Abstract
Ulcers in radiated skin continue to be a challenge for health care practitioners. Healing impairment in the setting of radiation‐damaged tissue will most of the time lead to chronic wounds that reduce the patient’s quality of life. In this review, we present an update of the pathophysiology of tissue damage caused by radiation that leads to chronic ulceration. We also explore the evidence available on the different prevention and treatment modalities that have been reported in the literature. The evidence for most preventive measures is inconclusive; however, sucralfate and amifostine seem to be the adequate recommendations for prophylaxis. As for treatment of ulcerated patients, the strongest level of evidence found was for the use of pentoxifylline, but proper trials are still scarce to be considered standard adjuvant therapy. Hyperbaric oxygen, cytokines and other growth factors and surgical interventions have shown some benefit in case reports and case series only. Other therapies show promise based on their mechanism of action but need to be tested in human studies and clinical trials.
Keywords: Radiation, Radiotherapy, Skin ulcer
Introduction
The term radiation injury refers to the morphological and functional changes that occur in non cancerous tissue as a direct result of ionising radiation (1). Tissue damage can lead to dermatitis and eventually skin necrosis and ulceration. In patients with ulceration and in those undergoing surgery, the structural damage caused by radiation may further delay healing.
In patients with cancer, good quality of life is cornerstone. Multidisciplinary treatment and support of these patients and their families are common practices, and this approach has improved the quality of life of patients, despite the frequent adverse reactions observed during their treatment. A wound in this setting can further impact patient’s everyday life.
The chronic complications depend on the total dose delivered, the dose per fraction, the energy and particles used, the interval between fractions, the volume of normal tissue that receives a high dose and the use of concomitant chemotherapy or biological modifier.
In the studies performed in the 1990s, the subcutaneous fibrosis grades 3 and 4 of the Radiation Therapy Oncology Group is estimated in 3% of patients. The induced malignancies appear in 0·25% 2, 3. The percentage of fibrosis after concomitant chemoradiation is reported in 10–60%. There have been very few reports of successful agents for prevention and treatment of radiation‐induced skin reactions or ulcers in radiated skin (URS). This clearly reflects the complexity of the problem.
In this manuscript, we intend to review the literature on the topic and analyse the available evidence by levels (Table 1), providing up‐to‐date information on the pathophysiology of radiation injury, the acute and chronic clinical manifestations, general recommendations on prevention and a general algorithm for the management of patients with URS. We also explore potential therapies based on their mechanism of action.
Table 1.
Levels of evidence used in the present review (103)
| Levels of evidence | |
|---|---|
| Ia | Evidence obtained from meta‐analysis or systematic review of randomised controlled trials |
| Ib | Evidence obtained from at least one randomised controlled trial |
| IIa | Evidence obtained from at least one well‐designed controlled study without randomisation |
| IIb | Evidence obtained from at least one other type of well‐designed, quasi‐experimental study without randomisation |
| III | Evidence obtained from well‐designed, non experimental, descriptive studies, such as comparative, correlation and case studies |
| IV | Evidence obtained from expert committee reports or opinions and/or clinical experiences of respected authorities |
Effects of radiation therapy
Ionising radiation causes damage to molecules by means of energy transference. This energy generates highly reactive chemical products such as free ion radicals that can subsequently combine with normal body chemicals and react with cellular components, ultimately causing intracellular and molecular damage.
Even though lipids and proteins are massively destroyed, the cellular DNA is implicated as the primary target for the biological and lethal effects of free radicals induced by ionising radiation 4, 5, 6. About 60–70% of this DNA damage is caused by hydroxyl radicals (OH•), which are considered the most damaging of all free radicals generated in organisms (7). Most of this damage can be repaired and enzymatic processes may continue; however, the radiobiological death when the cell loses its ability to divide and produce a clone is established 8, 9.
Morphological changes when using low doses of radiation occur mainly in the nucleus and are probably because of an apoptotic mechanism. In contrast, when using higher doses, the cell nucleus becomes dense and disfigured, and there may be loss of the nuclear membrane. These changes are probably caused by direct cellular necrosis. The cytoplasm may show distension, the mitochondria may be deformed and the endoplasmic reticulum may degenerate 6, 10, 11.
The success of the clinical application of radiation therapy rests on its lethal effects on cancer cells at sublethal levels for normal tissue (12).
Clinical manifestations
Acute effects
The most sensitive cells to radiation therapy are those that divide rapidly, such as skin, bone marrow and gastrointestinal tract cells (13).
Acute effects result from necrosis of the rapidly proliferating cell lines: erythema, as a result of dilatation of capillaries associated with an increased vascular permeability, and desquamation. Radiation inhibits mitotic activity in germinal cells of the epidermis, hair follicles and sebaceous glands. By the third week after exposure, erythema is localised to the radiation field and the skin is noticeably red, oedematous, with dry and moist desquamation, with loss of keratinised layers and depletion of the basal and stem cell population, scaly, hyper‐ or hypopigmented (14), warm and tender (6). All these are commonly referred to as radiodermatitis.
Ideally, surviving germinal cells regenerate to repopulate the epidermis and allow for healing, however, when this repopulation fails, an acute URS occurs (12).
An experimental study radiating rabbit hind limbs was able to show that there is only a transient impairment of tissue perfusion in the skin after acute irradiation, but after 11 weeks, subcutaneous tissue hypoxia returns above the level necessary for collagen synthesis. Nevertheless, in this study, light microscopy of the affected tissues showed fibrosis and blood vessel and bone marrow changes associated with progressive tissue hypoxia (15).
With modern megavoltage machines, the maximum deposited dose to the skin is at 0.5–4 cm below, which only causes hyperpigmentation and dry desquamation. The skin tolerance is well known with the conventional schemas of 2 Gy per fraction to a total dose of 66 Gy.
Long‐term effects
Two theories dominate current knowledge regarding the cause of late radiation injuries. One theory explains that late injury is a consequence of depletion of parenchymal and stromal elements (16). In contrast, the other theory 12, 14, 16, 17 supports hypoxia and ischaemia as responsible for late radiation damage. Proliferation of subendothelial connective tissue in small arteries induced by ischaemia causes marked narrowing and thrombosis of the microvasculature or ‘progressive obliterative endarteritis’(14). Functional changes by impaired blood flow further decrease tissue oxygenation 12, 17, 18. Other factors involved are excessive fibrosis and the direct cellular damage with chromosomal alterations that may further prevent normal cellular replication.
With high‐energy X rays, the late damage can occur in the dermis and is characterised by atrophy, contraction and induration (19).
