Abstract
The importance of temperature in the wound‐healing process is rapidly being recognised as a novel way in which to manipulate the wound‐healing environment. In this study, we aimed to investigate the direct effect of topical radiant heating (TRH), using a novel bandaging system (Warm‐Up™, Arizant Healthcare Inc., Eden Prairie MN, USA; Augustine Medical, USA), on wound healing at a physiological and cellular level. Experimental bandages were positioned over split‐thickness skin graft donor site wounds of 12 patients undergoing graft harvesting from the anterior thigh. The experimental group (n = 6) underwent intermittent heating for 5 hours (three 1‐hour heating cycles at 38°C, separated by two 1‐hour rest periods), whilst the control group (n = 6) received no radiant heating. Physiological blood‐flow recordings both in the control group and the topical radiant heat cohort were undertaken using Laser Doppler Imaging (LDI). Skin biopsies were obtained at identical time points, and immunohistochemical analysis was undertaken using antibodies against neutrophils (NP57), lymphocytes (CD3) and macrophages (CD68). We found that TRH significantly increased local dermal blood flow (P < 0·001) by up to 100% in both injured and intact skin. Furthermore, this increase in flow was associated with a significant (P < 0·05) increase in CD3 immunoreactivity on day 1 postoperatively. This study demonstrates that TRH increases local blood flow and lymphocyte (CD3) extravasation, and we postulate that these changes may enhance local innate immunity within the healing wound environment.
Keywords: TRH, Wound, Skin graft, LDI, Lymphocyte
Introduction
Although the success of wound healing is dependent on many variables, it is now recognised that the role of temperature may be important in the process of wound healing. Clinical studies have shown that peri‐operative hypothermia may be detrimental to successful wound healing (1) because of vasoconstriction and impaired immunity. Although the precise mechanism of this phenomenon is still unclear, two factors have been implicated. Primarily, at a purely chemical level, the effect of heat on accelerating chemical reactions is well known as Van't Hoff's Law and it has been shown, in vitro, that hyperthermia increases polymorph chemotaxis and phagocytosis, as well as fibroblast proliferation (2). Secondly, at a more physiological level, it has been shown that heating of acute wounds may increase local, dermal blood flow (3) and consequently subcutaneous oxygen tensions resulting in increased oxygen‐dependent nitroso free radical‐mediated microbial killing. Hypothermia resulting in decreased oxygen delivery to areas of wound healing has been shown to predispose to wound infections in animals (4, 5).
In addition to impaired local immunity, it is also thought that wound hypoxia during the process of healing may compromise wound structure and integrity leading to poor wound strength, independent of wound infection. Tensile strength in wounds is dependent on collagen cross‐linkage, a process catalysed by enzymes that are oxygen‐dependent 6, 7, 8) and so are also critically dependent on local blood flow.
In this study, we aimed to investigate the direct effect of topical radiant heating (TRH) on wound healing at three different levels: clinical, physiological and cellular. The heating of wounds was achieved with the use of a novel bandage that incorporates a thermostatically controlled, radiant heater (Warm‐Up©, Arizant Healthcare Inc., Eden Prairie MN USA; Augustine Medical, USA). Physiological blood‐flow recordings both in the control group and the local radiant heat cohort were undertaken using Laser Doppler Imaging (LDI). In addition, we studied the effect of TRH on inflammatory cell extravasation as evidenced by histological analysis of wound biopsy specimens.
Patients and experiments
Wound dressings
Topical radiant heat was applied to the skin of the ventral thigh using a novel therapeutic dressing (Warm Up™; Augustine Medical) (Figure 1) in both intact and injured skin models. This non contact dressing holds a warming card that delivers radiant heat at 38°C. The warming card is controlled by a temperature control unit, which can be operated from either an alternating current outlet or with a rechargeable battery pack. The dressing creates a humid environment, which is also known to increase the rate of reepithelialisation and collagen synthesis (9, 10). The dressing was applied on to the donor site wound in theatre. For the purposes of gaining access to the area being warmed, a window was made in the dressing enabling scanning of the area without removal the dressing. Between scans, the window was re‐sealed. The dressing was replaced every 1–2 days. Dressings were removed on discharge and replaced by bactigras dressings.
