Abstract
The use of antiseptics in wound care is often controversial and there is definitely a need for a non toxic, highly disinfective agent. This study assessed the efficacy of a neutral pH superoxidised aqueous solution (NpHSS) for infection control, odour reduction and surrounding skin and tissue damage on infected diabetic foot ulcerations. From November 2003 to March 2004, 45 patients with type 2 diabetes were randomised into a single‐blind clinical trial comparing NpHSS (intervention group; n = 21) versus conventional disinfectant (control group; n = 16). All patients received comprehensive care including surgical debridement as appropriate, moist wound care, intensive glucose control and broad spectrum antibiotics. Treatment groups were matched in terms of sex, age (61·9 ± 11·9 versus 67·8 ± 11·6), years of diabetes duration (16·4 ± 8·1 versus 17 ± 10·2), obesity, HgAlc (7·1 ± 2 versus 6·7 ± 1·8), initial fasting glycaemia (163 ± 59 versus 152 ± 65·8 mg/dl), ulcer duration/week (13·7 ± 24 versus 15·1 ± 16·3), B/A Index (0·9 ± 0·5 versus 1·14 ± 0·7), depth and extent of infection/periwound cellulitis (groups B and C of the Tampico Hospital Classification) as well as aetiology (P = 0·647). Odour reduction was achieved in all NpHSS patients (100% versus 25%; P < 0·01) and surrounding cellulitis diminished (P < 0·001) in 17 patients (80·9% versus 43·7%). Nineteen patients in the NpHSS group showed advancement to granulating tissue stage (90·4% versus 62·5%; P = 0·05) with significantly less tissue toxicity (94% versus 31·2%; P < 0·01). A non toxic, NpHSS, as part of a comprehensive care regimen, may be more efficacious in infection control, odour and erythema reduction than conventional disinfectants in treatment of diabetic foot infections.
Keywords: Antiseptics, Cellulitis, Diabetic foot, Infections, Wound healing
Introduction
Infected foot ulcerations are a frequent cause of morbidity, hospitalisation and amputations among persons with diabetes (1). The attributable cost of caring for a primary healing foot ulcer for 3 years after the initial diagnosis can amount to over $26 000 (2). The survival rate of patients who develop diabetic foot ulcers is reduced by about 15%, and at 5 years, these patients have approximately a 50% survival rate usually after arterial interventions and amputations 3, 4.
Diabetic patients are prone to develop foot ulcerations because of the lack of neurological sensation and cycles of repetitive stress from ambulation. Rates of ulceration, infection and amputation have declined dramatically in some centres that have developed programmes and teams for foot care. Infection still remains a common problem in diabetic patients 5, 6, 7, 8.
Ulcerations are pivotal events leading to diabetic foot infection, which has been shown to be the immediate cause of amputations in 25–50% of the cases (2). This is especially true of deep space infection, which is associated with an amputation rate of 52% (2). The resultant amputations are minor in 24–60% of the cases and major in 10–40% (2).
The spectrum of foot infections in diabetic patients ranges from simple superficial cellulitis to chronic osteomyelitis. Infections in patients with diabetes are often difficult to treat because of impaired microvascular circulation, which limits the access of phagocytic cells to the infected area and results in a poor concentration of antibiotics in the infected tissues (9). Wound healing is delayed when acute infection persists 10, 11, 12. Infection control along with appropriate debridement and good wound care are all essential factors in the treatment of diabetic foot ulcerations. Most of the chemicals disinfectants currently used in wound care can cause damage to the skin or granulating tissue. Topical antiseptics such as hypochlorite (Dakin’s solution), povidone iodine (betadine) and others can cause significant cell damage. This may not only interfere wound healing process (13), but also could be cytotoxic or deleterious for the underlying tissues or proximal skin (14). Furthermore, normal sterile saline or water, although non cytotoxic, do not adequately remove surface contaminates and does not have antiseptic properties. Some products as the wound cleansers help to break the bond between contaminates, necrotic tissue and the surface of the wound 15, 16.
An effective non toxic antiseptic agent that cleanses the wound and removes bacterial contaminants without damaging healthy tissue is therefore needed to help diminish the bacterial burden and prevent the substantial long‐term morbidity associated with amputations.