One year after radiation, epidermal atrophy can be seen, probably because of alteration of the ground substance 12, 14. The epidermis becomes thinner; drier; more semitranslucent, with telangiectasias and vessels easily visible; hypovascularised; extremely painful and injured without difficulty by slight trauma or infection (6). Hair follicles and sebaceous glands are usually absent and granulation tissue is scattered and unhealthy 8, 14 (Figure 1). One of the predominant effects in the skin is cutaneous and subcutaneous fibrosis. In the dermis, elastic fibres are destroyed and much of the collagen and the subcutaneous adipose tissue are replaced by atypical fibroblasts and massive production of extracellular matrix, causing so‐called irreversible, radiation‐induced fibrosis and keratoses. The transforming growth factor (TGF)‐β1 and ‐β3 are the critical mediators of this fibrosis. There is sustained expression in cells such as miofibroblasts, endothelial cells and keratinocytes that perpetuate this fibrotic reaction. Cutaneous damage can therefore be summarised by the ‘3 Hs’, hypovascular, hypoxic and hypocellular (Figure 2). Heterotopic calcification is a late radiation damage that occurs infrequently in regions with previous malignant involvement and in previously normal tissue (20). Increased vulnerability of the affected area may be complicated by the risk of secondary ulceration and impaired joint mobility as well as malignancies 8, 21. The most common of these malignancies are basal and squamous cell carcinomas that tend to be aggressive 6, 22, 23, 24, 25, 26.
Figure 1.

Radiation‐induced damage where atrophy, lack of adnexa, skin fragility and ulceration developed after treatment for breast cancer in this male patient.
Figure 2.
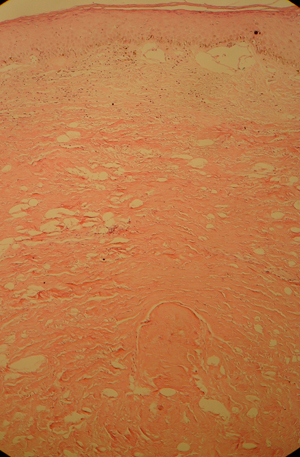
Microscopic chronic changes associated with radiodermits. Note epidermal atrophy, dilated blood vessels, loss of hair and glands and sclerosis.
The lymphatics are absent or blocked by fibrous tissue. Myocytes develop vacuoles and the muscles become scarred containing very few vessels. The bone shows marked obliteration of the vessels in the periosteum and becomes devitalised (8).
Evidence supporting a direct inhibitory effect of ionising irradiation on wound healing is increasing 27, 28. Delayed ulcers are more common than acute ulcers; they heal slowly and may persist for several years. The time difference between acute‐ and long‐term effects is because of the proliferation velocity and reproductive cycles of the diverse cell populations irradiated (18). Morbidity of radiation depends on the dose received, time over which the dose was received, volume of tissue irradiated and quality and type of radiation (10).
Prevention of skin damage
Modern radiotherapy focuses around giving an effective enough dose to rid the tissues of malignant cells while trying to avoid undesirable tissue damage that in turn will lead to skin breakdown. Some research has been carried out trying to minimise the effects of radiation on tissues to allow for normal healing.
Aistars (29) published a systematic review on skin care products used during and prior to breast cancer radiation. The author found no differences in the studies testing specific interventions (e.g. aqueous cream, aloe vera gel, washing the treatment area previously) in the prevention of radiation‐induced skin toxicity. In a systematic review by Bolderston et al. on the prevention of acute skin reactions (mainly desquamation and dermatitis), a variety of treatments that have been used for radiated skin are reported. Based on this review, they established practice guidelines for skin care and prevention of acute skin reactions (Table 2) (30).
Table 2.
Practice guidelines for skin care and prevention of acute skin reactions. Modified from Bolderston et al. (30).
| Practice guidelines |
|---|
| 1 Do not restrict skin washings (i.e. showers, baths). Use mild soap and water or water alone |
| 2 Use mild shampoo for scalp in patients receiving radiation therapy to the head |
| 3 Do not limit personal hygiene practices as this may lead to psychosocial distress for the patient |
| 4 In patients with breast cancer, limited evidence suggests that calendula ointment may decrease the occurrence of dermatitis |
| 5 The use of topical agents (corticosteroids, sucralfate cream, Biafine®, ascorbic acid, aloe vera, chamomile cream, almond ointment or polymer adhesive skin sealant) cannot be supported or refuted (lack of evidence). |
| 6 The use of systemic agents (enzymes, sucralfate, amifostine*) cannot be supported or refuted on the same grounds |
New evidence suggests that amifostine may be of benefit especially for mucositis (41).
Among the diverse physiological functions of Zinc (Zn), it maintains the membrane structure and function through promoting cell growth and suppressing apoptosis and protecting against free radical damage during inflammation, making it a potential radioprotector. To evaluate this potential effect, Ertekin et al. (31) performed a study in 37 rats showing that Zn prevents epidermal atrophy, dermal degeneration and hair follicle atrophy. The authors state that Zn may protect uninjured tissues against the prejudicial effects of chemotherapy and radiotherapy, without inhibiting the healing effects (31). With these results, the authors suggest that Zn could be used for reducing radiation‐induced toxicity in humans.
Sucralfate octasulfate
Sucralfate octasulfate (SOS) is a persulfated disaccharide that was introduced as an anti‐ulcer drug more than 20 years ago. Although its mode of action is not yet understood, in animal models, sucralfate stimulates the regenerative processes in the skin and accelerates wound healing.
Maiche et al. (32) conducted a randomised controlled study with sucralfate cream versus placebo in 50 patients receiving radiotherapy to the chest wall. They found that the sucralfate cream significantly prevented the acute skin reactions, such as erythema and moist desquamation, and that the recovery of the skin was significantly faster; however, another randomised prospective study was not able to show differences between SOS and placebo in 60 patients (33). Similar results have been found in patients with mucositis 32, 34, 35, 36, 37, 38, 39, 40 caused by radio‐ and chemotherapy.
The available research is therefore inconclusive, and more trials are needed to prove the efficacy of SOS to prevent acute radiation damage.
Amifostine
One of the most promising agents in radiodamage prevention is amifostine, the only radioprotector that has been clinically approved by the Food and Drug Administration for mitigating side‐effects in patients undergoing radiotherapy. Amifostine protects almost all normal tissues from the cytotoxic effects of some chemotherapeutic agents and radiation therapy. Its complex mechanism of action involves free radicals scavenging, DNA protection and repair acceleration and induction of cellular hypoxia (41).
In a study performed by Kouvaris et al. (42), a group of patients with pelvic tumours treated with radiotherapy and amifostine were compared with a control group that did not receive amifostine. Patients from the first group showed 77% lower risk for radiation‐induced dermatitis. Another study performed in 40 patients with pelvic tumours showed that subcutaneous amifostine applied before each radiotherapy fraction significantly reduces the incidence of acute perineal skin (43).