Figure 1.

The topical radiant heating (TRH) dressing.
Laser Doppler Imaging and dermal microvascular blood flow studies
Laser Doppler Imaging (LDI) was used to scan the ventral thigh before, during and after the application of TRH. The scan area was fixed by the boundaries of the TRH dressing and scanning was carried out with a fixed distance of 72 cm between LDI probe and skin by a single operator.
Study 1 – uninjured skin
To assess the effect of TRH on uninjured skin, dressings were applied to both thighs of healthy volunteers (n = 20). According to an established protocol, the right thigh was heated for 3 hours in total, in an intermittent fashion (three 1‐hour cycles of heating interspersed with 2 hours of no heating). The non heated left thigh was used as a control. Blood flow in both thighs was measured before the commencement of TRH and at hourly intervals thereafter. Biopsies were not harvested from this study group and Aylesbury Vale Local Research Ethics Committee gave approval for the two studies.
Study 2 – injured skin
To assess the effect of TRH on acute wounds, an experimental protocol was constructed such that patients who were already undergoing partial thickness skin grafting for a skin defect were invited to take part in the study. Written consent was obtained from all those who volunteered, and prior approval from the Aylesbury Vale Local Research Ethics Committee had already been obtained. In total, 12 patients were recruited into the study, six of whom were allocated to the experimental arm of the trial whilst the remainder acted as negative controls. All grafts were of medium thickness and harvested by the same surgeon from the right or left ventral thigh. The dressings were applied to the donor site wounds in theatre (n = 6), and heating was commenced within 3 hours into the postoperative period. The heating regimen used was identical to that used in study 1. In the control cohort (n = 6), identical wound dressings were applied with the omission of TRH card. Blood flow measurements were carried out at those time points already described in study 1.
Immunocytochemistry
Full‐thickness skin biopsies of the donor site were harvested in the immediate postoperative period, before the dressing was applied and at 24 and 48 hours after the dressing had been in place. Tissue biopsies were obtained under general anaesthetic, if indicated for surgery, or under local anaesthetic using 2% lignocaine with adrenaline 1 : 200 000. Full‐thickness biopsies were harvested using a sharp punch (4 mm diameter; Stiefel Laboratories Ltd, Sligo, Ireland), and primary closure was achieved with interrupted Prolene 4/0 (Ethicon, Edinburgh, UK) sutures. Specimens were immediately placed in formalin for preservation. Sections of the specimen embedded in paraffin were cut at 4‐µm thickness using a Leitz (Rankin Biomed, Clarkson MI, USA) microtome and mounted on to vectabond‐coated glass slides. An immunocytochemical staining protocol employing the Streptavidin–Biotin (Dakocytomation Ltd, Ely, UK) system was used for the identification of leucocyte cell markers. Antibodies to neutrophils (NP57), T‐lymphocytes (CD3) and monocytes and macrophages (CD68) were used to identify cells, all obtained from Dako, Bucks, UK.
The levels of NP57, CD68 and CD3 immunoreactivity was quantified in sections by manual cell counts by a single observer examining 40 random high‐power fields (×40 objective) per biopsy. Control tissue was obtained of donor site wounds dressed with opsite dressing. Samples were matched for age, gender and wound depth.
Measurements and statistical analysis
Dermal microvascular blood flow was expressed as mean perfusion units as described by other authors (11). Further analysis was performed and data was expressed as percentage change of blood flow from baseline. Statistical analysis was performed on LDI values using the Student's t‐test for unpaired samples to compare mean values and the paired t‐test for comparing changes within groups. P < 0·05 was considered significant.
