanical stresses lead to microdeformations of the wound milieu with resultant upregulation of TGF‐β. Furthermore, the mechanical strain exerted on collagen fibres and other piezoelectric tissues leads to development of piezoelectric current in the wound site, which acts synergistically with TGF‐β. TGF‐β and mechanical strain regulate the orientation of collagen fibres parallel with the skin surface, which minimises the induction of piezoelectricity through the action of internal forces because of improper angulation of collagen fibres and these forces. The resulting dominance of external forces guides the contractile activity towards restoration of the original unwounded tissue architecture and functional activity of the previously wounded milieu. The aforementioned contractile activity proceeds into the remodelling phase of wound healing as the level of TGF‐β is reduced and myofibroblasts undergo apoptosis.
Keywords: Mechanical strain, Piezoelectricity, Transforming growth factor‐β, Wound contraction
Introduction
Restoration of the anatomical integrity of full‐thickness excisional cutaneous wounds in adult mammals is a highly orchestrated phenomenon demanding harmonised interactions of various cellular and extracellular elements. Wound contraction, the centripetal movement of the wound periphery, plays a key role in the efficient closure of dermal wounds. However, aberrant or excessive contraction patterns are both undesirable because of impaired healing response and cosmetic and functional concerns. Wound contraction accounts for approximately a 20–30% reduction in wound size in humans and 80–90% in animals with mobile skin like rats 1, 2. Several mechanisms have been proposed for the explanation of this phenomenon, each with potential strengths and drawbacks.
The most widely accepted hypothesis proposes that the cellular elements of granulation tissue – fibroblasts and myofibroblasts – generate contractile forces within the wound milieu, leading to direct inward movement of wound edges by means of cellular and extracellular connections 3, 4, 5, 6. As an alternative, some authors believe that fibroblasts act as single units producing cell locomotion forces, leading to reorganisation of fibrous collagen lattices, which in turn causes an indirect reduction in size of the wounded area by facilitating the transmission of granulation tissue forces (7). However, the fact that blocking of collagen production has been shown to have no effect on the rate of closure contradicts the aforesaid view (8). The purse‐string mechanism attributes wound contraction to some structural or functional entity operating circumferentially analogous to a muscular sphincter (9). Gross et al. (10) showed that interruption of wound continuity through circumferential resection of a thin strip of the wound edge does not alter the rate of closure, which poses a serious argument against the purse‐string mechanism. The most recent studies suggest that polarised coordinated migration of a rim of densely packed proliferative fibroblasts underlying wound edges, or so‐called ‘picture frame’, is responsible for the centripetal movement of the wound periphery, reducing the wound surface area 10, 11. Yet, the picture‐frame theory cannot explain the extant difference between its own concept and the in vitro experiments underscoring the key role of central granulation tissue in the process of contraction.
The hypothesis of dynamic biophysical matrix contraction
The wound milieu is a composite structure comprising various microenvironments with different bioanatomical and mechanical properties (12). For example, collagen fibres in vascular wall and in extracellular matrix – which are two different microenvironments – when affected by equivalent external stress vectors are strained differently and exhibit dissimilar biological responses. Evidence from finite element analysis of microdeformations of wound tissue during vacuum‐assisted closure therapy confirms this notion (13). The assembly of vital/cellular and non vital/extracellular microenvironments gives rise to organised and functional macroenvironments or matrices, for example granulation tissue, which is composed of several vital and non vital elements. These functional matrices are dynamic because their structure and composition are altered over time being replaced with different functional macroenvironments. A paradigmatic example would be the substitution of granulation tissue in a dermal burn wound, partly with original cutaneous tissue and partly with scar tissue.
