Abstract
The chronic and relapsing nature of hidradenitis suppurativa leads to physical and psychological damage. The absence of a proven cure further worsens the scenario. Patient 1 was a 28‐year‐old woman with a 10‐year history of abscesses and non healing sinuses with foul‐smelling discharge from her axilla, submammary and groin areas. This led to an episode of self‐harm due to severe depression. After failed medical therapy, she was referred for surgery with wide excision of the skin and healing by secondary intention. Her wounds were managed by our specialist wound clinic with the use of topical and systemic antibiotics and thus remained free from symptoms. Patient 2 was a 32‐year‐old woman with a similar history for 15 years. Patient 3 was a 41‐year‐old man with a 20‐year history of discharging sinuses and abscesses. All the patients had endured a long period of medical treatment and subsequently required surgery for a long‐term relief of symptoms. This has undoubtedly led to psychological symptoms and a decrease in quality of life.
Keywords: Hidradenitis suppurativa, Surgical treatment
Introduction
Hidradenitis suppurativa (HS) is a chronic, relapsing inflammatory disease of skin, characterised by recurrent draining sinuses and abscesses, predominantly in skin folds carrying terminal hairs and apocrine glands (1). HS leads to significant morbidity in sufferers. Their quality of life is highly affected by the recalcitrant, painful lesions and malodorous discharge that requires regular dressings 2, 3 Initially, there is UHW, formation of non inflamed nodules, which progress to painful, deep‐seated abscesses and subsequently lead to scarring and suppuration (2). This commonly occurs in the axilla, inguinal, perianal, perineal, mammary and inframammary regions (3). Healing occurs with substantial scarring. Furthermore, following inefficient treatments, depression and social isolation can result 2, 3. A significant amount of time is spent with visits to primary care and district nursing; a variety of hospital specialists in outpatients such as dermatologists, gynaecologists and general and plastic surgeons and as an inpatient for surgical intervention.
Standard management includes the use of topical and systemic antibiotics such as clindamycin and tetracyclin and topical antimicrobials. Other treatment options include the use of oral glucocorticoids, isotretinoin, cyproterone acetate, cyclosporin and infliximab (4). As spontaneous resolution is rare and progressive disability is more likely to occur, early definitive surgical intervention is advisable. The surgical procedure of choice in most cases is wide local excision and healing by secondary intention.
A series of cases of patients who attended our specialist wound healing clinic with HS are presented here, whose disease was refractory to medical treatment and subsequently managed surgically.
Case report
Case I
A 28‐year‐old premenopausal woman presented after wide excision of HS with open wounds undertaken at another centre. She had a 10‐year history of non healing draining sinuses in the right and left axilla, in the groin crease on the right side extending over to her mons pubis and in the skin fold under the right breast. She was a smoker and was overweight. There was no history of diabetes, and she was otherwise fit and healthy.
Her disease started about 10 years ago, with a boil in her right axilla, with multiple recurrences leading to chronic infection, and later on, this involved other parts of the body. For many years, she had been unsuccessfully treated with local hygiene, sitz baths and multiple antibiotic regimens. She also developed abscesses during the course of her disease, which needed incision and drainage. During the course of the disease, she developed persistent draining sinuses, with malodorous discharge mainly on the right side of her axilla and groin. More recently, she has been unable to have sexual relationships and experienced difficulty in walking. This led to depression and attempts at self‐harm, and she was given antidepressants.
After failed medical therapy, she was referred for surgery. Preoperatively, she was treated with aggressive local hygiene and antibiotics. The patient underwent wide excision of the involved skin from the right axilla, submammary fold and groin. The wounds were left open to heal by secondary intention, and later, she was referred to the specialist Wound Healing Research Unit (WHRU) for further management.
In the clinic, she was treated for persisting wound infection with topical antimicrobials and oral flucloxacillin. She was regularly seen by the district nurse and followed up in wound healing clinics with advice on the management of her wounds that healed subsequently, and she became symptom free for the first time after many years. She was also referred for physiotherapy as she started to develop contractures in the axilla (Figure 1A–D).
Figure 1.
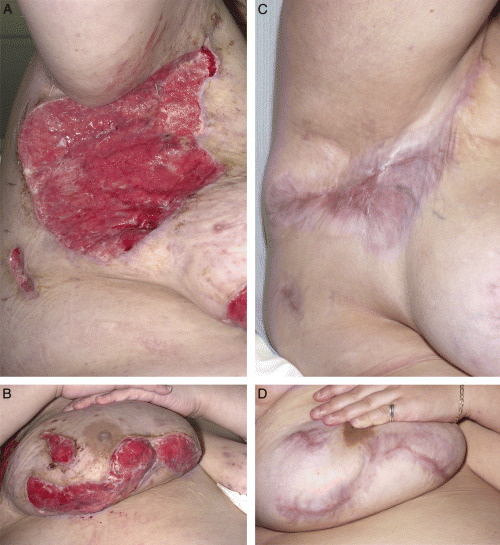
(A, B) Postoperative wound over right axilla and submammary area. (C, D) Healed wound over axilla and submammary area.