Results
Study 1 – normal skin
The application of intermittent TRH on to intact skin resulted in significant increases in dermal microvascular blood flow (Table 1 and Figure 3a). During the first two heating cycles, blood flow as measured by LDI increased by 83% (P < 0·001) and 103% (P < 0·001), respectively.
Table 1.
Effect of TRH on local blood flow in uninjured skin
| Blood flow/mean perfusion units | ||||
|---|---|---|---|---|
| Time/hour | Controls | Experimental | % change | P‐value |
| 0 | 84·3 (73·6–94·8) | 85·5 (71·7–99·3) | 1·5 | |
| 1 | 97·4 (84·3–110·5) | 178·0 (156·0–200·0) | 83·0 | <0·001 |
| 2 | 96·9 (81·5–112·3) | 109·3 (98·2–120·3) | 12·7 | |
| 3 | 92·7 (78·5–107·0) | 187·9 (157·7–219·1) | 103 | <0·001 |
| 4 | 102·0 (86·5–117·5) | 111·9 (92·0–131·5) | 9·6 | |
| 5 | 102·3 (83·9–120·8) | 161·4 (140·4–182·4) | 57·8 | <0·001 |
| 6 | 85·9 (74·4–97·4) | 87·7 (77·5–97·9) | 2·1 | |
Values in brackets indicate 95% confidence intervals.
Figure 3.
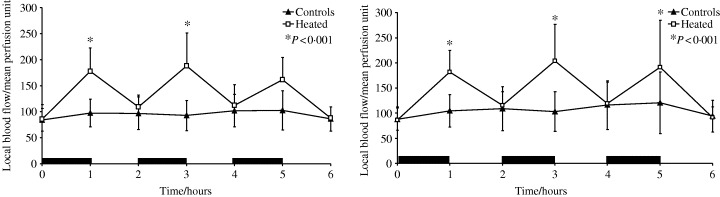
(a) The effect of TRH on blood flow in uninjured skin. (b) The effect of TRH on blood flow in injured skin. (Black bars represent periods during which TRH was applied)
Study 2 – injured skin
The application of TRH on to injured skin resulted in significant increases in dermal blood flow (Table 2 and Figure 3b). Once again blood flow was seen to increase by 97% (P < 0·001).
Table 2.
Effect of TRH on local blood flow in injured skin
| Blood flow/mean perfusion units | ||||
|---|---|---|---|---|
| Time/hour | Controls | Experimental | % change | P‐value |
| 0 | 87·9 (69·9–105·9) | 86·1 (65·2–107·0) | −2·09 | |
| 1 | 104·4 (79·0–129·9) | 182·0 (147·5–216·4) | 74·3 | <0·001 |
| 2 | 108·5 (73·5–143·5) | 115·3 (93·7–136·9) | 6·30 | |
| 3 | 102·8 (71·4–134·2) | 203·3 (144·7–261·9) | 97·8 | <0·001 |
| 4 | 115·7 (76·8–154·6) | 118·9 (84·9–152·8) | 2·77 | |
| 5 | 120·2 (71·1–169·2) | 191·2 (116·6–266·0) | 59·2 | <0·001 |
| 6 | 94·0 (68·7–119·4) | 91·2 (73·9–108·4) | −3·07 | |
Values in brackets indicate 95% confidence intervals.
Comparison of blood flow in unheated intact skin and unheated injured skin showed no significant difference (P > 0·05; Figure 4a). Comparison of blood flow in intact and injured skin following TRH showed no significant difference (P > 0·05; Figure 4b).
Figure 4.