As wound healing progresses through overlapping phases, the quality and also the quantity of various constituent elements of wound milieu are modified 14, 15. For example, during angiogenesis, the number of newly formed blood vessels increases and subsequently the structural maturation of the vessels takes place. This time‐dependent wound dynamism leads to the simultaneous alteration of physical properties of the wound tissues, with ongoing biological transformation of micro‐ and macroenvironments, for example maturation of collagen fibres and vasculature, respectively (biophysical coupling). Biophysical coupling is the reciprocal interactions of physical and biological properties of wound milieu throughout the healing period. For instance, physical modulation of the biological characteristics of wound has been suggested (16). Moreover, physical properties of wound are modified parallel with ongoing biological transformation (14). Consequently, the distribution of mechanical stresses throughout the wound would show a dynamic trend (mechanophysical coupling). In contrast, the structural elements of the microenvironments – for instance endothelial cells or collagen fibres of blood vessels – which are the sensory units of the produced strain, would show dissimilar biological responses to the exerted mechanical stimuli in various time points throughout the healing period (biomechanical coupling). Biomechanical coupling has two major causes. First, generated strains are a major determinant of dynamic mechanical properties of living tissues. For instance, while high strain rates decrease the stiffness of skin, lower rates enhance it (17). Also, the strain detection by the sensory units, for example fibroblasts, myofibroblasts, which determines their biological response, varies over time owing to altered mechanical attributes. The biological–physical–mechanical axis interactions delineate a complicated scenario through which the changes in one element of the continuum parallel and mutually affect the others in healing wounds. Of particular importance and relevance to the present hypothesis is the dissection of the aforementioned wound‐associated mechanical stimuli.
The wound site is subjected to various mechanical forces that can be categorised either as extrinsic or as intrinsic based on their origin (18). Extrinsic forces are generated in the deep and superficial periwound tissues and are transmitted through connecting elements, such as extracellular fibres running between wounded and normal tissue, to the wound site. These forces vary considerably with reference to their quality and quantity. For example, the magnitude of these forces exhibits a broad range, and variations in their natural tensile, compressive or shear direction; frequency; duration and other characteristics add to their relative non uniformity. The intrinsic forces, which have their origin within the wound milieu (the immediate periwound tissues), either are passive like the interstitial fluid pressure 19, 20 and pressure from the percolation of the inflammatory exudates to the extra‐cellular matrix (ECM) (21) or exhibit an active nature like the intrinsic contractile stress caused by cell–matrix interactions 22, 23. Intrinsic forces exhibit more uniformity in quality and quantity compared with extrinsic forces. Moreover, in contrast to extrinsic forces, intrinsic ones correlate with specific temporal and spatial patterns as a reflection of biological–physical–mechanical axis events. Biological–physical–mechanical axis reflects the aforementioned interactions of biological, physical and mechanical domains in healing wounds. Myofibroblasts are the main contractile units within wound tissues. Around the seventh day after wounding, the differentiation of these cells begins (24). This coincides with the upregulation of transforming growth factor (TGF)‐β1. This growth factor in the presence of fibronectin (FNX) stimulates the differentiation of the myofibroblasts from precursor cells. The presence of FNX is required for mechanical loading – which is the development of isometric tension – of the myofibroblasts, unloading – release of mechanical strain – of which would result in the consequent apoptosis. The mean contractile force produced by myofibroblasts and fibroblasts, when cultured on a substratum with low elastomer stiffness, approximated 2·2 and 2·0 μN/cell, respectively (25). What is more, the forces produced by fibroblasts were unaffected by augmentation of elastomer stiffness, but forces measured for myofibroblasts increased to a mean value of 4·1 μN/cell (25). The implication is that myofibroblasts are the main mechanoresponsive cells that possess a high degree of biophysical plasticity in the wound milieu.
