Abstract
Silver sulfadiazine has been used as a topical burn wound treatment for many years. Pain associated with dressing changes is a common problem in burn wounds. Aquacel Ag, a hydrofiber dressing coated with ionic silver has been reported to reduce burn wound infection and promote antimicrobial activity. The purpose of this study was to show the benefits of Aquacel Ag for the treatment of partial thickness burns. This prospective randomized study was conducted in 70 patients who had partial thickness burns less than 15% of total body surface area and were treated at Siriraj outpatient burn clinic during December 2006–February 2008. Patients were divided into two groups: Aquacel Ag‐treated group with dressing changes every 3 days (35 patients) and 1% silver sulfadiazine‐treated group, with daily dressing changes (35 patients). There was no difference in demographic data including age, gender, burn percentage between groups. Time‐to‐wound healing pain score during dressing change and cost of treatment were compared between both groups. Time‐to‐wound closure was significantly shorter in the Aquacel Ag‐treated group (10 ± 3 versus 13.7 ± 4 days, P < 0·02) as well as pain scores at days 1, 3 and 7 (4·1 ± 2·1, 2·1 ± 1·8, 0·9 ± 1·4 versus 6·1 ± 2·3, 5·2 ± 2·1, 3·3 ± 1·9, respectively, P < 0·02). Total cost of treatment was 52 ± 29 US dollars for the Aquacel Ag‐treated group versus 93 ± 36 US dollars for the silver sulfadiazine‐treated group. This study showed that Aquacel Ag increased time to healing, decreased pain symptoms and increased patient convenience because of limiting the frequency of replacement of the dressing at lower total cost. This study confirms the efficacy of Aquacel Ag for the treatment of partial thickness burns at an outpatient clinic.
Keywords: Burns, Dressing, Hydrofiber, Silver sulfadiazine
INTRODUCTION
Superficial second‐degree burns are common injuries in the outpatient department (1). The treatment algorithm for these burns remains controversial, whereas deep second‐degree burns and third‐degree burns are best managed with early excision and grafting (2). Silver sulfadiazine has been used as a topical burn treatment for several decades (2). However, disadvantages of silver sulfadiazine include the need for frequent dressing changes at least once daily, pain during dressing change, local maceration, cytotoxic to keratinocyte as well as fibroblast and bacterial resistance (2). Recent advances in local wound care products have led to the development of moisture‐retentive dressings that have the advantages of reducing pain symptoms, reducing the frequency of dressing changes, and improving exudate management (3).
Aquacel® Ag dressing (ConvaTec) is a Hydrofiber® dressing that consists of sodium carboxmethyl cellulose, which forms a gel on contact of body fluid, and 1·2% w/w ionic silver, which contains broad spectrum antimicrobial properties against vancomycin‐resistant enterococci (VRE) and methicillin‐resistant Staphylococcus aureus (MRSA) for up to 14 days (4). In a previous study, Aquacel® Ag was reported to produce a greater rate of re‐epithelialization, reduced pain, and nursing time 1, 4.
The purpose of this study was to compare the efficacy of 1% silver sulfadiazine versus Aquacel® Ag dressing in the treatment of superficial second‐degree burns. Endpoints included time to healing, pain during dressing changes, and cost‐effectiveness.
MATERIALS AND METHODS
Patients with burn injuries at the Trauma Division of Siriraj Hospital were considered eligible for the study entry if they had a burn injury within 24 hours of enrollment, had a superficial second‐degree burn and the burn was <15% of the total body surface area (TBSA). Main exclusion criteria included concomitant trauma, chemical or electrical burns, inhalation injuries, facial burns, underlying diseases that interfered with wound healing (poor‐controlled diabetes mellitus, cancer, COPD, ESRD, post‐radiation, on immunosuppressive drugs, immunocompromised disease) and unavailability for regular visits, recent systemic antibiotic use in a week, pregnancy, and history of wound dressing allergy.
This prospective, randomized study included 70 patients treated at Siriraj Hospital outpatient burn clinic during December 2006–February 2008. This study was approved by the Research Ethics Board of our institution. All patients provided informed written consent to participate in the study. Patients were randomized by computer and assigned into two groups according to the burn wound treatment: Aquacel® Ag‐treated group (35 patients) and 1% silver sulfadiazine‐treated group (35 patients). Demographic data were collected for both groups including age, gender, type of burn, TBSA and transportation cost. Efficacy data collected included day of wound closure, pain scores at each dressing change, hospital charges, patient's transportation cost, time of dressing change and burn wound infection.
Initial evaluation of burns was determined by the burn specialists. Burn wound cleansing was performed with normal saline and any blisters were removed. Clinical judgment was used to determine depth of injuries. TBSA was calculated with the Lund and Browder chart and adjusted with age.