Superoxidized aqueous solution is non toxic, neutral pH water that contains reactive oxygen species (ROS) generated by the electrolysis of sodium chloride and water. Components of this solution includes superoxide – neutral pH water, chlorine <85 parts per million (ppm), ROS/free radicals, oxidised water H2O; 99·99%, sodium hypochlorite (NaOCl) <50 ppm, hypochlorous acid (HOCl) <60 ppm, hydrogen peroxide (H2O2) <4 ppm, ozone (O3) <0·2 ppm, chlorine dioxide (ClO2) <1·5 ppm, sodium hydroxide (NaOH) <8 ppm, sodium carbonate (Na2CO3) <21 ppm, sodium chloride (NaCl) <110 ppm. The bactericidal advantages and wound healing properties of ROS has been documented (13). Superoxidised water has been shown to be a powerful non toxic bactericidal against a variety of aerobics Gram‐negative and Gram‐positive rods (17) and is highly effective in treating infectious skin conditions and refractory ulcers associated with diabetes mellitus or peripheral circulatory insufficiency 18, 19.
In this randomised, controlled, single‐blind study, our aim was to assess the efficacy of a non‐toxic, neutral pH superoxidised aqueous solution (NpHSS) in terms of infection control, odour reduction and periwound skin affection in infected diabetic foot ulcerations.
Materials and methods
Patients and baseline evaluations
Between November 2003 to March 2004, 45 consecutive patients with type 2 diabetes and infected, deep diabetic foot ulcers who presented for care at the San Elian Diabetic Foot Salvage and Prevention Center (SEDFSPC) in Veracruz, México were initially selected for the study by use of a randomly alternate assignment to either NpHSS or standard management (control group). All patients gave a written informed consent, and the study was reviewed and approved by the human subjects SEDFSPC Ethics Committee.
The inclusion criteria involved patients with type 2 diabetes older than 18 years of age with infected, deep wounds at or distal to the malleoli, presence of mal odour, active periwound cellulites, loss of protective sensation and at least one dopplerable pedal pulse. Wound size was evaluated by measuring the maximum length by the maximum width. Exclusion criteria included severe arterial disease (diagnosed by the criteria listed above based on the absence of both foot pulses on the affected extremity), brachial/ankle index below 0·5, the diagnosis of osteomyelitis total gangrene of the study foot or forefoot, severe cardiovascular and renal failure and severe neurological problems that would make the patient a poor candidate for the study (e.g. confined to the bed). Patients with no family assistance were also excluded from the study.
Baseline demographic measurements were performed at the first visit. The diagnosis of diabetes was made prior to enrolment and confirmed by either communication with primary care providers or review of medical records. Doppler studies were performed and branch/ankle index calculated. Neuropathy was evaluated by the Michigan (20), and the Rydel‐Seiffer scores. The Michigan test included vibratory and 10 g Semmes‐Weinstein sensitivity protective response (21). Neuropathic, vascular assessments and wound characteristics were included in the Tampico Hospital Diabetic Foot Classification system, which grades wounds according to depth, the extent of periwound cellulitis and the aetiology of injury.
Stage A ulcer corresponds to skin surface disruption, without cellulitis or bone affection, stage B is a deep wound with probable bone involvement and 2 cm of periwound cellulitis extension and a stage C ulcer encompasses all foot depths with abscess, necrosis, bone infection and cellulitis greater than 2 cm in width. This is in correlation to the Definitions and Criteria of the International Working Group on the Diabetic Foot International Consensus (2). All patients in the study had either a stage B or stage C ulcers. The second part of the grading system defines the aetiology, be it vascular, neuropathic or both. X‐ray radiography of the affected foot was also performed in order to help with the assessment of osteomyelitis.
Standard of care treatment and therapeutic intervention
Patients were randomised using randomly alternate assignment to either NpHSS or standard management (control group). Both groups received comprehensive care and the control group included the use of chemical antiseptics such as soap or povidone iodine. Patients were blinded about the differences in treatment.
All patients were treated using an outpatient ambulatory model, which included appropriate surgical debridement, aggressive parenteral/intramuscular broad spectrum antimicrobial administration, appropriate off loading and strict glycaemic control.
Broad spectrum antimicrobial use was started empirically and immediately at the first visit to cover polymicrobial infection. Appropriate changes or adjustments were made after microbiological assessments of deep wound cultures were determined 2, 22, 23 Antibiotics were used for a minimum of 10 days in all patients in both groups. Antibiotics were used for more that 10 days if clinical signs of infection continued to be present. All patients received pentoxyphylline at a dose of 1200 mg/day as a haemorheologic as part of the San Elian’s outpatient ambulatory model irrespective of their degree of vascular compromise.
Patients in both treatment groups were initially followed on a daily basis and depending on the condition of the wound, were seen every third day or once a week.
Patients randomised to the intervention group received an initial 15 to 20‐minute immersion of the affected foot in NpHSS. Following appropriate debridement, the affected foot soak was repeated either weekly or biweekly depending on the severity of the periwound cellulites followed by subsequent wound cleansings with NpHSS spray between immersions. NpHSS was also used instead of saline solution to remove gauzes. Foot immersions were discontinued on clinical improvement of wound and surrounding skin or when the first sign of maceration was observed. NpHSS spray applications were continued until either complete resolution of wound infection or the end of the 20‐week study.