The exertion of mechanical stress to the healing wound produces microdeformations in the wound space. These deformations are transferred to the extant cells. It has been shown that mechanically loaded living cells can proliferate in the presence of soluble growth factors, whereas unloading leads to cell cycle arrest and eventual apoptosis. Also, microdeformations of the strained collagen fibres increase the negative electrical charge in situ (22). It has been shown that the enhanced negative charge stimulates proliferative activity of soft tissue cells in the vicinity of the mechanically loaded area (22). In this environment, the mechanical stress is distributed throughout the wound milieu and is transferred to the different elements of the extant microenvironments. Some of these elements, for example collagen fibres and DNA, possess piezoelectric properties. The contraction of these elements would result in the production of piezoelectricity. However, as mentioned previously, the amount of force exerted to these elements and thus the quantity of the piezoelectricity would be proportional to the mechanical properties of the aforesaid micro‐ and macroenvironments. Regarding the concept of wound dynamics, we predict that the quality and quantity of piezoelectric current may change concurrently with the tissue changes as the healing processes progress. Piezoelectricity has an important role in the healing of live tissues (23). The strain‐induced electrical current modulates biological events at the cellular and the molecular level (23). It is now evident that TGF‐β and electric current act synergistically to enhance the effect of each other 26, 27. Electrical current has also been suggested to regulate the signalling pathway of TGF‐β(28). Falanga et al. (29) have shown that electrical stimulation (ES) upregulates receptors for TGF‐β on human dermal fibroblasts in culture. Microdeformations of the wound milieu and the resultant shear strain upregulate the expression of TGF‐β(30) and also produce piezoelectricity. Hence, mechanical strain may regulate wound contraction and also extracellular matrix remodelling through modulation of the biological–physical–mechanical axis and the synergistic interaction of TGF‐β and piezoelectricity. The previously mentioned web of events may provoke a biomechanical cycle whereby mechanical stimulation regulates production of piezoelectricity, which in turn modulates expression and signalling of TGF‐β. Subsequently, this growth factor affects the production of piezoelectricity through alteration of the extracellular matrix, especially the mechanical properties of collagen fibres.
The biological effects of these exogenous mechanical stimuli necessitate a modulation of previous wound treatment modalities. As exogenous and endogenous mechanical stimulation enhance tissue repair, we encourage practitioners to avoid immobilisation of wound and periwound tissues during the proliferative and remodelling phases of healing. Moreover, the existence of an intrinsic natural control mechanism in the target or the wounded area, to control the level of exerted mechanical stimuli, seems plausible. A second important question is what is the mechanism through which similarity of structural and functional properties of repaired tissue is restored to its original state? Considering temporal correlation, a single mechanism must fulfil both demands.
It has been shown that a constant pretension approximating 1 MP exists in normal skin, which arises from interactions of cells with their extracellular matrix as well as from the tension, which has been incorporated into the collagen fibril network during development (18). This pretension increases the coefficient of elasticity of the skin and therefore decreases its mobility. Thus, a specific stress would produce lower strain in the wound milieu, limiting the biological effects such as generation of hydraulic signals and induction of piezoelectricity. It seems that after wounding, release of pretension at the wound site may exaggerate the impact of external mechanical stimuli. Hence, we predict that coincident with the healing process, a ‘biphasic force shift’; during primary phase, both extrinsic and intrinsic forces exert their biological effect. However, during the second phase, extrinsic forces mask the effect of intrinsic forces. The mechanism for development of biphasic force has been elucidated below.
Initially, after deposition of collagen fibres, both endogenous and exogenous mechanical stresses affect the wound macroenvironment. It has been shown that an angle of 45° between applied forces and collagen fibres is necessary for maximal induction of piezoelectricity 31, 32. Dynamic mechanical properties of the wound site are another regulatory mechanism. The anisotropy of collagen and other piezoelectric generating tissue elements leads to proper angulation of various force vectors within the wound tissues. However, as wound healing progresses, the orientation of collagen fibres takes a more uniform pattern. It has been suggested that the application of TGF‐β reduces the anisotropy of collagen fibre orientation by 29% after 14 days in healing cutaneous wounds compared with normal unwounded skin (33). Thus, the blocking of anisotropy enhances the directional non uniformity of collagen fibres by 16·2% compared with TGF‐β‐treated wounds, which are 12·8% more