Case II
The second patient, a woman aged 32 years, was seen in the WHRU postoperatively following wide surgical excision of skin from right axilla and both groins. She had extensive HS involving axillae, submammary folds and groins. This has also spread to the skin over her mons pubis with proximal extension. She was premenopausal, with no children, overweight and non smoker. Her elder sister also suffered from similar symptoms.
She had a 15‐year history of chronic inflammation, ulceration and multiple draining sinuses in the axillae, pubic region, groins and submammary folds. This led to scarring, with persistent malodorous discharging sinuses. Antibiotics and other measures provided temporary relief initially, and the disease gradually became chronic and she was put on long‐term antibiotics, but with no cure.
After a prolonged period of physical and psychological trauma, she was referred for surgical review and opted for surgical excision. She had extensive disease and was treated with wide local excision from both axillae and groin, which were left open. She was treated in the WHRU with dressings of topical antiseptics, and now her wounds are healing successfully (Figure 2A–E).
Figure 2.
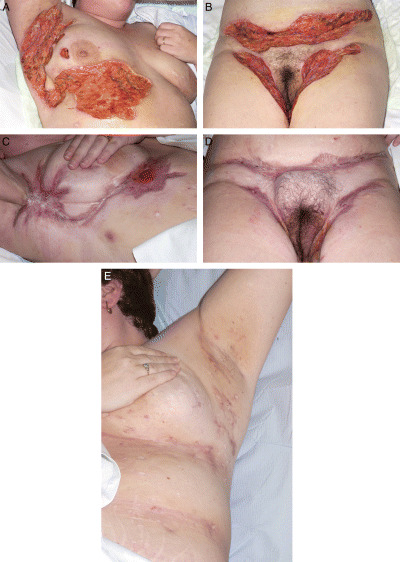
(A) Postoperative wound over right axilla and submammary area. (B) Postoperative wound over abdominal fold and groin. (C, D) Wound healed over right axilla, submammary, abdominal fold and groin area. (E) Scarring and fibrosis over left axilla and submammary area.
Case III
Mr C, a 41‐year‐old cab driver, was seen in the WHRU after surgery for HS. He was a smoker and was non diabetic. His problem started at the age of 21. His symptoms were recurrent discharging abscesses and sinuses in both the inguinoperineal and the axillary regions. In recent years, his axillae were the more affected, with debilitating pustular eruptions up to five times a week and secondary scarring. These symptoms lasted for a period of 20 years and greatly affected his family and social life.
He was eventually referred for surgical treatment and underwent wide excision of the involved skin from his right axilla and groin. The wounds were left open to heal by secondary intention. Postoperatively, he had a good recovery and the wounds healed. Now he is waiting for surgery on left axilla and groin (Figure 3).
Figure 3.
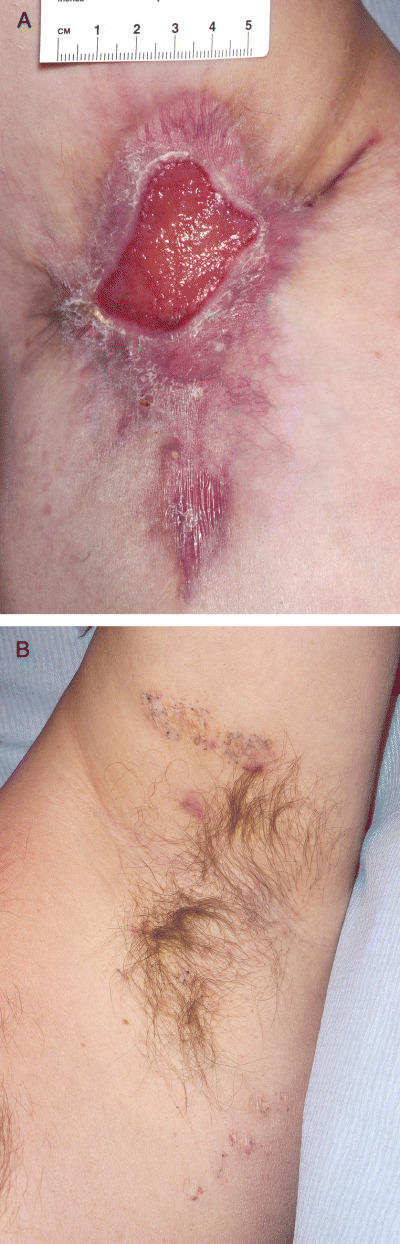
(A) Postoperative wound over right axilla. (B) Nodules and scarring over left axilla.