The control group received the same treatment with the exception that saline was used in place of NpHSS. Povidone iodine was initially used after debridement to cleanse the wound. When the infection resolved and formation of granulation tissue was observed, the patient was switched to a surgical soap (Dermo Clean) with saline rinse to minimise the cytotoxic effects of povidone iodine. If clinical signs of infection returned, the use of povidone iodine was resumed.
Both groups received conventional standard method of wound care consisting of gauze saturated with Triticum vulgare (Italdermol, Italmex, S.A., Mexico) to moisten the wound followed by adhesive covering. Heavily exudating wounds were dressed with a calcium alginate (e.g. Kaltostat, Bristol‐Mayer Squib, Mexico).
All patients in both groups were instructed to reduce weight bearing on the affected foot by using a wheelchair or crutches and by resting as much as possible. We are aware that most patients have difficulty complying with these modalities. Compliance was assessed by directly questioning the patient and his/her caretaker and by inspection of the dressings by the health care team.
Primary objective and measurements
The primary outcomes were odour reduction, infection control as indicators of antiseptic efficacy and wound safety in the treatment of diabetic foot infections. Infection control was assessed in terms of periwound cellulitis reduction and the percentage of patients advancing from infected, necrotic tissue to wound granulation. Cellulitis reduction was considered effective when the affected area of erythema decreased by more than 50% during the infection phase. Two members of the staff assessed odour reduction independently at wound level. Kappa agreement index was performed via 2 × 2 tables to detect differences between the two observers for odour perception. A kappa value (K = Po−Pe/1−Pe; Po = observed agreement, Pe = expected agreement by random) between 0·61 and 1·0 was representative of substantial to excellent agreement.
Granulating tissue is the pink/red, moist tissue comprised of new blood vessels, connective tissue, fibroblast and inflammatory cells, which fills an open wound when it starts to heal.
Erythema of surrounding skin and granulating tissue was assessed by direct clinical observation. Abnormal periulcer skin conditions such as dryness, erythema, induration, rash, epidermolysis or blister formation may be caused by the use of chemical disinfectants. The absence of this damage along with the presence of healthy tissue surrounding the ulcers was considered as clinical signs of no or minimal tissue toxicity.
Daily pictures and weekly measurements of the wound, periwound cellulitis, local skin affection and granulation tissue were recorded in a database as part of systematic data collection previously designed independently of this study.
For statistical analysis, significance was considered at P < 0·05. Values of chi‐squared with Yate’s correction or Fisher exact for 2 × 2 tables and of variance ratios for natural and treatment analysis of variance were calculated.
Results
Forty‐five patients were evaluated at baseline. Eight patients had severe arterial disease and did not meet the inclusion criteria. They were sent to a vascular surgeon for either vascular intervention or amputation, and their data were not included in the report. From the remaining 37 patients, 21 received the NpHSS and 16 received control treatment. All subjects completed the full length of the 20‐week study. Subjects in both treatment groups were matched in terms of age, sex, years of diabetes duration, obesity, mean HbA1c, ulcer duration/week and Yao B/A index (Table 1). No differences were noted in the staging of ulcers (Table 2) of the Diabetic Foot Tampico Hospital Classification and the wounds were similar in aetiology (P = 0·873). Mean average of oral antibiotics treatment was of 26 ± 3·1 days for intervention versus 30 ± 5·2 for control group (NS).
Table 1.
Baseline demographic and clinical characteristics of NIDDM patients with severe infected foot ulcers
| Characteristic | NpHSS (n = 21) | Control (n = 16) | P value |
|---|---|---|---|
| Age (years)* | 61·9 ± 11·9 | 67·8 ± 11·6 | NS |
| Sex† | |||
| Male | 9 (45) | 8 (50) | NS |
| Female | 12 (55) | 8(50) | |
| Years of diabetes duration* | 16·4 ± 8·1 | 17 ± 10·2 | NS |
| Mean of HbA1c* | 7·1 ± 2 | 6·7 ± 1·8 | NS |
| Mean of fasting glycaemia* (mg/dl) | 163 ± 59 | 152 ± 65·8 | NS |
| Obesity† | 6 (30) | 4 (25) | NS |
| Ulcer duration/week* | 13·7 ± 24 | 15·1 ± 16·3 | NS |
| B/A index (Yao)* | 0·9 ± 0·5 | 1·14 ± 0·7 | NS |
Obesity is defined as body mass index >27 kg/m2.
HbA1c, haemoglobin A1c; NS, non significant; NIDDM, non insulin‐dependent diabetes mellitus; NpHSS, neutral pH superoxide solution.
Values are given as mean ± SD or actual (per cent).