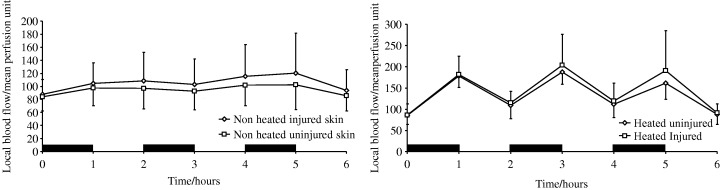
(a) The effect of dermal injury on local blood flow in unheated skin. (b) The effect of dermal injury on local blood flow in heated skin. (Black bars represent periods during which TRH was applied)
Immunohistochemical analysis
CD68 and NP57
Cell counts for CD68 and NP57 showed no temporal variation in immunoreactivity and no difference between heated and non heated cohorts postoperatively.
CD3
Analysis of T‐lymphocyte activity in both heated and unheated samples revealed an increase of CD3‐positive immunoreactivity (2, 5) in samples that had undergone heating (P < 0·05), as compared with controls, on postoperative day 1. This relationship was not found to persist after postoperative day 1.
Figure 2.
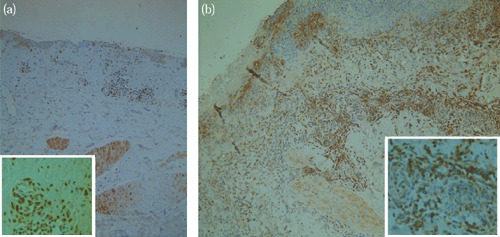
(a) CD3 Immunoreactivity prior to the application of TRH (x100 and x400 (inset)). (b) CD3 immunoreactivity after the application of three 1‐hour cycles of TRH (x100 and x400 (inset)).
Figure 5.
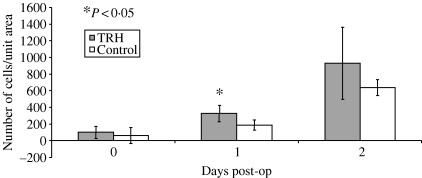
Temporal changes in CD3 immunoreactivity in skin biopsies from split‐thickness skin graft donor sites.
Discussion
Wound healing relies on the presence of a local wound milieu that can support and facilitate tissue regeneration. Whilst some factors involved in wound healing, such as infection, are well recognised (12) to be detrimental to the quality of healing, others, such as temperature, have been late in being acknowledged as being influential in the process at all. It is only relatively recently that the role of temperature has been investigated. There is now a growing body of evidence to suggest that temperature could significantly affect wound and patient outcomes (13). Peri‐operative hypothermia has been shown to be associated with increased rates of wound infections, blood transfusion and prolonged hospital inpatient stay (1). Studies (1, 18) that have investigated the effect of peri‐operative normothermia have shown that those patients who are actively warmed during surgery are less at risk of developing postoperative infections and are discharged earlier. Similarly, in urological patients undergoing surgery such as transurethral resection of prostate, it has been shown that peri‐operative hypothermia is associated with adverse haemodynamic consequences postoperatively (14). Such evidence regarding the role of temperature on postoperative outcomes has been the impetus behind the now routine clinical use of extra‐corporeal warming during surgery, for example, the Bair© (Arizant Healthcare Inc., Eden Prairie MN, USA) Hugger heated blanket and warmed solutions during transurethral prostatic surgery (15, 16).
In comparison, the majority of studies on local wound warming have focused primarily on physiological parameters, such as blood flow, local oxygen tensions and immunohistochemical analysis of wound collagen deposition. It is known that TRH of wounds is associated with an increase in estimated local blood flow (as measured by Fick's principle) and local tissue oxygen tensions (3). Although it has been shown that increased local oxygen tensions are associated with increased collagen deposition (7, 8), no direct relationship between TRH and collagen deposition has been proven (17). In clinical studies comparing postoperative outcomes in normothermic and hypothermic patients, those patients receiving either systemic or local wound heating were at less risk of developing postoperative wound infections (18). Analysis of outcomes between the two heating regimens (that is, local or systemic) showed no overall benefit of one paradigm over the other; however, it was suggested that local heating was associated with a lower incidence of wound infection, although this difference was not found to reach statistical significance.