Case discussion
The three cases described here had advanced HS, which was refractory to the medical treatment, and the patients opted for surgery after a long period of failed medical therapies. All of them were treated by wide excision and healing by secondary intention as this method provides better results and is generally well tolerated by the patients. Two of the patients started to develop postoperative contractures in the axilla and were referred for physiotherapy early in the postoperative period. Different kinds of dressings, topical antiseptics and antibiotics were used accordingly. Generally, the procedure was well tolerated by the patients and no local recurrence was noted.
Discussion and review of the literature
Historical background
Hidradenitis suppurativa (from the Greek hidros, sweat and aden, glands), also known as acne inversa 1, 3, 5, was first described by Velpeau, a French physician in 1839, who reported a peculiar inflammation of the skin with the formation of superficial abscesses in the axillary, mammary and perianal areas (6). In 1854, this condition was termed ‘hidrosadénite phlegmoneuse’ by Verneuil 7, 8, a French surgeon who also suggested an association between HS and sweat glands, which had been described by Purkinje in 1833 (9). However, conclusions by Verneuil were based purely on the clinical observation of the characteristic distribution of HS as he did not conduct any histopathological studies (8). After numerous studies, HS is now accepted to be an acneform disorder, which begins with follicular occlusion, rather than infection of sweat glands 10, 11, 12, 13.
Epidemiology
Although the exact prevalence of the disease is not known, it has been estimated at 1 in 100 (14), 1 in 300 (15) or 1 in 600 (16). A point prevalence of 4·1% has been reported (14), but this may be falsely elevated as the patients were from an at‐risk age range (15). It has been asserted that HS is two to five times more common in women than in men (17). The age of onset varies from childhood to middle age, peaking in the second and third decades of life 3, 18 but rarely before puberty (19). An endocrine component in the aetiology of HS has been considered (20). In women, the onset of HS after menopause is rare (3).
Genitofemoral lesions are more common in women, but there does not seem to be a difference between the sexes in axillary involvement (14).
Pathological features and distribution
HS is a misnomer. The key feature is not a suppurative inflammation of the apocrine glands but an occlusion of the hair follicles. The use of the term ‘acne inversa’ has now replaced HS 11, 21.
Apocrine glands are compound sweat glands, which secrete opalescent and malodorous sweat, related to effects of bacteria, onto the surface of the skin (18). They are most commonly found in the axilla, genitofemoral, gluteal folds, perianal, pubic, periareolar, submammary, intermammary and periumbilical areas, as well as in the scalp, ear canal (ceruminal glands) and eyelids (Moll’s glands).
Because of the anatomical correlation between the distribution of these apocrine glands and the clinical presentation of HS lesions in these areas, it was concluded that HS was a primary disorder of these glands. The term ‘apocrinitis’ has also been used to describe HS. However, apocrine gland involvement has been shown to be incidental but not essential to the pathogenesis (21). The primary event is the occlusion of hair follicles by keratinised, stratified, squamous epithelium, which then leads to dilatation and rupture of the follicles, spilling their contents, which include keratin and bacteria, into the surrounding dermis 3, 10, 12. Polyporous comedones are a characteristic feature of burnt out acne inverse (1).
Following dermal rupture, an inflammatory cascade is triggered, leading to the accumulation of neutrophils, lymphocytes and histiocytes. The abscesses are formed, leading to the destruction of the pilosebaceous unit. Histological evidence shows that the dermis contains inflammatory cells, giant cells, sinus tracts and fibrosis (3).
Aetiology
The exact aetiology of HS still remains unclear, and few studies have attempted to clarify this (17). Genetic factors may play a role as a positive family history has been elicited in 26% of patients with HS (14). Some studies have suggested an autosomal‐dominant mode of inheritance, but the identification of an exact genetic abnormality is yet to be discovered 15, 22.
The role of endocrine factors in the aetiology of HS has been controversial. The observation that HS is associated with acne vulgaris, comedones, hirsutism (23), rare prepubertal onset, premenstrual flare‐ups (24) and the use of oral contraceptive pill (25) strongly suggests the influence of sex hormones. However, a study of 66 women with HS did not find any evidence of hyperandrogenism (26). Nevertheless, the use of antiandrogens has been reported to improve the symptoms 27, 28.
Although exogenous factors such as the use of deodorants and shaving are thought to be causal, they have not been shown to be significantly responsible in a retrospective comparison of 40 patients with HS (29).
Smoking is more common in patients with HS, but the aetiological basis is unknown (30). Wiltz et al. reported an association between smoking and perianal HS in 70% of patients (24). Patients should probably be advised to stop smoking.
Obesity is an exacerbating factor (31), and weight loss can help control the disease severity, as shearing forces in the skin are increased in the obese (32).