Chi‐squared and Yates’ correction.
Table 2.
Stage severity of diabetic foot tampico hospital grading
| Stage | NpHSS (n = 21) | Control (n = 16) | P value |
|---|---|---|---|
| B | 12 | 9 | 0·873 |
| Vascular | 4 | 1 | NS |
| Neuropathic | 7 | 3 | |
| Both | 1 | 5 | |
| C | 9 | 7 | |
| Vascular | 2 | 1 | |
| Neuropathic | 2 | 2 | |
| Both | 5 | 4 |
All patients (100%) in the NpHSS group versus only 25% (n = 4) of the control group showed odour reduction (Table 3). The NpHSS group also showed statistically significant reduction in cellulites, 81% compared with 44% for the control group. The periwound cellulitis remained unchanged or worsened for four patients in the NpHSS group and for nine patients in the control group. Nineteen of the 21 patients (90·4%) in the NpHSS group showed good outcomes advancing from infected, necrotic tissue to granulating tissue; this was seen in only 62·5% of the control group.
Table 3.
Total of patients with good outcomes with NpHSS versus control group and clinical efficacy assessment by NNT
| Outcome | NpHSS (n = 21) | Control (n = 16) | P value* | NNT | 95% CI (min–max) |
|---|---|---|---|---|---|
| Fetid odour reduction | 21 (100) | 4 (25) | 0·001 | 2 | 1–1·9 |
| Infection control | |||||
| Cellulitis reduction | 17 (80·9) | 7 (43·7) | 0·01 | 3 | 1·5–13·1 |
| Advances from infection to granulating tissue | 19 (90·4) | 10 (62·5) | 0·05 | 4 | 1·8–87·9 |
| Improvement of skin around the ulcer | 19 (90·4) | 5 (31·2) | 0·001 | 2 | 1·2–3 |
Percentage values are given in parentheses.
NNT, number needed to treat. NNT significant clinical efficacy range = 2–4.
Yates correction for chi‐squared; significant results for P < 0·05.
Tissue damage was observed to be less severe in the intervention versus control group. Ninety per cent (n = 19) of patients in the NpHSS group showed improvements in periwound conditions compared with 31% (n = 5) of controls (Table 3). The majority of the patients in the control group showed granulation as well as skin damage. Two and six patients in the intervention and control group, respectively, showed no change in the percentage of granulation tissue at the start and at end of the 20‐week study. Seventy‐five per cent of control subjects had a malodorous wound at the end of the study compared with zero patients in the NpHSS group. The absolute risk reduction (ARR) is 75% [95% confidence interval (95% CI): 53·78–96·22] with a number needed to treat (NNT) of 2. In 56% of the control subjects, periwound cellulitis either worsened or remained unchanged; this was seen in only 19% of NpHSS subjects. The ARR is 37% (95% CI: 7·66–66·75). The NNT was 3, meaning that one in every three patients with periwound cellulitis will benefit from this treatment. Thirty‐seven per cent of control subjects showed either no improvement of infected tissue to granulating tissue or presented worsening of the wound (Table 3), while only 9·5% of the NpHSS group had similar results. The ARR was 27·9% with a 95% CI for this difference ranges from 1·14% to 54·82% and the NNT of 4. Sixty‐nine per cent of control subjects had tissue toxicity, while only 9·5% of NpHSS subjects had skin and tissue damage around the ulcer. The difference or the ARR was 59·2 (95% CI: 33·28–85·18) with a NNT of 2.
Discussion
The use of antiseptics in wound care is often controversial and there is definitely a need for a non toxic, highly disinfective agent. In this randomised, controlled, single‐blind study, we found that the use of a non toxic, NpHSS, is more effective in infection control, odour reduction and cause less skin affection than conventional disinfectants in treatment of diabetic foot infections.
Patients with diabetes have a higher risk of developing deleterious infections 24, 25. Defects in host immune responses are at least partially responsible for their susceptibility and may include diminished polymorphonuclear leucocyte functions such as abnormalities of migration, phagocytosis (26), intracellular killing and chemotaxis (27). Some evidence suggests that cellular immune responses may be reduced as well (28). Poor granulation tissue formation, prolonged persistence of abscesses and impaired wound healing may also help predispose diabetic persons to infectious complications. Many of these immunodeficiencies are related to the metabolic perturbations caused by poorly controlled diabetes 28, 29, 30.
Deleterious wound healing complicated by infections are often a challenge to clinicians even with the use of antibiotics. One must determine whether the failure to heal is secondary to persistent infection, antiseptic chemical damage or because of the persistence of abnormal inflammatory response. Antibiotics are usually continued for 10 days or longer even though clinical symptoms of infection may disappear earlier. Prompt empirical treatment with antibiotics can prevent the infection from spreading rapidly and reaching the blood and organs 2, 21, 23, 31. Symptoms of cellulitis usually disappear after a few days of antibiotic therapy in non diabetic patients, but in diabetic foot infections, not only are broad spectrum antimicrobials necessary, adequate debridement and the use of local antiseptics for wound cleansing are mandatory.