Assessment and treatment
URS very often fail to progress at the expected healing rate, and they easily become chronic wounds. Local wound care consists of the debridement of necrotic tissue, control of bacterial burden and inflammation and moisture balance 8, 44 (Figure 3).
Figure 3.
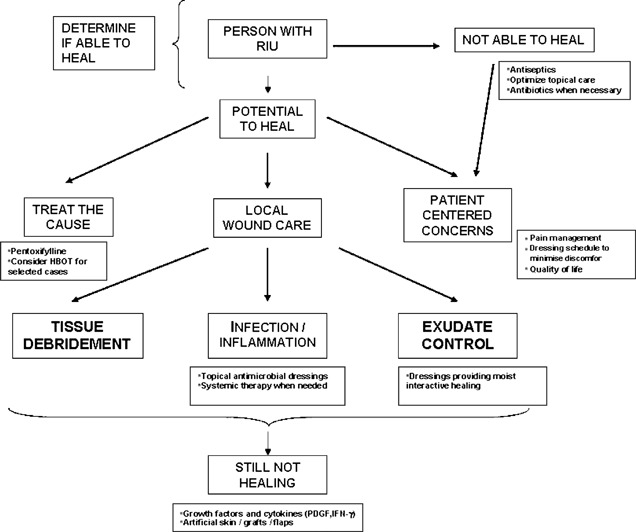
Modified wound care paradigm for the management of patients with URS. Adapted from Sibbald et al. (44). HBOT, hyperbaric oxygen therapy; IFN‐γ, interferon‐gamma; PDGF, platelet‐derived growth factor.
Debridement should only remove dead (necrotic) tissue, avoiding damage to living tissue (bleeding). If pain is of concern, methods that are not painful or even soothing are indicated such as the use of hydrogels or enzymatic ointments. Maggots have been described anecdotally in the literature for palliative care (45). Other forms of debridement may be necessary for debulking large amounts of necrotic tissue, but proper pain management and anaesthesia are mandatory.
The skin should be smoothly cleansed with saline or water and rinsed to avoid irritation. Discomfort, such as dryness and scaliness, can be reduced by applying hydrophilic preparations that act as lubricants, ointments that protect dry lesions, colloidal oatmeal baths and/or steroids that decrease itching (6). Sunlight exposure must be avoided (14). These and other general interventions can be helpful for radiodermatitis.
Topical antimicrobial therapy in the form of antiseptics should be used to prevent infection when host resistance is decreased (i.e. chemotherapy, systemic infection, diabetes). If the wound is infected using agents such as silver sulfadiazine or antimicrobial dressings with ionic silver, iodine (povidone or cadexomer) or even chlorhexidine 8, 12 may be indicated. Drosou et al. published a review of a variety of antiseptics applied on wounds and examined their effects on reepithelialisation and their efficacy on reducing bacterial number and incidence of wound infections (46). Most of these antiseptics did not decrease healing rate.
Systemic antibiotics may be indicated in wounds with deeper infection and cellulitis. Particularly in URS, infection may further increase tissue hypoxia, causing additional ulcers in the surrounding tissue; however, systemic therapy may not reach the damaged tissue because the ulcer is isolated as a result of ischaemia 4, 7.
Dressings preserve a moist environment that intensify reepithelialisation by allowing enzymes to lyse necrotic tissue, facilitating phagocytosis of bacteria and debris by inflammatory cells and allowing fibroblast and keratinocyte migration to the wound (6). Dressings providing moist interactive healing are also useful to protect areas that are exposed to trauma (14). A variety of dressings can be used, such as films, hydrogels, hydrocolloids, foams and calcium alginates among others (6). Pain has been recognised as a major problem for patients with radiation injury (47). Dressings with silicone contact layers that detach without harming the wound bed provide less traumatic changes, improving patient’s quality of life (48).
Although most wounds heal with proper wound bed preparation (49), URS very often fail to resolve and adjunctive therapies have been explored for that purpose.
Adjunctive therapies
Table 3 summarises the existing evidence of the most important papers published on each of the following adjunctive therapies.
Table 3.
Evidence level of trials of interventions for URS
| Therapy | Evidence level | References |
|---|---|---|
| Pentoxifylline | IIa | 16, 17, 50, 51, 104 |
| Hyperbaric oxygen | III | 12, 52, 53, 54, 55, 56, 57, 58 |
| Platelet‐derived growth factor | III | 65, 66, 67, 68, 69 |
| Interferon‐gamma | III | 22, 82 |
| Surgery | III | 8, 14 |
| Ginkgo biloba | Not rated† | 70, 71, 72, 73, 74, 75, 76, 80, 81 |
Only trials in animals are available.
Pentoxifylline
The prophylactic administration of this antifibrotic in the postirradiation period can reduce late radiation injury by (i) increasing red blood cell deformability and prostacyclin release, (ii) inhibiting the activation of neutrophils and (iii) inhibiting the production of thromboxane (50). In some studies, it has decreased the rate of late radiation reaction, but no proven effect has been observed for acute radiation reactions 50, 51.
Futran et al. performed a study in 30 patients with radiotherapy complications who received pentoxifylline for at least 3 months and obtained data, which suggest that this medication may accelerate healing of certain late radiation‐related injuries (soft tissue necrosis and mucosal injury) (50).
Dion et al. (16) studied radiation‐related soft tissue injury in 12 patients with 15 sites of late radiation necrosis who were treated with pentoxifylline 400 mg t.i.d. for 6 months. At the time of analysis, 87% of the necroses had healed completely, and one was partially healed. The author mentions that the time needed for healing with pentoxifylline was significantly less than the duration of non healing prior to starting the drug (average of 9 versus 30·5 weeks). This study suggests that this agent decreased the incidence and severity of late radiation necrosis and improved healing of chronic radiation‐induced ulcers, but no comparison group was included and results may be biased. In a similar study, Dasgeb and Phillips (17) found a 50% regression of progressive osteoradionecrosis at 6 months with a combination of pentoxifylline, tocopherol and clodronate.
Hyperbaric oxygen therapy
Hyperbaric oxygen therapy (HBOT) means the administration of inspired 100% oxygen under higher than atmospheric pressure (2–3 absolute atmospheres), and it is a form of treatment that has been used for 30 years to treat chronic and delayed radiation injuries (52) and nercotising soft tissue infections, among others 12, 53.
HBOT makes the oxygen gradient severely steeper, so that the body can recognise the damaged tissue and support angiogenesis (53). It improves fibroblast growth, collagen formation, neovascularisation, epithelialisation and leukocyte bactericidal activity, and also reduces tissue oedema. All these effects contribute to more rapid wound healing in ischaemic tissue (54). HBOT improves acute radiation injury by preventing tissue hypoxia. It has also been proposed to reverse radiotherapy side‐effects in a limited amount (55).