Our study differed from previous studies on the effect of TRH on wound healing because dermal microvascular blood flow was measured directly using the Laser Doppler Imager (LDI, Moor Instruments Ltd, UK; helium‐neon laser, wavelength 632.8 nm). Laser Doppler technology is based on the phase shift of reflected light as it encounters moving particles (mainly erythrocytes) and non moving components of a limited tissue volume. After signal‐processing data are stored and a perfusion image is generated using a laptop (Dell Latitude Cpi D266XT). The perfusion image is colour‐coded in a scale ranging from dark blue to red, reflecting increasing blood flow. The use of LDI in skin blood flow measurement was first reported in burns patient (19) and since then its validity as a research tool has been well documented and investigated (20). The use of LDI in the measurement of blood flow in normal and pathological skin follows on from the use of Laser Doppler Flowmetry (LDF). However unlike LDF, laser doppler imaging represents a non contact technology that is able to rapidly measure perfusion within a scan area as opposed to a single scan point (20).
In this study, we aimed to show that TRH of wounds is associated with an increase in local blood flow, as shown by LDI. We have demonstrated that TRH of the skin increase dermal microvascular blood flow in both normal and injured skin. Our results also show that this increase in local flow is not simply an artefact of skin injury (Figure 4a) nor is the response to heating augmented in injured skin (Figure 4b). Furthermore, we have also shown that this response is associated with a transient increase in lymphocyte CD3 antigen immunoreactivity (Figure 5).
There is little known about the effect of heat on blood flow in acute wounds. Methods for the study of blood flow generally employ the measurement of either the physical movement of blood as in LDF or the oxygen content which is correlated with tissue perfusion. Laser Doppler technology has been well described for the measurement of dermal blood flow. The development of LDI has enabled rapid non contact measurement of dermal blood flow over larger anatomical areas 21, 22, 23, 24). It has already proven to be of diagnostic value in the determination of the depth of burns (25, 26). In a recent study, Ikeda et al. estimated the response of blood flow to heating by measuring the subcutaneous oxygen tension as a correlate of tissue perfusion. They reported an increase in subcutaneous oxygen tensions of approximately 50% when wounds were heated at a temperature of 38°C over 2 hours. This study reports a similar increase in blood flow using LDI. However, whereas subcutaneous oxygen tension remained elevated for up to 3 hours, blood flow subsided to levels just higher than baseline within an hour after the cessation of TRH.
At a cellular level, the role of TRH on immune cell activation and extravasation has not previously been described. We found that wounds receiving TRH displayed a 195% increase in CD3‐positive lymphocytes after 24 hours compared to non heated wounds. Although a formal explanation of the mechanism behind this phenomenon is beyond the scope of this study, these findings support previous studies that suggest that the presence of pyrexia increases the ability of organisms to mount an immune response (27). In particular, it is known that the presence of fever is associated with an increase in interleukin secretion and production of nitric oxide and reactive oxygen intermediates, the biochemical tools of the cell‐mediated immune response. Furthermore, the presence of higher temperatures is associated with increased expression of heat shock proteins in lymphocytes only, which are immuno‐protective against the stressful conditions of the immune repsonse itself (28). The authors of these two studies argue for an augmentative effect of temperature on innate immunity, and on the basis of the findings presented in this article, we propose that TRH has an augmentative and catalytic effect on local innate immunity.
Previous histological studies (29) investigating the role of immune cell traffic in the skin of burns victims has shown that deep dermal burns, that is those associated with destruction of the superficial venous plexus, are associated with a predominantly neutrophil‐rich immune cell infiltrate. In comparison, more superficial burns, where the superficial venous plexus is left intact, is associated with a lymphocyte‐predominant infiltrate. The study also found that progressive neutrophil‐mediated injury, as a result of chaotic nitroso‐free radical and cytokine release, was only seen in deeper burns where there was no lymphocytic infiltrate, implying that the presence of lymphocytes in the acute inflammatory environment may exert a regulating influence over neutrophil activity. Our findings show the presence of an early lymphocytic infiltrate in acute wounds receving TRH, and we postulate that this may result in a better prognosis for wound healing.