Prior to the introduction of superoxidised solution to our facilities, the use of antiseptic agents had been a topic of controversy and debate. There are pros and cons to the use of cytotoxic but potent antiseptic solutions like povidone iodine or less cytotoxic but weak antiseptics like soap. The resultant dry, dark, indurated skin from povidone iodine applications made it difficult to distinguish whether the delayed wound healing was secondary to infection or chemical damage. Consequently povidone iodine is often employed at the initial surgical debridement for severe deep infections with the primary focus on infection treatment, while the possible associated cytotoxicity and tissue damage becomes a secondary issue until this phase ends. This study showed that the use of NpHSS as compared with conventional chemical antiseptic agents provides higher infection control while minimising skin and tissue damage. Once the severity of the infection has been reduced, more patients advanced to the granulating tissue phase with NpHSS treatment as compared with those who received conventional chemical antiseptics.
The subjects in the two groups were matched in terms of their metabolic status, severity of wound depth, cellulitis extension and vascular/neuropathic aetiology. This prevented bias and showed that NpHSS, compared with conventional disinfectants, was an effective antiseptic agent with greater odour reduction and less tissue toxicity. The absence of malodour following cellulitis reduction was seen in 100% of the patients who were treated with NpHSS. The reduction of odour can be explained by the action of free radicals in the NpHSS that react highly with putrefaction substances from necrotic tissues and anaerobes. Hydrogen peroxide, chlorine, hypochlorite and hypochlorous acid all have known deodorant properties. NpHSS’ bactericidal actions and minimal cytotoxicity over granulating tissue increased the number of patients advancing to the next wound healing stage, while patients in the control group had clinical signs of wound infection for a longer period of time. The transition stage between inflammatory and granulating phases where ulcers although apparently uninfected are not granulating are often highly time and resource consuming. Unlike invasive infection, which produces the classic signs of erythema, oedema and induration, indicators for surface level infections are much more subtle: persistent high volume exudate, sudden deterioration in the quality or quantity of granulation tissue, continual formation of a thin layer of avascular tissue and increased pain (32). The reinforcement of patients care with NpHSS along this transition stage showed better control of this subtle infection, increasing the percentage of patients that advanced to the granulating phase.
The preliminary NpHSS success in this trial could be explained by its ROS properties that simultaneously act to kill bacteria, react with odour putrefaction substances while causing less cytotoxicity and tissue damage to granulating tissue, and surrounding healthy tissue.
NpHSS contains ROS and free radicals similar to those produced and released during the respiratory burst inside the mitochondria to produce energy (adenosine triphosphate, ATP), CO2 and water. Microbial killing requires the ability of leucocytes to generate ROS, as well as the action of various microbicidal enzymes and peptides contained in leucocyte secretory granules. Superoxide anion and granule microbicidal enzymes are the mechanisms phagocytic leucocytes use to kill their targets. Superoxides act as degradative oxygen free radicals killing directly by their oxidising capacity, they are highly reactive with bacterial cell wall and membrane components, and ‘signalling’ protease activation via the highly pH‐dependent nicotinamide adenine dinucleotide phosphate (NADPH) oxidase electrogenic process (32).
Dismutation of the superoxide generates OH−, the accumulating negative charge must be compensated by an influx of H+ and/or K+. The hypertonicity resulting from K+ transport promotes the release of inactive cationic granule proteases bound to an anionic sulphated proteoglycan matrix. The highly K+ hypertonic environment solubilises bacterial proteases and allows them to participate in the killing response.
Superoxide anion dismutes to form H2O2 and several other ROS and reactive intermediates (Figure 1). The reactive intermediates are products of nitrogen or oxygen reactions. The reactive nitrogen intermediates derivate from endothelial cell nitric oxide (NO) metabolism. The properties of NO on wound healing and immune response become deleterious when it reacts with superoxide to produce peroxynitrite, a compound involved in DNA damage to cell and bacteria wall. Fortunately, NO must compete to react with superoxide molecules that are continuously producing reactive oxygen intermediates (ROI), thereby reducing the production of peroxynitrite. Instead of reacting with NO to form peroxinitrate, superoxide produces H2O2, other ROI, and ROS that are scavenged or reversed by primary and secondary antioxidants defences. Catalysed by the granule enzyme myeloperoxidase (MPO), H2O2 combines with chloride to form the highly microbicidal chemical, hypochlorous acid. Organisms such as Staphylococcus aureus and Candida albicans require NADPH oxidase activity to be effectively killed and are particularly susceptible to the action of ROS and MPO in both in vitro and in vivo experiments (33). Both oxidase activity and protease action are necessary to destroy these organisms.