Marx et al. (56) showed a dose‐dependent increase in vascular density in irradiated rabbits treated with hyperbaric oxygen (HBO). Feldmeier et al. (57) showed evidence of decreased fibrosis in the abdominal organs of mice that received whole abdominal irradiation and then postirradiation HBOT compared with those that received the same radiation course without HBOT.
Yildiz et al. (58) reported two cases of radiation injury treated with HBOT (15 and 140 sessions each) resulting in improvement of tissue ‘nourishment’ (most likely the term refers to an increased perfusion and vascularisation), infection control and clearance of necrotic areas, without any side‐effects. The authors themselves recognise that the efficacy of HBOT cannot be fully proved in this report because of the absence of a control group.
HBOT has been contraindicated in the presence of malignant tumour, with the questionable rationale of HBOT increasing vascularity of tumours. Current research suggests that this is not a concern, and in some instances, it may even have a therapeutic effect 59, 60, 61, 62, 63. HBOT is contraindicated in combination with certain medications, such as cysplatinum, disulfiram and doxorrubicine used in oncological patients (64).
Growth factors
By producing growth factors with biomolecular techniques in yeast or bacteria, more efficient healing of tissue defects can be achieved through the manipulation of the wound’s environment.
The cells located in the wound or around it synthesise platelet‐derived growth factor (PDGF), TGF‐β and epidermal growth factor (EGF). The key element in wound healing is the relationship between these and other growth factors and the wound matrix. As fibroblasts produce extracellular matrix and several growth factors, such as PDGF and EGF, they can trigger tissue regeneration and the remodelling process. Fibroblast growth factor also stimulates the proliferation of capillary endothelial cells, making it a potent angiogenic factor 65, 66.
Platelets and PDGF are a major improvement in the treatment of chronic wounds. PDGF serves as a strong chemoattractant for neutrophils, monocytes and fibroblasts and stimulates mesenchymal cells into synthesising extracellular matrix components, collagenase and other growth factors (67). PDGF also controls the genetic expression of stem cells by modulating signal transduction pathways, which results in cellular division and differentiation. This technology has gained reputation in specialties such as orthopaedic, maxillofacial and plastic surgery. In addition, it is widely being approved as an important method for accelerating wound healing (68).
Wollina et al. also reported a case of a patient who developed chronic URS on her trunk after total body electron beam irradiation. The combination of PDGF gel and hydrophilic copolymer membranes markedly improved healing potency (granulation and reepithelialisation) and pain relief (69).
Ginkgo biloba
It is an herbal remedy that has been promoted as a treatment for a variety of ailments including memory loss, poor concentration, glaucoma, cerebral insufficiency and peripheral circulatory disturbances. The postulated mechanisms of action include increase blood flow, antagonism of platelet‐activating factor, modulation of neurotransmitter and receptor activity and prevention of membrane damage caused by free radicals. It has also been discussed that the inhibition of platelet aggregation is because of the increasing concentrations of endothelium‐derived thrombolytics, such as nitric oxide and prostacyclin. Flavonoids and terpene lactones present in the ginkgo leaf have antihypoxic, antiplatelet and anti‐oedema effects as free radical scavenging that possibly act together for protecting tissue from ischaemia 70, 71, 72, 73, 74, 75.
Sener et al. (76) performed a case–control study in rats that underwent whole‐body irradiation after a pre‐treatment with Gingko biloba extract (EGb). They assessed tissue damage by increased lipid peroxidation, neutrophil infiltration and fibrosis. They found that treatment with EGb depressed lipid peroxidation and neutrophil infiltration, verifying the protective effect of EGb against oxidative injury. EGb reduced radiation‐induced oxidative damage by its free radical scavenging and antioxidant properties, showing that EGb may have the capacity to ameliorate irradiation‐induced oxidative organ injury.
Other studies in rats have shown similar results 77, 78, 79; however, its security profile has been of concern 80, 81.
The benefit of this drug may be limited by its adverse effects such as gastrointestinal symptoms, headache, nausea and vomiting. It may also interact with anticoagulants by increasing their effects (81) and may cause spontaneous bleeding (80).
Interferon‐gamma therapy
It has been shown that interferon‐gamma therapy is a new treatment for radiation‐induced fibrosis, as well as URS and fistulas. It inhibits collagen production in normal dermal fibroblasts 22, 82. In five patients who suffered from radiation‐induced cutaneous fibrosis, Gottlöber et al. showed the positive impact of this therapy by means of reduction of fibrosis (22).
Surgical treatments
Many methods of wound closure have been used and studied for several years. Within the most simple, we can mention direct reapproximation of the wound edge, skin grafts and flap coverage. Musculocutaneous or vascularised free flap coverage has shown a number of advantages: they allow more motion in kinetic areas, cover laid open structures (large vessels, nerves, tendons, bones, pleura, etc.) and provide a cover through which future reconstructive surgeries can be performed. It is important to mention that these coverage must be a well‐vascularised flap 8, 14.
Treatments under investigation
The following therapies may be useful because of their mechanism of action of healing of chronic ulcers and are mentioned subsequently with the purpose of encouraging research on the topic.
Negative pressure therapy
Negative pressure wound therapy (NPWT) is the controlled application of subatmospheric pressure to a wound using an electrical pump and specialised wound dressings 83, 84, 85. NPWT removes the excess of interstitial oedema, thereby decompressing the small vessels and optimising local blood flow. It aids in the removal of bacteria from the wound by suctioning the excessive pro‐inflammatory exudate, it stimulates the proliferation of fibroblasts, endothelial cells and vascular smooth muscle by mechanically deforming the cells, thus increasing the rate of cell division and subsequent formation of granulation tissue 65, 85, 86. Because of this mechanism, NPWT is indicated in acute, chronic, traumatic and dehisced wounds, as well as diabetic and pressure ulcers, flaps, grafts and partial‐thickness wounds. In contrast, the favoured cellular proliferation and vascular proliferation have deemed NPWT contraindicated in wounds where malignancy is suspected (87). The authors could not find any solid evidence supporting this contraindication other than the recommendations by one of the NPWT manufacturers but should not be considered as standard therapy (88). The use of NPWT in URS where malignancy is not suspected has not been studied either.
Other growth factors
Vascular endothelial growth factor (VEGF) is a mitogen for endothelial cells. VEGF is an angiogenic factor and has been shown to improve wound healing in the setting of ischaemia (89). Unfortunately, there is still no data to support VEGF’s ability to promote wound healing in patients with URS (65).