The role of bacterial infection in pathogenesis is uncertain as half of cultures taken from early HS lesions were found to be negative in a study by Jemec et al. (33). It is thought that bacterial involvement occurs secondary to follicular occlusion. Staphylococcus aureus and coagulase‐negative Staphylococci are the organisms most frequently isolated from the lesions 33, 34, but a variety of other bacteria such as Streptococci and Propionibacterium acnes have also been isolated from the lesions (35).
Clinical presentation and natural history of disease
HS usually starts as ‘blind boils’, most commonly at or soon after puberty. Abscesses tend to be deep in the dermis or the subdermis, with no pointing or central necrosis. Diagnosis is based on the clinical presentation of tender subcutaneous nodules, which usually develop insidiously around the time of puberty. The axilla and anogenital regions are commonly affected first. Symptoms such as discomfort, pruritus, erythema and burning may be reported. Differential diagnosis includes carbuncle, dermoid cyst, furuncle, granuloma inguinale, pilonidal cyst and tuberculosis of the skin (5). These nodules may have a malodorous and purulent discharge. Biopsies are taken only from the HS in the perianal region to exclude Crohn’s disease and the possibility of a coexisting cancer (36). In a study of patients with HS in secondary care, individual boils were found to develop at a rate of about two per month and took an average of 1 week to resolve (9). The chronicity of HS is exemplified by the fact that 90% of patients in that study had an average disease duration of 19 years (9). However, it still remains unclear if it is always relentlessly progressive.
As the disease progresses, patients present with recurrent lesions with sinus tracts, incomplete healing and scarring. Flare‐ups may be exacerbated by stress, heat, sweat and tight clothing (9). One‐third of the patients reported worsening of their symptoms during the summer. In women, the condition frequently flares up premenstrually and following pregnancy (32) and tends to subside after their menopause (23). Children are never affected unless they have precocious puberty (32)
Treatment
A simple reliable cure for HS is yet to be found. Treatment of HS involves preventive, medical, surgical and psychological measures (5). Treatment strategies should be tailored to individual patients depending on the site and severity of the disease and the patients’ preferences.
Medical management
Initial management may be conservative. Patients are advised to avoid prolonged exposure to heat, shaving, deodorants and depilation if it causes irritation and tight clothing. However, these measures are only anecdotal and have not been scientifically proven in trials. Advice on weight loss and cessation of smoking may also be useful. Patients should also be reassured that HS is not due to poor hygiene and is not contagious (5).
Initial treatment in patients who present with mild disease, nodules with minimal pain, is with topical antiseptics and antibacterial soap. Antibiotics are the mainstay of medical treatment. Topical (37) and oral (38) clindamycin 300 mg twice a day have been shown to be effective. However, the disease often relapses on discontinuation (38). In severe and recurrent disease, long‐term antibiotic therapy for 2 months or more may be required to limit the progression (5).
-
1
Hormonal therapy: Hormonal therapy with cyproterone acetate has been shown to be useful in a randomised controlled trial (39). Finasteride, which is used in the treatment of benign prostatic hypertrophy, has been used successfully to treat severe HS in two patients (40). As mentioned above, oral contraceptives containing a high oestrogen to progesterone ratio are also an option (25).
-
2
HS is associated with acne; therefore, treatments for acne with retinoids such as isotretinoin and acitretin have been used for HS. Although isotretinoin has little effect on HS (41), a dosage of 0·5–1·0 mg/kg daily for a few months preoperatively has been recommended for its anti‐inflammatory effects (1). Acitretin, which is usually used to treat psoriasis, seems to be effective at a dose of 25 mg twice a day (42). The use of retinoids, which are known to be teratogenic, is contraindicated in pregnancy and patients should be advised to take adequate contraceptive precautions. However, further trials are needed to prove the efficacy of these treatments.
-
2
Immunosuppression: Immunosuppression with corticosteroids and other chemotherapeutic agents such as cyclosporin (43) may be effective. Infliximab, which is a chimeric monoclonal antibody with a high affinity for tumour necrosis factor alpha, is used for the treatment of rheumatoid arthritis and psoriasis (4). A retrospective analysis of five patients with HS who received infliximab suggested an improvement of symptoms (4). However, further adequate controlled trials are needed.
Surgical treatment
There is no evidence that treatment other than surgery has any effect on the natural history of severe HS (38). Surgical management should be a part of a multidisciplinary approach, which allows optimisation pre‐ and postoperatively.
Although there is general agreement that wide excision of all involved skin and soft tissue is key, both the extent of the skin excision and the management of the subsequent wound remain controversial 17, 44, 45.
The principle of surgical management of HS is a complete excision of affected tissue, leaving clear margins. Both the width and the depth of excision should be adequate as recurrences usually occurs due to inadequate excision. It has been recommended to map the apocrine‐gland‐bearing area with iodine–starch method to ensure adequate excision and prevent recurrence 18, 38.