Figure 1.
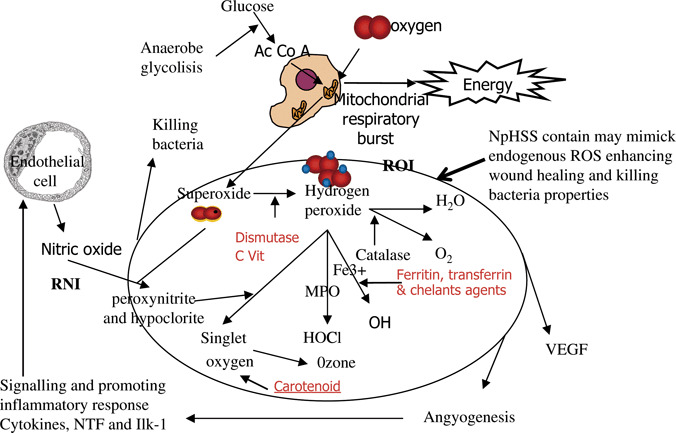
Oxidative stress and body primary and secondary defences. HOCl, hypochlorous acid; ILK‐1, interleukin‐1; MPO, myeloperoxidase; NpHSS, neutral pH superoxidised aqueous solution; NTF, necrosis tumor factor; ROI, reactive oxygen intermediates; ROS, reactive oxygen species; RNI, reactive nitrogen intermediates; VEGF, vascular endothelial growth factor.
The electrical energy of the superoxide solutions destroys microorganisms including fungi, viruses, mycobacteria, spirochetes and bacteria (32). Former acid solutions lose both its electrical potential and its germicidal action when it comes into contact with multicellular organic matter, and it reverts to ordinary water. Conversely, NpHSS appears to be more effective in retaining its germicidal action in this condition because of the trigger of ROS in a potassium‐ and pH‐dependant mechanism. Further studies are needed to assess if NpHSS has additional properties on wound healing plus its disinfectant action. We hypothesised that the increased granulating tissue patients proportion on the intervention group may be explained as an additional property of the ROS that might trigger early wound healing through fibroblast migration and proliferation (34). One basic study shows that the H2O2 neutrophil and macrophage production acts on human keratinocytes causing Rac1 gene overexpression to improve histological architecture, enhance deposition of connective tissue, higher cell density and a hyperproliferative epithelial region (13).
The success of treatment modalities must be determined in order to choose a new proposal of treatment, significant statistic results are not always enough to make clinical decisions. For this reason, we calculated the NNT to assess the clinical impact of our statistical outcomes. The relative benefit of an active treatment over a control is usually expressed as the relative risk, the relative risk reduction or the odds ratio. For clinical decision making, however, it is more meaningful to use the NNT (35). Of clinical importance in decision making is the ability of NpHSS to reduce odour in one patient of every three treated; its significant cellulitis reduction in one of every three patients, and advancement from infection to granulating stage with less tissue toxicity in one of every two patients.
Diabetic foot infections are a common and complex problem with serious economic and medical consequences. Fortunately, much progress has been made in the past two decades to treat and prevent diabetic foot infections. The results of this study have shown that patients with mild to severe, non limb‐threatening infections can be treated as outpatients with antibiotics and NpHSS local antiseptic therapy. Accumulating evidence suggests that infection may be controlled by proper wound care; optimal metabolic control; and early, aggressive, surgical and antibiotic therapy 36, 37.
All these factors or components work synergistically to help with limb preservation in the large majority of patients (2, 3). The continued development of new treatments like NpHSS may take us one step closer to a more efficacious and cost‐effective algorithm to treat diabetic foot ulcerations.
Figure 2.
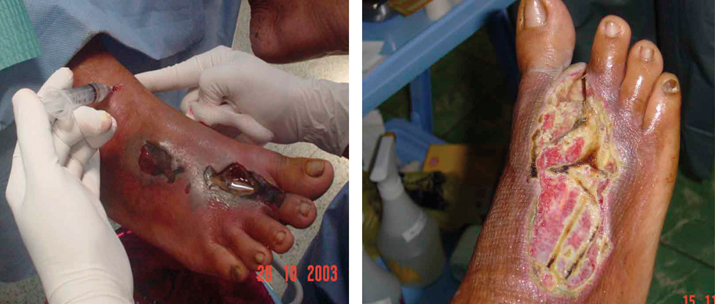
A 48‐year‐old woman with severe diabetic foot infection condemned previously to our assessment for below‐knee amputation. Left picture shows the patient foot at initial surgical debridement. Fetid odour, necrosis, cellulitis area of more than 10 cm deep abscess, necrotic injuries and ‘browned’ coloured purulent discharge were present. Right picture shows 2–3 weeks later that granulating tissue increased and improving of skin condition around ulcer after neutral pH superoxidised aqueous solution treatment.