Other growth factors that deserve further research, given their ability to increase wound healing, are insulin‐like growth factor (65), EGF, and granulocyte/macrophage colony‐stimulating factor (90). The TGF‐β, the Smad3‐signalling pathway (91) or the induction of connective tissue growth factor because of the recognition of long‐term sustained activity of cytokine networks could be blocked to interfere with the fibrotic events (92). More ample investigation of other growth factors continues to determine their interactions with proteins and enhance the healing of these lesions.
A number of new treatments (including innovative dressings and living skin equivalents) have been approved for treating difficult chronic wounds. However, specific research in URS of these therapies is absent. Literature and experience from non English publications deserves more attention for there are a number of trials in Russian suggesting usefulness of therapies such as laser, baliz, electrophoresis and others 93, 94, 95, 96, 97, 98, 99, 100.
Conclusions
URS remain a challenge for clinicians because radiated tissue has a tendency to heal slowly most likely because of hypoxic and fibrotic events. The pathophysiology of wound healing on radiated tissue is for the most part only theoretical and warrants further study. Prevention of radiation damage is an active field of research, but most interventions presently in use have not shown to protect from radiation damage conclusively. Sucralfate and amifostine studies have shown the greatest prophylactic effect in well‐designed studies. Proper wound bed preparation is mandatory and is the most valuable measure to achieve healing and improve the patient’s quality of life. Although the strongest level of evidence was for the use of pentoxifylline, it is still too low to be considered as a standard adjuvant therapy. HBO, cytokines and other growth factors, as well as surgical interventions, have shown to be useful in isolated reports and some case series (evidence level III), but solid evidence on their effectiveness is lacking. Although surgery is a consideration, the risks of reconstructing on radiated tissues are galore 101, 102. Promising treatments are under investigation but are yet to be tried in well‐controlled trials in URS.
References
- 1. Vuilleumier HA, Reis ED. Radiation injury. In: Marti MC, Givel JC, editors. Surgical management of anorectal and colonic disease. Berlin: Springer; 1998:423–35. [Google Scholar]
- 2. Calais G, Alfonsi M, Bardet E, Sire C, Germain T, Bergerot P, Rhein B, Tortochaux J, Oudinot P, Bertrand P. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced‐stage oropharynx carcinoma. J Natl Cancer Inst 1999;91:2081–6. [DOI] [PubMed] [Google Scholar]
- 3. Fu KK, Pajak TF, Trotti A, Jones CU, Spencer SA, Phillips TL, Garden AS, Ridge JA, Cooper JS, Ang KK. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys 2000;48:7–16. [DOI] [PubMed] [Google Scholar]
- 4. Agrawal A, Chandra D, Kale RK. Radiation induced oxidative stress: II studies in liver as a distant organ of tumor bearing mice. Mol Cell Biochem 2001;224:9–17. [DOI] [PubMed] [Google Scholar]
- 5. Daly JM, Bertagnoli M, De Cosse JJ, Morton DL. Oncology. In: Schwartz SI, editor. Principles of surgery. New York: McGraw‐Hill, 1999:335–45. [Google Scholar]
- 6. Mendelsohn FA, Divino CM, Reis ED, Kerstein MD. Wound care after radiation therapy. Adv Skin Wound Care 2002;15:216–24. [DOI] [PubMed] [Google Scholar]
- 7. Ward JF. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol 1988;35:95–125. [DOI] [PubMed] [Google Scholar]
- 8. Shack RB. Management of radiation ulcers. South Med J 1982;75:1462–6. [DOI] [PubMed] [Google Scholar]
- 9. Steel GG. Basic clinical radiobiology, 3rd edn. London: Hodder Arnold, 2002. [Google Scholar]
- 10. Mettler FA Jr, Moseley RD Jr. Medical effects of ionizing radiation. Orlando: Grune & Stratton, 1985. [Google Scholar]
- 11. Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys 1995;33:99–109. [DOI] [PubMed] [Google Scholar]
- 12. Luce EA. The irradiated wound. Surg Clin North Am 1984;64:821–9. [DOI] [PubMed] [Google Scholar]
- 13. Herndon DN. Total burn care. London: WB Saunders, 1996. [Google Scholar]
- 14. Miller SH, Rudolph R. Healing in the irradiated wound. Clin Plast Surg 1990;17:503–8. [PubMed] [Google Scholar]
- 15. Aitasalo K, Aro H. Irradiation‐induced hypoxia in bones and soft tissues: an experimental study. Plast Reconstr Surg 1986;77:256–67. [DOI] [PubMed] [Google Scholar]
- 16. Dion MW, Hussey DH, Doornbos JF, Vigliotti AP, Wen BC, Anderson B. Preliminary results of a pilot study of pentoxifylline in the treatment of late radiation soft tissue necrosis. Int J Radiat Oncol Biol Phys 1990;19:401–7. [DOI] [PubMed] [Google Scholar]
- 17. Dasgeb B, Phillips TJ. Diagnostic dilemmas: osteoradionecrosis. Wounds 2004;16:291–5. [Google Scholar]
- 18. Dion MW, Hussey DH, Osborne JW. The effect of pentoxifylline on early and late radiation injury following fractionated irradiation in C3H mice. Int J Radiat Oncol Biol Phys 1989;17:101–7. [DOI] [PubMed] [Google Scholar]
- 19. Hopewell JW. Mechanisms of the action of radiation on skin and underlying tissues. Br J Radiol Suppl 1986;19:39–47. [PubMed] [Google Scholar]
- 20. Carl UM, Hartmann KA. Heterotopic calcification as a late radiation effect: report of 15 cases. Br J Radiol 2002;75:460–3. [DOI] [PubMed] [Google Scholar]
- 21. Gottlober P, Kerscher MJ, Korting HC, Peter RU. Sonographic determination of cutaneous and subcutaneous fibrosis after accidental exposure to ionising radiation in the course of the Chernobyl nuclear power plant accident. Ultrasound Med Biol 1997;23:9–13. [DOI] [PubMed] [Google Scholar]