Studies have shown that the extent of surgical excision has a greater influence on the recurrence rates compared with the method of wound management 46, 47. Still, wider controversy exists about the management of wound postoperatively and method of closure, but studies show that healing by secondary intention is associated with less complications and produces better results.
The different methods to deal with the wound include the following given below.
-
1
Radical excision and healing by secondary intention: Radical excision and healing by secondary intention is the technique preferred by many surgeons. It permits adequate disease clearance and results in a cosmetically acceptable scar, with little limitation of movement (38), and is thought to provide the best outcome. However, good reports of the relative cure rates of the different surgical options are scarce and controlled trials non existent (38).
-
2
Harrison et al. (48) and Morgan et al. (49) have advocated excision and healing by secondary intention. Harrison et al. hypothesised that the low recurrence rate observed in his patients with axillary disease was the result of removal of the entire hair‐bearing area.
-
3
Morgan et al. did not graft the excised wounds, citing poor wound vascularity and bacterial contamination, and noted that many of his patients preferred secondary healing rather than the discomfort and scarring associated with skin grafts and their donor sites. He also noted that although healing is quicker following split skin grafting (SSG), patients are more comfortable with the silastic method, allowing early discharge back to the community, very little limitation in movement and, apparently, a surgically acceptable scar, superior to that obtained by SSG. Patients (seven of ten) preferred the silastic dressing method to SSG. Most importantly, adequate clearance was achieved, which was reflected in their low recurrence rates (49).
-
4
Also in our experience, patients perform well after excision and healing by secondary intention, and this is the preferred method in many centres.
-
2
Radical excision and SSG (+/− pressure dressings): Large defects can be covered by immediate or delayed SSG. Negative pressure dressings are particularly useful in the treatment of contoured wounds needing closure with skin grafts. Greeley (50), Paletta (51), Pollock et al. (52) and Anderson and Perry (53) have advocated excision and primary closure for localised axillary disease, noting decreased operative morbidity, complications, length of hospitalisation and postoperative disability compared with more extensive procedures. They have recommended split‐thickness skin grafting only when the disease is so extensive as to preclude primary closure. A relatively recent study has used vacuum‐assisted closure coupled with SSG for large, complex open wounds, with 95% graft take in all patients (54).
-
3
Radical excision and cover with local or distant flaps: Different types of flaps have also been used with varying degree of success. Fasciocutaneous flaps, pedicled flaps and free flaps have been described in the literature for defect closure in HS surgery.
-
4
Limited excision and primary closure: If the extent of skin involvement is limited, local excision and primary closure is a method advocated by some authors, giving lower morbidity but a higher recurrence (55).
-
5
Incision and drainage: or excision of skin with primary suture may be used for early disease that presents with abscesses. However, patients should be advised that this only provides short‐term relief and disease may recur, locally or at a new site (5).
Generally, healing by secondary intention is preferred, but skin grafts and flaps may be required for large contoured wounds. They should be used for severe recurrent disease not responding to simpler measures, and the patients should be referred to specialised units. Postoperatively, it is very important to use the right kind of dressing according to the condition of the wound. Non adherent dressing, silver‐impregnated dressing, alginates and hydrocolloid dressings are useful and should be used according to wound conditions. It is important to keep the wound clean and prevent infections to expedite healing. Local or systemic antibiotics maybe required to control wound infection. Physiotherapy is needed to prevent contractures and for early rehabilitation.
Other treatment options
Radiotherapy Radiotherapy has been used in a retrospective study of 231 patients with HS, and 38% reported complete relief of symptoms and 40% showed clear improvement (56). However, further research is required as the possibility of long‐term side effects is not clear.
Laser treatment Carbon dioxide laser treatment used along with healing by secondary intention is suitable for patients with mild to moderate disease (57). Advantages include improved haemostasis and better visualisation of diseased tissue (58). However, postoperative pain was a major problem in two patients who had to take extra time off work (57).
Prognosis
The course of the disease is variable in different patients. In some, a milder form runs a relatively benign course and is easily controlled with antibiotics. Eventually, the disease burns itself out, leaving scarred and fibrotic skin. However, in others, the course of HS lasts for years until the patient opts for surgery after a period of physical and psychological debility. Treatment with antibiotics is used to suppress active infection pre‐ and postsurgery, although most patients respond poorly. In a study of 110 patients (9), the average duration of individual boils was almost 7 days, which equals the length of a course of antibiotics. This may explain the benefit that some patients obtain from the use of antibiotics.
Most patients require surgery at some point during the course of the disease, and early radical excision is advocated. With an average age of onset of between 22 and 25 years and an average duration of disease of 19 years (9), HS probably burn out before the age of 50 in many patients.