In the Aquacel® Ag‐treated group, Aquacel® Ag was applied with 1 cm overlap to allow shrinkage, according to the manufacturer's direction, and covered with one layer of plain gauze. Dressings were evaluated in the burn clinic of Siriraj Hospital on post‐burn day 1 and then every 3 days until the wound healed. At each evaluation after the dressing was removed, the burn wound was inspected for wound healing and change in depth and infection. For the silver sulfadiazine group, the treatment was daily application and removal of 1% silver sulfadiazine (AgSD) and drying gauze dressing. Burn wounds were also observed daily by the experienced burn surgeon. After each burn dressing change in both groups, the performance characteristic photograph and questionnaire were recorded.
The primary endpoint of this study was time‐to‐wound healing, defined as spelling of the wound. Secondary endpoints included pain assessment by patients' pain scores during wound dressing. The pain scores were assessed and reported by patients with visual analogue pain score 1–10 if patient's age was over 6 years, and the face pain rating scale in small children (2). Total dressing cost was divided into hospital charges including hospital fee, dressing cost and pain medication and transportation cost that the patient had to pay for each hospital visit.
Statistic analysis
All statistical data analyses were performed with the use of SPSS version 15. Pain score, time to healing, hospital charges and total dressing costs were analysed by two‐tailed unpaired student T‐test while differences of gender and type of burn were compared with chi‐square test. Difference in time to healing was calculated with 95% confidence interval. The survival curves for each treatment group were determined using Kaplan‐Meier.
RESULTS
Seventy patients were enrolled in the study and randomly assigned into two groups, Aquacel® Ag and silver sulfadiazine group. The demographic data for both group are reported in Table 1. No significant differences were found between groups.
Table 1.
Demographic data of both groups
| Aquacel®Ag | AgSD | |
|---|---|---|
| %burn | 2.8 ± 2.4 | 2.7 ± 2.4 |
| Type of burn (Flame/Scalded) | 8 flame, 27 scalded | 7 flame, 28 scalded |
| Gender (Male/female) | 15(42.9%) | 17(48.6%) |
| Age | 34.9 ± 26.7 | 42.3 ± 23.5 |
| Transportation Cost/time(US$) | 2.4 ± 0.8 | 2.6 ± 1 |
Wound healing
The primary objective of the study was to evaluate healing time between the two dressing methods. Time‐to‐wound closure was significantly shorter in the Aquacel® Ag‐treated group versus the silver sulfadine‐treated group (10 ± 3 versus 13·7 ± 4·3 days, P < 0·02; Figure 1). Accumulative survival of wound healing is shown in Figure 2. The difference in wound healing time of both groups was 3·7 days respectively (95% confident interval 1·9–5·4 days) Number of hospital visits for dressing change was obviously lower in the Aquacel® Ag‐treated group (3·5 ± 1 versus 13·7 ± 4, P < 0·001).
Figure 1.
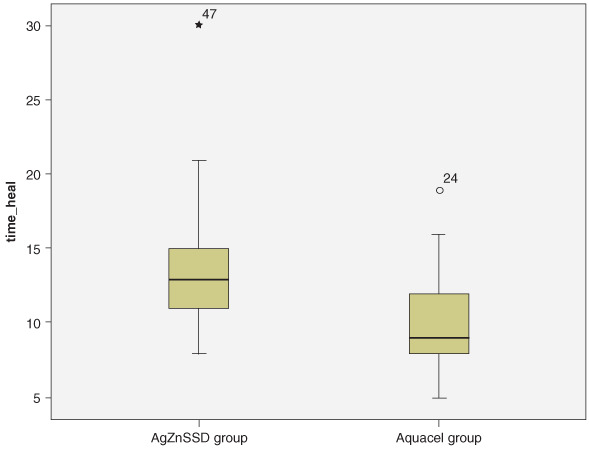
Time of wound healing.
Figure 2.
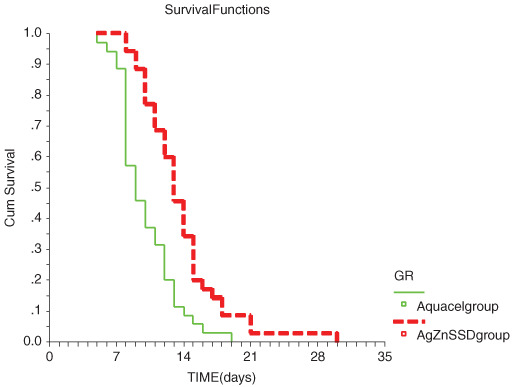
Cumulative wound healing of both the treated groups.
Pain during dressing changes
Average pain scores in the Aquacel® Ag‐treated group were significantly lower than the silver sulfadiazine‐treated group on days 1, 3 and 7 (4·1 ± 2·1, 2·1 ± 1·8, 0·9 ± 1·4 versus 6·1 ± 2·3, 5·2 ± 2·1, 3·3 ± 1·9 and, P < 0·02) (Figure 3). There were no complaints of pain with either dressing method.