- 22. Gottlober P, Steinert M, Bahren W, Weber L, Gerngross H, Peter RU. Interferon‐gamma in 5 patients with cutaneous radiation syndrome after radiation therapy. Int J Radiat Oncol Biol Phys 2001;50:159–66. [DOI] [PubMed] [Google Scholar]
- 23. Hall G, Goldberg L, Phillips T. Diagnostic dilemmas: chronic ulceration in a radiotherapy site. Wounds 2003;15:346–50. [Google Scholar]
- 24. Kirsner RS, Spencer J, Falanga V, Garland LE, Kerdel FA. Squamous cell carcinoma arising in osteomyelitis and chronic wounds. Treatment with Mohs micrographic surgery vs amputation. Dermatol Surg 1996;22:1015–8. [DOI] [PubMed] [Google Scholar]
- 25. Malkinson FD. Radiobiology of the skin. In: Freedberg IM, Eisen AZ, Wolff K, editors. Fitzpatrick’s dermatology in general medicine, 5th edn. New York: McGraw‐Hill, 1999:1514–23. [Google Scholar]
- 26. Trent JT, Kirsner RS. Wounds and malignancy. Adv Skin Wound Care 2003;16:31–4. [DOI] [PubMed] [Google Scholar]
- 27. Grant RA, Cox RW, Kent CM. The effects of gamma irradiation on the structure and reactivity of native and cross‐linked collagen fibres. J Anat 1973;115:29–43. [PMC free article] [PubMed] [Google Scholar]
- 28. Rudolph R, Vande BJ, Schneider JA, Fisher JC, Poolman WL. Slowed growth of cultured fibroblasts from human radiation wounds. Plast Reconstr Surg 1988;82:669–77. [DOI] [PubMed] [Google Scholar]
- 29. Aistars J. The validity of skin care protocols followed by women with breast cancer receiving external radiation. Clin J Oncol Nurs 2006;10:487–92. [DOI] [PubMed] [Google Scholar]
- 30. Bolderston A, Lloyd NS, Wong RK, Holden L, Robb‐Blenderman L. The prevention and management of acute skin reactions related to radiation therapy: a systematic review and practice guideline. Support Care Cancer 2006;14:802–17. [DOI] [PubMed] [Google Scholar]
- 31. Ertekin MV, Tekin SB, Erdogan F, Karslioglu I, Gepdiremen A, Sezen O, Balci E, Gundogdu C. The effect of zinc sulphate in the prevention of radiation‐induced dermatitis. J Radiat Res (Tokyo) 2004;45:543–8. [DOI] [PubMed] [Google Scholar]
- 32. Maiche A, Isokangas OP, Grohn P. Skin protection by sucralfate cream during electron beam therapy. Acta Oncol 1994;33:201–3. [DOI] [PubMed] [Google Scholar]
- 33. Evensen JF, Bjordal K, Jacobsen AB, Lokkevik E, Tausjo JE. Effects of Na‐sucrose octasulfate on skin and mucosa reactions during radiotherapy of head and neck cancers–a randomized prospective study. Acta Oncol 2001;40:751–5. [DOI] [PubMed] [Google Scholar]
- 34. Pfeiffer P, Madsen EL, Hansen O, May O. Effect of prophylactic sucralfate suspension on stomatitis induced by cancer chemotherapy. A randomized, double‐blind cross‐over study. Acta Oncol 1990;29:171–3. [DOI] [PubMed] [Google Scholar]
- 35. Pfeiffer P, Hansen O, Madsen EL, May O. A prospective pilot study on the effect of sucralfate mouth‐swishing in reducing stomatitis during radiotherapy of the oral cavity. Acta Oncol 1990;29:471–3. [DOI] [PubMed] [Google Scholar]
- 36. Carter DL, Hebert ME, Smink K, Leopold KA, Clough RL, Brizel DM. Double blind randomized trial of sucralfate vs placebo during radical radiotherapy for head and neck cancers. Head Neck 1999;21:760–6. [DOI] [PubMed] [Google Scholar]
- 37. Epstein JB, Wong FL. The efficacy of sucralfate suspension in the prevention of oral mucositis due to radiation therapy. Int J Radiat Oncol Biol Phys 1994;28:693–8. [DOI] [PubMed] [Google Scholar]
- 38. Lievens Y, Haustermans K, Van den WD, Van den BW, Scalliet P, Hutsebaut L, Fowler J, Lambin P. Does sucralfate reduce the acute side‐effects in head and neck cancer treated with radiotherapy? A double‐blind randomized trial. Radiother Oncol 1998;47:149–53. [DOI] [PubMed] [Google Scholar]
- 39. Makkonen TA, Bostrom P, Vilja P, Joensuu H. Sucralfate mouth washing in the prevention of radiation‐induced mucositis: a placebo‐controlled double‐blind randomized study. Int J Radiat Oncol Biol Phys 1994;30:177–82. [DOI] [PubMed] [Google Scholar]
- 40. Meredith R, Salter M, Kim R, Spencer S, Weppelmann B, Rodu B, Smith J, Lee J. Sucralfate for radiation mucositis: results of a double‐blind randomized trial. Int J Radiat Oncol Biol Phys 1997;37:275–9. [DOI] [PubMed] [Google Scholar]
- 41. Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: the first selective‐target and broad‐spectrum radioprotector. Oncologist 2007;12:738–47. [DOI] [PubMed] [Google Scholar]
- 42. Kouvaris J, Kouloulias V, Kokakis J, Matsopoulos G, Myrsini B, Vlahos L. The cytoprotective effect of amifostine in acute radiation dermatitis: a retrospective analysis. Eur J Dermatol 2002;12:458–62. [PubMed] [Google Scholar]
- 43. Koukourakis MI, Kyrias G, Kakolyris S, Kouroussis C, Frangiadaki C, Giatromanolaki A, Retalis G, Georgoulias V. Subcutaneous administration of amifostine during fractionated radiotherapy: a randomized phase II study. J Clin Oncol 2000;18:2226–33. [DOI] [PubMed] [Google Scholar]
- 44. Sibbald RG, Orsted H, Schultz GS, Coutts P, Keast D. Preparing the wound bed 2003: focus on infection and inflammation. Ostomy Wound Manage 2003;49:23–51. [PubMed] [Google Scholar]
- 45. Thomas S, Jones M, Andrews A. Special focus: tissue viability. The use of fly larvae in the treatment of wounds. Nurs Stand 1997;12:54, 57–54, 59. [DOI] [PubMed] [Google Scholar]
- 46. Drosou A, Falabella A, Kirsner RS. Antiseptics on wounds: an area of controversy. Wounds 2003;15:149–66. [Google Scholar]