Discussion
HS remains a challenging disease for both the patient and the physician. It is a chronic debilitating disease whose aetiology is still controversial. This has repercussions on the management of this condition as different treatment options are tried and tested on patients without clear responses and a definitive cure is yet to be discovered. Patients with HS are usually young, economically productive and have an active life. Delay in diagnosis causes considerable frustration and can affect family and social life. When properly diagnosed and adequately treated, the early stage of the disease may be controlled with medical measures. However, in established acne inversa (HS), there is no evidence that treatment other than surgery has any effect on the natural course of the condition (21). In the absence of significant research comparing treatment options, the choice of treatment should depend upon patient’s circumstances and preferences, the outcome of previous treatments, the experience of physicians and the chronicity and severity at presentation. Patient’s refractory to medical treatment should be identified early and referred for surgical treatment as this offers an early chance to control the disease. Ideally, this should be undertaken in a multidisciplinary environment, with the cooperation from surgeons, physicians and wound management teams (Table 1).
Table 1.
Current treatment trend for hidradenitis suppurativa
| Severity | Characteristics | Treatment |
|---|---|---|
| Mild | Minimally painful solitary nodules in the absence of abscesses | Conservative |
| Moderate | Moderately painful recurrent nodules and discharging abscesses | Medical? Surgical |
| Severe | Diffuse abscess formation with draining sinuses and scarred skin | Surgical |
Conservative treatment and incision and/or surgical removal of the abscesses and fistulas are usually futile. The method of choice is the early complete surgical excision of the involved skin extending into normal tissue both laterally and at the base. In most cases, healing of the defects by secondary intention is uneventful.
Conclusions
The aetiology of HS is unknown, and uniformly effective treatment is lacking. While surgery offers a cure, antibiotics, retinoids, hormonal treatment and sometimes immunosuppressive medication may offer benefit to some patients. It is important to diagnose HS early and treat promptly to reduce associated morbidity. HS may be reversible initially, but once follicular rupture has occurred and sinus tracts have formed, the disease enters a more pernicious and chronic stage. At this stage, only surgery offers a hope of cure and relief of patients’ symptoms. There have been many suggested approaches to the operative treatment of this disease, so a rational approach to treatment based on the extent and location of disease is needed. This allows the surgeon and the patient to individualise treatment based on the anatomical features of the disease and the patient’s personal and socio‐economic preferences. Regular follow‐up evaluation and a multidisciplinary approach are needed to treat the patient appropriately.
References
- 1. Jansen T, Plewig G. Acne inversa. Int J Dermatol 1998;37:96–100. [DOI] [PubMed] [Google Scholar]
- 2. Jemec GBE. What’s new in hidradenitis suppurativa? J Eur Acad Dermatol Venereol 2000;14:340–1. [DOI] [PubMed] [Google Scholar]
- 3. Slade DEM, Powell BW, Mortimer PS. Hidradenitis suppurativa: pathogenesis and management. Br J Plast Surg 2003;56:451–61. [DOI] [PubMed] [Google Scholar]
- 4. Sullivan TP, Welsh E, Kerdel FA, Burdick AE, Kirsner RS. Infliximab for hidradenitis suppurativa. Br J Dermatol 2003;149:1046–9. [DOI] [PubMed] [Google Scholar]
- 5. Shah N. Hidradenitis suppurativa: a treatment challenge. Am Fam Physician 2005;72:1547–52. [PubMed] [Google Scholar]
- 6. Velpeau A. Dictionnaire de Médecine, un Répertoire Général des Sciences Médicales sous la Rapport Théorique et Practique. Aiselle, Vol. 2, 2nd edition. Paris: Bechet Jeune, 1839. [Google Scholar]
- 7. Verneuil A. Études sur les tumeurs de la peau et quelques maladies des glandes sudoripares. Arch Gén Méd 1854;94:447–68, 693–705. [Google Scholar]
- 8. Verneuil A. De l’hidrosadénite phlegmoneuse et des abcés sudoripares. Arch Gén Méd 1864;2:537–572. [Google Scholar]