In summary, we have demonstrated that TRH at 38°C increases dermal microvascular blood flow in normal skin and injured skin. These increases in flow are associated with preferential lymphocytic infiltrate into the local environment. However, further work is needed to characterise precisely the role of temperature on immune cell activation and function in such situations.
Further research is also required into the clinical efficacy of TRH in preventing and dealing with already established wound infections. Nonetheless topical radiant heating of wounds represents a novel method of manipulating the local environment of the healing wound.
Presented at the European Tissue Repair Society, Annual Meeting, Amsterdam, September 2003.
References
- 1. Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical‐wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group [see comments]. N Engl J Med 1996;334: 1209–15.DOI: 10.1056/NEJM199605093341901 [DOI] [PubMed] [Google Scholar]
- 2. Xia Z, Sato A, Hughes MA, Cherry GW. Stimulation of fibroblast growth in vitro by intermittent radiant warming. Wound Repair Regen 2000;8: 138–44.DOI: 10.1046/j.1524-475x.2000.00138.x [DOI] [PubMed] [Google Scholar]
- 3. Rabkin JM, Hunt TK. Local heat increases blood flow and oxygen tension in wounds. Arch Surg 1987;122: 221–5. [DOI] [PubMed] [Google Scholar]
- 4. Sheffield CW, Sessler DI, Hunt TK. Mild hypothermia during isoflurane anesthesia decreases resistance to E. coli dermal infection in guinea pigs [see comments]. Acta Anaesthesiol Scand 1994;38: 201–5. [DOI] [PubMed] [Google Scholar]
- 5. Sheffield CW, Sessler DI, Hopf HW et al. Centrally and locally mediated thermoregulatory responses after subcutaneous oxygen tension. Wound Repair Regen 1996;4: 339–45.DOI: 10.1046/j.1524-475X.1996.40310.x [DOI] [PubMed] [Google Scholar]
- 6. De Jong L, Kemp A. Stoichiometry and kinetics of the prolyl 4‐hydroxylase partial reaction. Biochim Biophys Acta 1984;787: 105–11. [DOI] [PubMed] [Google Scholar]
- 7. Jonsson K, Jensen JA, Goodson WH, West JM, Hunt TK. Assessment of perfusion in postoperative patients using tissue oxygen measurements. Br J Surg 1987;74: 263–7. [DOI] [PubMed] [Google Scholar]
- 8. Jonsson K, Jensen JA, Goodson WH et al. Tissue oxygenation, anemia, and perfusion in relation to wound healing in surgical patients. Ann Surg 1991;214: 605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alvarez OM, Mertz PM, Eaglstein WH. The effect of occlusive dressings on collagen synthesis and re‐epithelialization in superficial wounds. J Surg Res 1983;35: 142–8.DOI: 10.1016/0022-4804(83)90136-1 [DOI] [PubMed] [Google Scholar]
- 10. Winters GD. Formation of scab and the rate of epithelialization of superficial wounds in the skin of the young domestic pig. Nature 1962;193: 293. [DOI] [PubMed] [Google Scholar]
- 11. Bornmyr S, Svensson H, Lilja B, Sundkvist G. Skin temperature changes and changes in skin blood flow monitored with laser Doppler flowmetry and imaging: a methodological study in normal humans. Clin Physiol 1997;17: 71–81. [DOI] [PubMed] [Google Scholar]
- 12. Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surigical‐site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol 1999;20: 725–30. [DOI] [PubMed] [Google Scholar]