- 47. Briggs M, Ferris FD, Glynn C, Harding K, Hofman D, Hollinworth H, Krasner DL, Lindholm C, Moffatt C, Price P, Romanelli M, Sibbald G, Stacey M, Teot L. Assessing pain at wound dressing‐related procedures. Nurs Times 2004;100:56–7. [PubMed] [Google Scholar]
- 48. Main N, Hatcher A, Meeks E. Dressing the discomfort: managing radiation therapy‐induced dermatitis. Ostomy Wound Manage 2005;51:12–3. [PubMed] [Google Scholar]
- 49. Ayello EA, Dowsett C, Schultz GS, Sibbald RG, Falanga V, Harding K, Romanelli M, Stacey M, Teot L, Vanscheidt W. TIME heals all wounds. Nursing 2004;34:36–41. [DOI] [PubMed] [Google Scholar]
- 50. Futran ND, Trotti A, Gwede C. Pentoxifylline in the treatment of radiation‐related soft tissue injury: preliminary observations. Laryngoscope 1997;107:391–5. [DOI] [PubMed] [Google Scholar]
- 51. Aygenc E, Celikkanat S, Bilgili H, Aksaray F, Orhun S, Kaymakci M, Ozdem C. Pentoxifylline effects on acute and late complications after radiotherapy in rabbit. Otolaryngol Head Neck Surg 2001;124:669–73. [DOI] [PubMed] [Google Scholar]
- 52. Jain KK. Hyperbaric oxygen therapy in the management of radionecrosis. In: Jain KK, editor. Textbook of hyperbaric medicine, 4th edn. Germany: Hogrefe & Huber Publishers Inc, 2004:67–79. [Google Scholar]
- 53. Anderson DW. Using hyperbaric oxygen therapy to heal radiation wounds. Nursing 2003;33:50–3. [DOI] [PubMed] [Google Scholar]
- 54. Niinikoski JH. Clinical hyperbaric oxygen therapy, wound perfusion, and transcutaneous oximetry. World J Surg 2004;28:307–11. [DOI] [PubMed] [Google Scholar]
- 55. Filntisis GA, Moon RE, Kraft KL, Farmer JC, Scher RL, Piantadosi CA. Laryngeal radionecrosis and hyperbaric oxygen therapy: report of 18 cases and review of the literature. Ann Otol Rhinol Laryngol 2000;109:554–62. [DOI] [PubMed] [Google Scholar]
- 56. Marx RE, Ehler WJ, Tayapongsak P, Pierce LW. Relationship of oxygen dose to angiogenesis induction in irradiated tissue. Am J Surg 1990;160:519–24. [DOI] [PubMed] [Google Scholar]
- 57. Feldmeier JJ, Jelen I, Davolt DA, Valente PT, Meltz ML, Alecu R. Hyperbaric oxygen as a prophylaxis for radiation‐induced delayed enteropathy. Radiother Oncol 1995;35:138–44. [DOI] [PubMed] [Google Scholar]
- 58. Yildiz S, Cimsit M, Ilgezdi S, Uzun G, Gumus T, Qyrdedi T, Dalci D. Hyperbaric oxygen therapy used to treat radiation injury: two case reports. Ostomy Wound Manage 2006;52:14–6, 18, 20. [PubMed] [Google Scholar]
- 59. Daruwalla J, Christophi C. Hyperbaric oxygen therapy for malignancy: a review. World J Surg 2006;30:2112–31. [DOI] [PubMed] [Google Scholar]
- 60. Daruwalla J, Christophi C. The effect of hyperbaric oxygen therapy on tumour growth in a mouse model of colorectal cancer liver metastases. Eur J Cancer 2006;42:3304–11. [DOI] [PubMed] [Google Scholar]
- 61. Raa A, Stansberg C, Steen VM, Bjerkvig R, Reed RK, Stuhr LE. Hyperoxia retards growth and induces apoptosis and loss of glands and blood vessels in DMBA‐induced rat mammary tumors. BMC Cancer 2007;7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hoogsteen IJ, Marres HA, Van Der Kogel AJ, Kaanders JH. The hypoxic tumour microenvironment, patient selection and hypoxia‐modifying treatments. Clin Oncol (R Coll Radiol) 2007;19:385–96. [DOI] [PubMed] [Google Scholar]
- 63. Haroon AT, Patel M, Al‐Mehdi AB. Lung metastatic load limitation with hyperbaric oxygen. Undersea Hyperb Med 2007;34:83–90. [PubMed] [Google Scholar]
- 64. Mathur NN, Prince M. Hyperbaric oxygen. Emedicine 2006. http://www.emedicine.com/ent/TOPIC733.HTM [Google Scholar]
- 65. Giurini J, Rich J. Unlocking the secrets to growth factors. Podiatry Today 2001;14(7):28–36. [Google Scholar]
- 66. Pham HT, Rich J, Veves A. Wound healing in diabetic foot ulceration: a review and commentary. Wounds 2000;12:79–81. [Google Scholar]
- 67. Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A. Role of platelet‐derived growth factor in wound healing. J Cell Biochem 1991;45:319–26. [DOI] [PubMed] [Google Scholar]
- 68. Barrett SL. A new approach to using growth factors in wound healing. Wounds 2003;16:44–50. [Google Scholar]
- 69. Wollina U, Liebold K, Konrad H. Treatment of chronic radiation ulcers with recombinant platelet‐derived growth factor and a hydrophilic copolymer membrane. J Eur Acad Dermatol Venereol 2001;15:455–7. [DOI] [PubMed] [Google Scholar]
- 70. Chung KF, Dent G, McCusker M, Guinot P, Page CP, Barnes PJ. Effect of a ginkgolide mixture (BN 52063) in antagonising skin and platelet responses to platelet activating factor in man. Lancet 1987;1:248–51. [DOI] [PubMed] [Google Scholar]
- 71. Cupp MJ. Herbal remedies: adverse effects and drug interactions. Am Fam Physician 1999;59:1239–45. [PubMed] [Google Scholar]
- 72. DeFeudis FV. Ginkgo biloba extract: pharmacological activities and clinical applications. Paris: Elsevier, 1991. [Google Scholar]
- 73. Evans JR. Ginkgo biloba extract for age‐related macular degeneration. Cochrane Database Syst Rev 2000;CD001775. [DOI] [PubMed] [Google Scholar]
- 74. Hoyer S, Lannert H, Noldner M, Chatterjee SS. Damaged neuronal energy metabolism and behavior are improved by Ginkgo biloba extract (EGb 761). J Neural Transm 1999;106:1171–88. [DOI] [PubMed] [Google Scholar]
- 75. Maitra I, Marcocci L, Droy‐Lefaix MT, Packer L. Peroxyl radical scavenging activity of Ginkgo biloba extract EGb 761. Biochem Pharmacol 1995;49:1649–55. [DOI] [PubMed] [Google Scholar]
- 76. Sener G, Kabasakal L, Atasoy BM, Erzik C, Velioglu‐Ogunc A, Cetinel S, Gedik N, Yegen BC. Ginkgo biloba extract protects against ionizing radiation‐induced oxidative organ damage in rats. Pharmacol Res 2006;53:241–52. [DOI] [PubMed] [Google Scholar]