Figure 3.
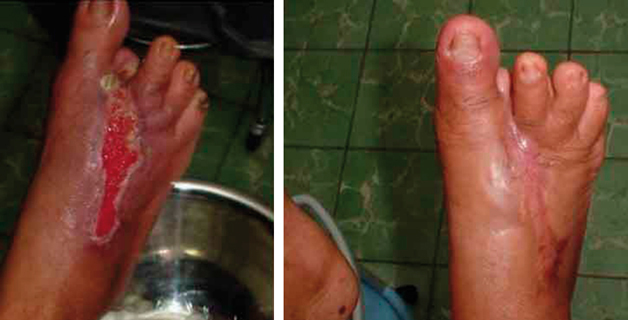
Nine weeks later, after use of neutral pH superoxidised aqueous solution. Granulating tissue, wound contraction and healthy skin around the ulcer. Right picture shows total wound healing in a patient visit 4 months later.
Acknowledgement
The authors thank Nicolas Martínez Montañez for statistical support and assistance in data collection and analysis.
References
- 1. Lipsky BA. Medical treatment of diabetic foot infections. Clin Infect Dis 2004;39:104–14. [DOI] [PubMed] [Google Scholar]
- 2. International Working Group on the Diabetic Foot . International consensus on the diabetic foot. Maastricht: International Working Group on the Diabetic Foot, 1999. [Google Scholar]
- 3. Andros G. Diagnostic and therapeutic arterial interventions in the ulcerated diabetic foot. Diabetes Metab Res Rev 2004;20:29–33. [DOI] [PubMed] [Google Scholar]
- 4. Santos D, Carline T. Examination of the lower limb in high risk patients. J Tissue Viability 2000;10:97–105. [DOI] [PubMed] [Google Scholar]
- 5. Martinez DA, Aguayo J, Morales G, Aguiran M, Illan F. Impact of a clinical pathway for the diabetic foot in a general hospital. An Med Interna 2004;21:420–4. [DOI] [PubMed] [Google Scholar]
- 6. Bartus CL, Margolis DJ. Reducing the incidence of foot ulceration and amputation in diabetes. Curr Diab Rep 2004;4:413–8. [DOI] [PubMed] [Google Scholar]
- 7. Van Houtum WH, Rauwerda JA, Ruwaard D, Schaper NC, Bakker K. Reduction in diabetes‐related lower‐extremity amputations in the Netherlands: 1991‐2000. Diabetes Care 2004;27:1042–6. [DOI] [PubMed] [Google Scholar]
- 8. Birke JA, Patout CA Jr, Foto JG. Factors associated with ulceration and amputation in the neuropathic foot. J Orthop Sports Phys Ther 2000;30:91–7. [DOI] [PubMed] [Google Scholar]
- 9. Raymakers JT, Houben AJ, Van Der Heyden JJ, Tordoir JH, Kitslaar PJ, Schaper NC. The effect of diabetes and severe ischaemia on the penetration of ceftazidime into tissues of the limb. Diabet Med 2001;18:229–34. [DOI] [PubMed] [Google Scholar]
- 10. Ovington L. Bacterial toxins and wound healing. Ostomy Wound Manage 2003;49(7A Suppl):8–12. [PubMed] [Google Scholar]
- 11. Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis 2004;17:91–6. [DOI] [PubMed] [Google Scholar]
- 12. Robson MC. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am 1997;77:637–50. [DOI] [PubMed] [Google Scholar]
- 13. Fowler E, Vesely N, Pelfrey M, Jordan S, Amberry T. Wound care for persons with diabetes. Home Healthc Nurse 1999;17:437–44. [DOI] [PubMed] [Google Scholar]
- 14. Nakae H, Inaba H. Effectiveness of electrolyzed oxidized water irrigation in a burn‐wound infection model. J Trauma 2000;49:511–4. [DOI] [PubMed] [Google Scholar]
- 15. Kirsner R, Froelich C. Soaps and detergents: understanding their composition and effect. Ostomy Wound Manage 1999;44:62S–70S. [PubMed] [Google Scholar]
- 16. Rodeheaver GT. Wound cleansing, wound irrigation, wound disinfection. In: Krasner D, Kane D, editors. Chronic wound care: a clinical source book for healthcare professionals, 2nd edn. Wayne, PA: Health Management Publications, Inc., 1997:97–108. [Google Scholar]
- 17. Sen CK, Khanna S, Babior BM, Hunt TK, Ellison EC, Roy S. Oxidant‐induced vascular endothelial growth factor expression in human keratinocytes and cutaneous wound healing. J Biol Chem 2002;277:33284–90. [DOI] [PubMed] [Google Scholar]
- 18. Tanaka H, Hirakata Y, Kaku M, Yoshida R, Takemura H, Mizukane R, Ishida K, Tomono K, Koga H, Kohno S, Kamihira S. Antimicrobial activity of superoxidized water. J Hosp Infect 1996;34:43–9. [DOI] [PubMed] [Google Scholar]