- 13. Harper CM, McNicholas T, Gowrie‐Mohan S. Maintaining perioperative normothermia. BMJ 2003;326: 722–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gravenstein D. Transurethral resectio of the prostate (TURP) syndrome: a review of the pathophysiology and management. Anesth Analg 1997;84: 438–46.DOI: 10.1097/00000539-199702000-00037 [DOI] [PubMed] [Google Scholar]
- 15. Carpenter AA. Hypothermia during transurethral resection of the prostate. Urology 1984;23(2):122–4. [DOI] [PubMed] [Google Scholar]
- 16. Hugh Evans JW, Singer M, Coppinger SWV, Macartney N, Malcolm Walker J, Milroy EJG. Cardiovascular performance and core temperature during transurethral prostatectomy. J Urol 1994; 152: 2025–9. [DOI] [PubMed] [Google Scholar]
- 17. Ikeda T, Tayefeh F, Sessler DI et al. Local radiant heating increases subcutaneous oxygen tension. Am J Surg 1998;175: 33–7.DOI: 10.1016/S0002-9610(97)00237-7 [DOI] [PubMed] [Google Scholar]
- 18. Melling AC, Ali B, Scott EM, Leaper DJ. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet 2001;358: 876–80.DOI: 10.1016/S0140-6736(01)06071-8 [DOI] [PubMed] [Google Scholar]
- 19. Niazi ZB, Essex TJ, Papini R, Scott D, McLean NR, Black MJ. New laser Doppler scanner, a valuable adjunct in burn depth assessment. Burns 1993;19(6):485–9. [DOI] [PubMed] [Google Scholar]
- 20. Pape SA, Skouras CA, Byrne PO. An audit of the use of laser Doppler imaging (LDI) in the assessment of burns of intermediate depth. Burns 2001;27(3):233–9. [DOI] [PubMed] [Google Scholar]
- 21. Essex TJ, Byrne PO. A laser Doppler scanner for imaging blood flow in skin. J Biomed Eng 1991;13: 189–94. [DOI] [PubMed] [Google Scholar]
- 22. Wårdell K, Braverman IM, Silverman DG, Nilsson GE. Spatial heterogeneity in normal skin perfusion recorded with laser Doppler imaging and flowmetry. Microvasc Res 1994;48: 26–38.DOI: 10.1006/mvre.1994.1036 [DOI] [PubMed] [Google Scholar]
- 23. Wårdell K, Jakobsson A, Nilsson GE. Laser Doppler perfusion imaging by dynamic light scattering. IEEE Trans Biomed Eng 1993;40: 309–16.DOI: 10.1109/10.222322 [DOI] [PubMed] [Google Scholar]
- 24. Svedman P, Svedman C, Njalsson T. Epithelialization and blood flow in suction blister wounds on healthy volunteers. J Invest Surg 1991; 4(2):175–89. [DOI] [PubMed] [Google Scholar]
- 25. Park DH, Hwang JW, Jang KS, Han DG, Ahn KY, Baik BS. Use of laser Doppler flowmetry for estimation of the depth of burns. Plast Reconstr Surg 1998;101: 1516–23. [DOI] [PubMed] [Google Scholar]
- 26. Yeong EK, Mann R, Goldberg M, Engrav L, Heimbach D. Improved accuracy of burn wound assessment using laser Doppler. J Trauma 1996; 40: 956–2. [DOI] [PubMed] [Google Scholar]
- 27. Gothard LQ, Ruffner ME, Woodward JG, Ok‐Kyong P‐S, Sarge KD. Lowered temperature set point for activation of the cellular stress response in T‐lymphocytes. J Biochem 2003;278(11):9322–6. [DOI] [PubMed] [Google Scholar]
- 28. Rosenspire JA, Kindzelskii AL, Petty HR. Cutting Edge: fever‐associated temperatures enhance neutrophil responses to lipopolysaccharide: a potential mechanism involving cell metabolism. J Immunol 2002;169: 396–400. [DOI] [PubMed] [Google Scholar]
- 29. Tyler MPH, Watts AMI, Perry ME, Roberts AHN, McGrouther DA. Burn depth and its histological measurement. Burns 2001;27(5):433–8. [DOI] [PubMed] [Google Scholar]