- 77. Hedayat I, Salam OM, Baiuomy AR. Effect of Ginkgo biloba extract on carrageenan‐induced acute local inflammation in gamma irradiated rats. Pharmazie 2005;60:614–9. [PubMed] [Google Scholar]
- 78. Lamproglou I, Boisserie G, Mazeron JJ, Bok B, Baillet F, Drieu K. [Effect of Ginkgo biloba extract (EGb 761) on rats in an experimental model of acute encephalopathy after total body irradiation]. Cancer Radiother 2000;4:202–6. [DOI] [PubMed] [Google Scholar]
- 79. Aoui‐Youssefi A, Lamproglou I, Drieu K, Emerit I. Anticlastogenic effects of Ginkgo biloba extract (EGb 761) and some of its constituents in irradiated rats. Mutat Res 1999;445:99–104. [DOI] [PubMed] [Google Scholar]
- 80. Bent S, Goldberg H, Padula A, Avins AL. Spontaneous bleeding associated with ginkgo biloba: a case report and systematic review of the literature. J Gen Intern Med 2005;20:657–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ernst E. The risk‐benefit profile of commonly used herbal therapies: Ginkgo, St. John’s Wort, Ginseng, Echinacea, Saw Palmetto, and Kava. Ann Intern Med 2002;136:42–53. [DOI] [PubMed] [Google Scholar]
- 82. Jimenez SA, Freundlich B, Rosenbloom J. Selective inhibition of human diploid fibroblast collagen synthesis by interferons. J Clin Invest 1984;74:1112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Armstrong DG, Lavery LA, Abu‐Rumman P, Espensen EH, Vazquez JR, Nixon BP, Boulton AJ. Outcomes of subatmospheric pressure dressing therapy on wounds of the diabetic foot. Ostomy Wound Manage 2002;48:64–8. [PubMed] [Google Scholar]
- 84. McCallon SK, Knight CA, Valiulus JP, Cunningham MW, McCulloch JM, Farinas LP. Vacuum‐assisted closure versus saline‐moistened gauze in the healing of postoperative diabetic foot wounds. Ostomy Wound Manage 2000;46:28–32, 34. [PubMed] [Google Scholar]
- 85. Venturi ML, Attinger CE, Mesbahi AN, Hess CL, Graw KS. Mechanisms and clinical applications of the vacuum‐assisted closure (VAC) Device: a review. Am J Clin Dermatol 2005;6:185–94. [DOI] [PubMed] [Google Scholar]
- 86. Fleck CA, Frizzell LD. When negative is positive: a review of negative pressure wound therapy. Wounds 2004;92:20–5. [Google Scholar]
- 87. Paul JC. Vacuum assisted closure therapy: a must in plastic surgery. Plast Surg Nurs 2005;25:61–5. [DOI] [PubMed] [Google Scholar]
- 88. KCI . V.A.C. therapy clinical guidelines. A reference source for clinicians. 2005. San Antonio, Kinetic Concepts Inc. [Google Scholar]
- 89. Corral CJ, Siddiqui A, Wu L, Farrell CL, Lyons D, Mustoe TA. Vascular endothelial growth factor is more important than basic fibroblastic growth factor during ischemic wound healing. Arch Surg 1999;134:200–5. [DOI] [PubMed] [Google Scholar]
- 90. Cianfarani F, Tommasi R, Failla CM, Viviano MT, Annessi G, Papi M, Zambruno G, Odorisio T. Granulocyte/macrophage colony‐stimulating factor treatment of human chronic ulcers promotes angiogenesis associated with de novo vascular endothelial growth factor transcription in the ulcer bed. Br J Dermatol 2006;154:34–41. [DOI] [PubMed] [Google Scholar]
- 91. Sumiyoshi K, Nakao A, Setoguchi Y, Okumura K, Ogawa H. Exogenous Smad3 accelerates wound healing in a rabbit dermal ulcer model. J Invest Dermatol 2004;123:229–36. [DOI] [PubMed] [Google Scholar]
- 92. Martin M, Lefaix J, Delanian S. TGF‐beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys 2000;47:277–90. [DOI] [PubMed] [Google Scholar]
- 93. Bardychev MS, Vaisberg GE, Givsktalv NI. [Complex treatment of late radiation injuries of the skin by use of prodigiozan]. Antibiotiki 1969;14:943–7. [PubMed] [Google Scholar]
- 94. Bardychev MS, Kazantseva NA, Kurpesheva AK, Dunaeva OS, Kumskova LA. [Proteolytic enzyme and heparin electrophoresis in treating late radiation skin lesions]. Med Radiol (Mosk) 1978;23:23–6. [PubMed] [Google Scholar]
- 95. Bardychev MS. [Late, local radiation injuries and their treatment]. Med Radiol (Mosk) 1981;26:40–4. [PubMed] [Google Scholar]
- 96. Bardychev MS. [Local radiation injuries and their combined treatment]. Med Radiol (Mosk) 1982;27:44–9. [PubMed] [Google Scholar]
- 97. Bardychev MS. [Treatment of the radiation injuries occurring in the radiotherapy of malignant tumors]. Vopr Onkol 1984;30:89–97. [PubMed] [Google Scholar]
- 98. Bardychev MS, Katsalap SN. [Local radiation damage: the characteristics of its pathogenesis, diagnosis and treatment]. Vopr Onkol 1995;41:99. [PubMed] [Google Scholar]
- 99. Kim I, Klimanov ME, Bardychev MS. [Treatment of late radiation‐induced skin ulcers with laser radiation]. Med Radiol (Mosk) 1985;30:71–3. [PubMed] [Google Scholar]
- 100. Petrik VD, Bardychev MS. [Use of baliz for the treatment of late radiation skin ulcers]. Med Radiol (Mosk) 1979;24:48–51. [PubMed] [Google Scholar]
- 101. Ruvalcaba‐Limon E, Robles‐Vidal C, Poitevin‐Chacon A, Chavez‐Macgregor M, Gamboa‐Vignolle C, Vilar‐Compte D. Complications after breast cancer surgery in patients treated with concomitant preoperative chemoradiation: a case‐control analysis. Breast Cancer Res Treat 2006;95:147–52. [DOI] [PubMed] [Google Scholar]
- 102. Vilar‐Compte D, Jacquemin B, Robles‐Vidal C, Volkow P. Surgical site infections in breast surgery: case‐control study. World J Surg 2004;28:242–6. [DOI] [PubMed] [Google Scholar]
- 103. Registered Nurses Association of Ontario (RNAO) . Promoting continence using prompted voiding, 2nd edn. Toronto (Ontario): Registered Nurses Association of Ontario (RNAO), 2007. [Google Scholar]
- 104. Okunieff P, Augustine E, Hicks JE, Holden L, Robb‐Blenderman L. Pentoxifylline in the treatment of radiation‐induced fibrosis. J Clin Oncol 2004;22:2207–13. [DOI] [PubMed] [Google Scholar]