- 19. Sekiya S, Ohmori K, Harii K. Treatment of infectious skin defects or ulcers with electrolyzed strong acid aqueous solution. Artif Organs 1997;21:32–8. [DOI] [PubMed] [Google Scholar]
- 20. Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two‐step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–9. [DOI] [PubMed] [Google Scholar]
- 21. Birke JA, Rolfsen RJ. Evaluation of a self‐administered sensory testing tool to identify patients at risk of diabetes‐related foot problems. Diabetes Care 1998;21:23–5. [DOI] [PubMed] [Google Scholar]
- 22. Lobmann R, Ambrosch A, Seewald M, Dietlein H, Zink K, Kullman KH. Antibiotic therapy for diabetic foot infections: comparison of cephalosporines with chinolones. Diabetes Nutr Metab 2004;17:156–62. [PubMed] [Google Scholar]
- 23. Lipsky BA, Itani K, Norden C. Treating foot infections in diabetic patients: a randomized, multicenter, open‐label trial of linezolid versus ampicillin‐sulbactam/amoxicillin‐clavulanate. Clin Infect Dis 2004;38:17–24. [DOI] [PubMed] [Google Scholar]
- 24. Senneville E. Antimicrobial interventions for the management of diabetic foot infections. Expert Opin Pharmacother 2005;6:263–73. [DOI] [PubMed] [Google Scholar]
- 25. Naguib G, Al‐Mashat H, Desta T, Graves DT. Diabetes prolongs the inflammatory response to a bacterial stimulus through cytokine dysregulation. J Invest Dermatol 2004;123:87–92. [DOI] [PubMed] [Google Scholar]
- 26. Yu Y, Liang J. [Study on phagocytosis of human neutrophils in diabetics]. Hua Xi Yi Ke Da Xue Xue Bao 1994;25:134–7. [PubMed] [Google Scholar]
- 27. Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med 1997;14:29–34. [DOI] [PubMed] [Google Scholar]
- 28. Patino R, Ibarra J, Rodriguez A, Ruiz‐Yagüe M, Pintor E, Fernandez‐Cruz A, Figueredo A. Circulating monocytes in patients with diabetes mellitus, arterial disease, and increased CD14 expression. Am J Cardiol 2000;85:1288–91. [DOI] [PubMed] [Google Scholar]
- 29. Wierusz‐Wysocka B, Wykretowicz A, Byks H, Sadurska K, Wysocki H. Polymorphonuclear neutrophils adherence, superoxide anion (O2‐) production and HBA1 level in diabetic patients. Diabetes Res Clin Pract 1993;21:109–14. [DOI] [PubMed] [Google Scholar]
- 30. Savagnone E. Multicenter study of the risk of infection in diabetics. I. Correlation between altered glucose metabolism and cutaneous reactivity to the Multitest IMC. Minerva Endocrinol 1991;16:119–25. [PubMed] [Google Scholar]
- 31. Lipsky BA. Medical treatment of diabetic foot infections. Clin Infect Dis 2004;39 Suppl 2:S104–S14. [DOI] [PubMed] [Google Scholar]
- 32. Thomson PD. Immunology, microbiology, and the recalcitrant wound. Ostomy Wound Manage 2000;46(1A Suppl):77S–82S; quiz 83S–84S. [PubMed] [Google Scholar]
- 33. Bokoch GM. Microbial killing: hold the bleach and pass the salt! Nat Immunol 2002;3:340–2. [DOI] [PubMed] [Google Scholar]
- 34. Yahagi N, Kono M, Kitahara M, Ohmura AO, Sumita O, Sashimoto T, Hori K. Effect of electrolyzed water on wound healing. Artif Organs 2000;24:984–7. [DOI] [PubMed] [Google Scholar]
- 35. Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med 1988;318:1728–33. [DOI] [PubMed] [Google Scholar]
- 36. Bowler PG. Wound pathophysiology, infection and therapeutic options. Ann Med 2002;34:419–27. [DOI] [PubMed] [Google Scholar]
- 37. Jirkovska A. Basic questions in therapy of the diabetic foot. Vnitr Lek 2002;48:542–8. [PubMed] [Google Scholar]