isotropic than unwounded skin. The uniform arrangement of collagen fibres throughout the healing period call forth a second phase. In this new arrangement, collagen fibres are parallel with the skin surface. This happens because collagen fibres became rapidly oriented in the direction of the force exerted on them (34). Thus, the majority of multiplanar contractile (intrinsic) forces are perpendicular to cutaneous wounds or parallel with the skin surface. This angulation of force vectors and collagen fibres – which are the major piezoelectric element of skin – decreases the amount of intrinsic‐strain‐related piezoelectricity. Moreover, the increasing stiffness of granulation tissue and constant nature of intrinsic forces attenuate the production of piezoelectricity by these forces. Additionally, other molecules, for example DNA, contribute to generation of low levels of intrinsic‐stress‐induced piezoelectricity. At the same time, several parameters contribute to the dominancy of the extrinsic forces including cyclical and intermittent nature, higher intensity and axial variability (multiaxial nature, Figure 1) of extrinsic force vectors. Multiaxial property of extrinsic forces is the result of variation in source – adjacent tissues – and direction. Therefore, during the second phase of ‘biphasic force shift’, extrinsic stresses are dominant and exert their biological effects by masking the attenuated intrinsic forces. The extrinsic (exogenous) mechanical stresses are proportional to the tissue function, anatomy and structure. So we predict that bioanatomical properties of the wound milieu and adjacent tissues determine the characteristics of piezoelectric potentials, which in turn modulate healing of wounded tissue, and this cycle proceeds through the remodelling phase. Consistent with the latter, Burgess et al. (35) found that mechanical stresses at the wound site may play a role in guiding collagen fibrillogenesis because altered tensions during wound closure affect the extent of scarring. Furthermore, Reger et al. (36) found that electrical stimulation may orient new collagen formation in a pattern similar to normal skin even in the absence of neural influences. The additional confirmative evidence comes from study of Osaki (37) who showed that the degree of orientation of collagen fibres in calf skin was greater in areas where skin motions were marked. Gradually, the cycle is coalesced with normal ongoing remodelling of repaired tissue similar to that which occurs in unwounded tissues. Through this mechanism (i) wound contraction is controlled, (ii) original tissue structure is restored and (iii) the influence of unnecessary interfering mechanical forces (peripheral mechanical fog) is minimised. Mechanical fog describes the unfavourable extrinsic mechanical stimuli developed as a result of mobility of adjacent tissues with adverse effects on the healing procedure because of their direction or magnitude. Irion et al. (38) have recently reported that mechanically stimulated 4‐mm biopsy wounds in rats reduced time to closure by nearly 50% compared with sham‐stimulated wounds.
Figure 1.
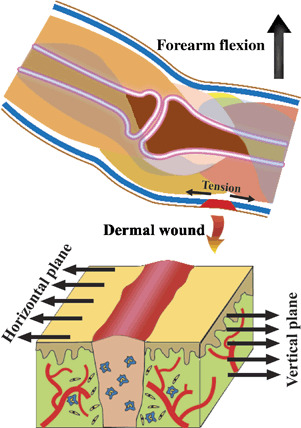
Development of extrinsic force vectors and multiaxial nature of these forces.
The proposed hypothesis has important clinical implications, especially in conditions in which wound contraction is considered a serious challenge, for example plastic and reconstructive surgery and the conservative management of chronic wounds. The use of electrical stimulation for the enhancement of healing should be customised for application to various regions of human body according to mechanical properties, for example functional mobility and anatomical features such as surface curvature. Furthermore, alteration of biological–physical–mechanical axis modules through therapeutic approaches may influence the quality and quantity of wound contraction and resultant scarring.
We have described the phenomenon of tensgrity in which the cell itself behaves as a mechanical transducer, which allows mechanical events that distort the cell to be detected and routed to intracellular signalling mechanisms that produce rapid adaptation to the mechanical stress 39, 40, 41.
The future research may be directed towards evaluation of present hypothesis by in vitro and in vivo models. The investigation of effect of piezoelectricity on wound contraction through implantation of piezoelectric sensors in wounded regions seems interesting. Finally, reverse piezoelectric phenomenon as a therapeutic modality to diminish contraction and scarring may prove useful.
Glossary
Piezoelectric effect: The generation of electricity or electric polarity in dielectric crystals subjected to mechanical stress, or the generation of stress in such crystals subjected to an applied voltage.
Angulation of collagen fibres: The geometric spatial arrangement of collagen fibres that leads to formation of an angle between these fibres.
Microdeformations: Microscopic deformations of tissue.
Tensgrity: Tensgrity describes a structural‐relationship principle in which structural shape is guaranteed by the finitely closed, comprehensively continuous, tensional behaviours of the system and not by the discontinuous and exclusively local compressional member behaviours. Tensgrity provides the ability to yield increasingly without ultimately breaking or coming asunder.