- 9. Von Der Worth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2000;14:389–92. [DOI] [PubMed] [Google Scholar]
- 10. Yu CCW, Cook MG. Hidradenitis suppurativa: a disease of follicular epithelium, rather than apocrine glands. Br J Dermatol 1990;122:763–9. [DOI] [PubMed] [Google Scholar]
- 11. Plewig G, Steger M. Acne inversa (alias acne triad, acne tetrad or hidradenitis suppurativa). In: Marks R, Plewig G, editors. Acne and related disorders. London: Martin Dunitz, 1991:345–57. [Google Scholar]
- 12. Attanoos RL, Appleton MAC, Douglas‐Jones AG. The pathogenesis of hidradenitis suppurativa: a closer look at apocrine and apo‐eccrine glands. Br J Dermatol 1995;133:254–8. [DOI] [PubMed] [Google Scholar]
- 13. Jemec GBE, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol 1996;34:994–9. [DOI] [PubMed] [Google Scholar]
- 14. Jemec GBE, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol 1996;35:191–4. [DOI] [PubMed] [Google Scholar]
- 15. Fitzsimmons JS, Guilbert PR, Fitzsimmons EM. Evidence of genetic factors in hidradenitis suppurativa. Br J Dermatol 1985;113:1–8. [DOI] [PubMed] [Google Scholar]
- 16. Harrison BJ, Mudge M, Hughes LE. The prevalence of hidradenitis suppurativa in South Wales. In: Marks R, Plewig G, editors. Acne and related disorders. London: Martin Dunitz, 1989:365–6. [Google Scholar]
- 17. Wiseman MC. Hidradenitis suppurativa: a review. Dermatol Ther 2004;17:50–4. [DOI] [PubMed] [Google Scholar]
- 18. Parks RW, Parks TG. Pathogenesis, clinical features and management of hidradenitis suppurativa. Ann R Coll Surge Engl 1997;79:83–9. [PMC free article] [PubMed] [Google Scholar]
- 19. Mengesha YM, Holcombe TC, Hansen RC. Prepubertal hidradenitis suppurativa: two case reports and review of the literature. Pediatr Dermatol 1999;16:292–6. [DOI] [PubMed] [Google Scholar]
- 20. Harrison BJ, Kumar S, Read GF, Edwards CA, Scanlon MF, Hughes LE. Hidradenitis suppurativa: evidence for an endocrine abnormality. Br J Surg 1985;72:1002–4. [DOI] [PubMed] [Google Scholar]
- 21. Jansen T, Plewig G. What’s new in acne inversa (alias hidradenitis suppurativa)? J Eur Acad Dermatol Venereol 2000;14:342–3. [DOI] [PubMed] [Google Scholar]
- 22. Fitzsimmons JS, Fitzsimmons EM, Bilbert G. Familial hidradenitis suppurativa: evidence in favour of single gene transmission. J Med Genet 1984;21:281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mortimer PS, Dawber RPR, Gales M, Moore RA. Mediation of hidradenitis suppurativa by androgens. BMJ 1986;292:245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wiltz O, Schoetz DJ, Murray JJ, Roberts PL, Coller JA, Veidenheimer MC. Perianal hidradenitis suppurativa: the Lahey clinic experience. Dis Colon Rectum 1990;33:731–4. [DOI] [PubMed] [Google Scholar]
- 25. Stellon AG, Wakeling M. Hidradenitis suppurativa associated with use of oral contraceptives. BMJ 1989;298:28–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barth JH, Layton AM, Cunliffe WJ. Endocrine factors in pre‐ and postmenstrual women with hidradenitis suppurativa. Br J Dermatol 1996;134:1057–9. [PubMed] [Google Scholar]
- 27. Sawers RS, Randall VA, Ebling FJG. Control of hidradenitis suppurativa in women using combined antiandrogen (cyproteron acetate) and oestrogen therapy. Br J Dermatol 1986;115:269–74. [DOI] [PubMed] [Google Scholar]
- 28. Goldsmith PC, Dowd PM. Successful therapy of the follicular occlusion triad in a young woman with high does oral antiandrogens and minocycline. J R Soc Med 1993;86:729–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morgan WP, Leicester G. The role of depilation and deodarants in hidradenitis suppurativa. Arch Dermatol 1982;118:101–2. [PubMed] [Google Scholar]
- 30. König A, Lehman C, Rompel R, Happel R. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology 1999;198:261–4. [DOI] [PubMed] [Google Scholar]
- 31. Jemec GBE. Body weight in hidradenitis suppurativa. In: Marks R, Plewig G, editors. Acne and related disorders. London: Martin Dunitz, 1989: 375–6. [Google Scholar]
- 32. Mortimer PS, Lunniss PJ. Hidradenitis suppurativa. JR Soc Med 2000;93:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jemec GBE, Faber M, Gutschick E, Wedelboe P. The bacteriology of hidradenitis suppurativa. Dermatology 1996;193:203–6. [DOI] [PubMed] [Google Scholar]