References
- 1. Rudolph R. Location of the force of wound contraction. Surg Gynecol Obstet 1979;148:547–51. [PubMed] [Google Scholar]
- 2. McGrath MH, Simon RH. Wound geometry and the kinetics of wound contraction. Plast Reconstr Surg 1983;72:66–72. [DOI] [PubMed] [Google Scholar]
- 3. Krummel TM, Ehrlich HP, Nelson JM, Micna BA, Thomas BL, Haynes JH, Cohen IK, Diegelmann RF. In vitro and in vivo analysis of the inability of fetal rabbit wounds to contract. Wound Repair Regen 1993;1:15–21. [DOI] [PubMed] [Google Scholar]
- 4. Clark RA. Regulation of fibroplasia in cutaneous wound repair. Am J Med Sci 1993;306:42–8. [DOI] [PubMed] [Google Scholar]
- 5. Billingham RE, Russell RS. Studies on wound healing, with special reference to the phenomenon of contracture in experimental wounds in rabbits’ skin. Ann Surg 1956;144:961–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gabbiani G, Ryan GB, Majno G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971;27:549–50. [DOI] [PubMed] [Google Scholar]
- 7. Ehrlich HP, Rajaratnum JB. Cell locomotion forces versus cell contraction forces for collagen lattice contraction: an in vitro model of wound contraction. Tissue Cell 1990;22:407–17. [DOI] [PubMed] [Google Scholar]
- 8. Grillo HC, Gross J. Studies in wound healing. III. Contraction in vit. C. deficiency Proc Soc Exp Biol Med 1959;101:268–70. [DOI] [PubMed] [Google Scholar]
- 9. Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature 1992;360:179–83. [DOI] [PubMed] [Google Scholar]
- 10. Gross J, Farinelli W, Sadow P, Anderson R, Bruns R. On the mechanism of skin wound “contraction”: a granulation tissue “knockout” with a normal phenotype. Proc Natl Acad Sci U S A 1995;92:5982–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grillo HC, Watts GT, Gross J. Studies in wound healing: I. Contraction and the wound contents. Ann Surg 1958;148:145–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cook TH. Mechanical properties of human skin with aging. In: Balin AK, Kligman AM, editors. Aging and the skin. New York: Raven Press, 1989:205–25. [Google Scholar]
- 13. Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum‐assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114:1086–96. [DOI] [PubMed] [Google Scholar]
- 14. Fung YC. Biomechanics: mechanical properties of living tissues, 2nd edn. New York: Springer‐Verlag, 1993. [Google Scholar]
- 15. Dunphy JE. The fibroblast: a ubiquitous ally for the surgeon. N Engl J Med 1963;268:1367–77. [Google Scholar]
- 16. Farahani RM. Wound healing in the context of mechanical strain: “coupled pendulum” hypothesis. Med Hypotheses 2007;69:711 [DOI] [PubMed] [Google Scholar]
- 17. Edsberg LE, Mates RE, Baier RE, Lauren M. Mechanical characteristics of human skin subjected to static versus cyclic normal pressures. J Rehabil Res Dev 1999;36:133–41. [PubMed] [Google Scholar]
- 18. Silver FH, Siperko LM, Seehra GP. Mechanobiology of force transduction in dermal tissue. Skin Res Technol 2003;9:3–23. [DOI] [PubMed] [Google Scholar]
- 19. Wiig H, Noddeland H. Interstitial fluid pressure in human skin measured by micropuncture and wick‐in‐needle. Scand J Clin Lab Invest 1983;43:255–60. [PubMed] [Google Scholar]
- 20. Shimizu S, Tanaka H, Sakaki S, Yukioka T, Matsuda H, Shimazaki S. Burn depth affects dermal interstitial fluid pressure, free radical production, and serum histamine levels in rats. J Trauma 2002;52:683–7. [DOI] [PubMed] [Google Scholar]
- 21. Myers MB, Cherry G, Heimburger S, Hay M, Haydel H, Cooley L. The effect of edema and external pressure on wound healing. Arch Surg 1967;94:218–22. [DOI] [PubMed] [Google Scholar]
- 22. Tanaka T. Gels. Sci Am 1981;244:124–38. [DOI] [PubMed] [Google Scholar]
- 23. Turchaninov R. Research & massage therapy. Massage Bodywork 2000;October/November: 60–71. [Google Scholar]
- 24. Clark RAF., editor. The molecular and cellular biology of wound repair. New York: Plenum, 1996. [Google Scholar]
- 25. Wrobel LK, Fray TR, Molloy JE, Adams JJ, Armitage MP, Sparrow JC. Contractility of single human dermal myofibroblasts and fibroblasts. Cell Motil Cytoskeleton 2002;52:82–90. [DOI] [PubMed] [Google Scholar]
- 26. Lee PY, Chesnoy S, Huang L. Electroporatic delivery of TGF‐beta1 gene works synergistically with electric therapy to enhance diabetic wound healing in db/db mice. J Invest Dermatol 2004;123:791–8. [DOI] [PubMed] [Google Scholar]
- 27. Zhuang H, Wang W, Seldes RM, Tahernia AD, Fan H, Brighton CT. Electrical stimulation induces the level of TGF‐beta1 mRNA in osteoblastic cells by a mechanism involving calcium/calmodulin pathway. Biochem Biophys Res Commun 1997;237:225–9. [DOI] [PubMed] [Google Scholar]
- 28. Ugarte G, Brandan E. Transforming growth factor β(TGF‐β) signaling is regulated by electrical activity in skeletal muscle cells. J Biol Chem 2006;281:18473–81. [DOI] [PubMed] [Google Scholar]
- 29. Falanga V, Bourguignon G, Bourguignon L. Electrical stimulation increases the expression of fibroblast receptors for transforming growth factor‐beta. J Invest Dermatol 1987;88:488–92. [Google Scholar]
- 30. Song RH, Kocharyan HK, Fortunato JE, Glagov S, Bassiouny HS. Increased flow and shear stress enhance in vivo transforming growth factor‐β1 after experimental arterial injury. Arterioscler Thromb Vasc Biol 2000;20:923. [DOI] [PubMed] [Google Scholar]
- 31. Facade E, Yasuda I. Piezoelectric effects in collagen. J Appl Physiol 1964;3:117. [Google Scholar]
- 32. Noris‐Suárez K, Lira‐Olivares J, Ferreira AM, Feijoo JL, Suárez N, Hernández MC, Barrios E. In vitro deposition of hydroxyapatite on cortical bone collagen stimulated by deformation‐induced piezoelectricity. Biomacromolecules 2007;8:941–8. [DOI] [PubMed] [Google Scholar]
- 33. Bowes LE, Jimenez MC, Hiester ED, Sacks MS, Brahmatewari J, Mertz P, Eaglstein WH. Collagen fiber orientation as quantified by small angle light scattering in wound treated with transforming growth factor‐β2 and its neutralizing antibody. Wound Repair Regen 1999;7:179–86. [DOI] [PubMed] [Google Scholar]
- 34. Noorlander ML, Melis P, Jonker A, Van Noorden CJF. A quantitative method to determine the orientation of collagen fibers in the dermis. J Histochem Cytochem 2002;50:1469–74. [DOI] [PubMed] [Google Scholar]
- 35. Burgess LP, Morin GV, Rand M, Vossoughi J, Hollinger JO. Wound healing. Relationship of wound closing tension to scar width in rats. Arch Otolaryngol Head Neck Surg 1990;116:798–802. [DOI] [PubMed] [Google Scholar]
- 36. Reger SI, Hyodo A, Negami S, Kambic H, Sahgal V. Experimental wound healing with electrical stimulation. Artif Organs 1999;23:460. [DOI] [PubMed] [Google Scholar]
- 37. Osaki S. Distribution map of collagen fiber orientation in a whole calf skin. Anat Rec 1999;254:147–52. [DOI] [PubMed] [Google Scholar]
- 38. Irion GL, Stone S, Fischer T, Finch VP, Phillips LR, Frederickson C. Accelerated closure of biopsy‐type wounds by mechanical stimulation. Adv Skin Wound Care 2006;19:97–102. [DOI] [PubMed] [Google Scholar]
- 39. Ingber DE. Tensgrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol 1997;59:575–99. [DOI] [PubMed] [Google Scholar]
- 40. Coughlin MF, Stamenovic D. A tensgrity model of the cytoskeleton in spread and round cells. J Biomech Eng 1998;120:770–7. [DOI] [PubMed] [Google Scholar]
- 41. Chen CS, Ingber DE. Tensgrity and mechanoregulation: from skeleton to cytoskeleton. Osteoarthritis Cartilage 1999;7:81–94. [DOI] [PubMed] [Google Scholar]