- 34. Lapins J, Jarstrand C, Emtestam L. Coagulase‐negative staphylococci are the most common bacteria found in cultures from the deep portions of hidradenitis suppurativa lesions, as obtained by carbon dioxide laser surgery. Br J Dermatol 1999;140:90–5. [DOI] [PubMed] [Google Scholar]
- 35. Brown TJ, Rosen T, Orengo IF. Hidradenitis suppurativa. South Med J 1998;91:1107–14. [DOI] [PubMed] [Google Scholar]
- 36. Townsend CM, Sabiston DC. Sabiston textbook of surgery: the biological basis of modern surgical practice. 16th edition. Philadelphia: WB Saunders, 2001. [Google Scholar]
- 37. Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol 1983;22:325–8. [DOI] [PubMed] [Google Scholar]
- 38. Banerjee AK. Surgical treatment of hidradenitis suppurativa. Br J Surg 1992;79:863–6. [DOI] [PubMed] [Google Scholar]
- 39. Mortimer PS, Dawber RPR, Gales MA, Moore RA. A double‐blind cross‐over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol 1986;115:263–8. [DOI] [PubMed] [Google Scholar]
- 40. Farrell AM, Randall VA, Vafaee T, Dawber RP. Finasteride as a therapy for hidradenitis suppurativa [letter]. Br J Dermatol 1999;141:1138–9. [DOI] [PubMed] [Google Scholar]
- 41. Boer J, Mirjan JP, Gemert V. Long‐term results of isotretinoin in the treatment of 68 patients with hidradenitis suppurativa. J Am Acad Dermatol 1999;40:73–6. [DOI] [PubMed] [Google Scholar]
- 42. Chow ETY, Mortimer PS. Successful treatment of hidradenitis suppurativa and retroauricular acne with etretinate. Br J Dermatol 1992;126:415. [DOI] [PubMed] [Google Scholar]
- 43. Buckley DA, Rogers S. Cyclosporin‐responsive hidradenitis suppurativa. J R Soc Med 1995;88:289–90. [PMC free article] [PubMed] [Google Scholar]
- 44. Cornldeet T. Pregnancy and apocrine gland diseases: hidradenitis, Fox‐Fordyce disease. Arch Sermatol Syph 1952;65:12–20. [DOI] [PubMed] [Google Scholar]
- 45. Bohn J, Svensson H. Surgical treatment of hidradenitis suppurativa. Scand J Plast Reconstr Hand Surg 2001;5:305–9. [DOI] [PubMed] [Google Scholar]
- 46. Soldin MG, Tulley P, Kaplan H, Hudson DA, Grobbelaar AO. Chronic axillary hidradenitis – the efficacy of wide excision and flap coverage. Br J Plast Surg 2000;53:434–6. [DOI] [PubMed] [Google Scholar]
- 47. Rompel R, Petres J. Long‐term results of wide surgical excision in 106 patients with hidradenitis suppurativa. Dermatol Surg 2000;26:638–43. [DOI] [PubMed] [Google Scholar]
- 48. Harrison BJ, Mudge M, Hughes LE. Recurrence after surgical treatment of hidradenitis suppurativa. BMJ 1987;294:487–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morgan WP, Harding KG, Hughes LE. A comparison of skin grafting and healing by granulation, following axillary excision for hidradenitis suppurativa. Ann R Coll Surg Engl 1983;65:235–6. [PMC free article] [PubMed] [Google Scholar]
- 50. Greeley PW. Plastic surgical treatment of chronic suppurative hidradenitis. Plast Reconstr Surg 1951;7:143–6. [DOI] [PubMed] [Google Scholar]
- 51. Paletta FX. Hidradenitis suppurativa: a pathologic study and the use of skin flaps. Plast Reconstr Surg 1963;31:307–15. [PubMed] [Google Scholar]
- 52. Pollock WJ, Virnelli FR, Ryan RF. Axillary hidradenitis suppurativa. Plast Reconstr Surg 1972;49:22–7. [PubMed] [Google Scholar]
- 53. Anderson DK, Perry AW. Axillary hidradenitis. Arch Surg 1975;110:69–72. [DOI] [PubMed] [Google Scholar]
- 54. Blackburn JH, Boemi L, Hall WW, Jeffords K, Hauck RM, Banducci DR, Graham WP. Negative‐pressure dressings as a bolster for skin grafts. Ann Plast Surg 1998;40:453–7. [DOI] [PubMed] [Google Scholar]
- 55. Jemec GBE. Effect of localized surgical excisions in hidradenitis suppurativa. J Am Acad Dermatol 1988;18:1103–7. [DOI] [PubMed] [Google Scholar]
- 56. Frohlich D, Baaske D, Glatzel M. Radiotherapy of hidradenitis suppurativa – still valid today? Strahlenther Onkol 2000;176:286–9. [PubMed] [Google Scholar]
- 57. Laplins J, Sartorius K, Emtestam L. Scanner‐assisted carbon dioxide laser surgery: a retrospective follow‐up study of patients with hidradenitis suppurativa. J Am Acad Dermatol 2002;47:280–5. [DOI] [PubMed] [Google Scholar]
- 58. Finley EM, Ratz JL. Treatment of hidradenitis suppurativa with carbon dioxide laser excision and second intention healing. J Am Acad Dermatol 1996;34:465–9. [DOI] [PubMed] [Google Scholar]