Figure 3.
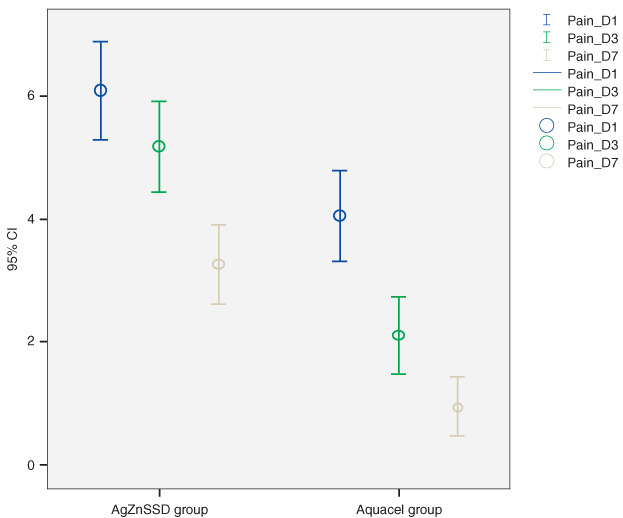
Average pain scores in Aquacel® Ag and silver sulfadiazine‐treated group.
Total cost of treatment
The Aquacel® Ag‐treated group was more cost‐effective than the silver sulfadiazine‐treated group in both hospital and total treatment cost of burn care. Total costs were 52 ± 29 US$ for the Aquacel® Ag‐treated group versus 93 ± 36 US$ for the silver sulfadiazine‐treated group. (Table 2)
Table 2.
Total cost of treatment between both groups
| Aquacel®Ag | SSD | P value | |
|---|---|---|---|
| Hospital cost(US$) | 43 ± 28 | 57 ± 25 | 0.03 |
| Travel cost(US$) | 9 ± 4 | 36 ± 14 | <0.01 |
| Total cost(US$) | 52 ± 29 | 93 ± 36 | <0.01 |
DISCUSSION
Superficial second‐degree burns are challenging to manage (2). Silver in its numerous forms has been used for over 200 years in the treatment of burn injury (5). Tissue irritation by silver nitrate and inactivation of much of the silver by wound fluid and formation of a pseudo‐eschar for silver sulfadiazine are some limitations of silver products in burn topical treatment (3).
Aquacel® Ag is a moisture‐retention dressing with sustained released of 1·2% w/w silver ion. Ionic silver exhibits antimicrobial activity against a broad range of microorganisms including MRSA,VRE and pseudomonas (6). Silver exerts its antimicrobial effects by the interfering with the respiratory chain at cytochrome level, and microbial electron transport system 6, 7, 8, 9. However, concerns associated with the overuse of silver and consequent emergence of bacterial resistance have been raised since 1998 (10). Despite the sporadic evidence of bacterial resistance to silver ion, there have been very few studies undertaken to document its prevalence (11). Silver ion also has low mammalian cell toxicity and does not induce resistance if used at adequate levels 9, 10.
Very few randomized prospective studies on the use of silver have been published. Large prospective, randomized trials of the product do not exist. In 2006, Caruso et al. conducted a randomized controlled study with 84 patients for large burn wound size (5–40% TBSA). In this study, the primary objective was cost‐effectiveness. The study showed that Aquacel® Ag was associated with less pain, anxiety, less nursing time and tended to have lower total treatment cost (12). This study was not designed to show actual wound healing but showed a greater rate of reepithelialization (12).
The current prospective, randomized study was conducted in Thailand to evaluate wound healing, pain and cost‐effectiveness in the outpatient clinic. The percent of total body surface area was approximately 3% TBSA. Aquacel® Ag dressing was associated with rapid wound healing. Pain is also an important clinical consideration in the management of burns. Aquacel® Ag dressing was also associated with less pain symptoms in various ages and, fewer dressing changes. A potential limitation in the economic analysis was the focus on direct medical resources and transportation costs without consideration of non medical or additional costs such as daily work, cost of surgical complications and psychic trauma. Cost‐effectiveness in the outpatient clinic was shown with Aquacel® Ag dressing.
CONCLUSIONS
Our data suggest that Aquacel® Ag is an effective burn dressing in superficial second‐degree burns. Aquacel® Ag promoted time‐to‐wound healing, decreased pain symptoms and increased patient convenience by limiting the frequency of hospital visit for dressing changes and lower total cost. This study confirms the efficacy of Aquacel® Ag for the treatment of partial thickness burns.
ACKNOWLEDGEMENTS
The authors wish to thank Mrs Supaparn Suvanchote and Miss Rachanee Benjathanang for helping to conduct the study and Faculty of Medicine Siriraj Hospital for funding support.
REFERENCES
- 1. Caruso DM, Foster KN, Hermans MH, Rick C. Aquacel® Ag in the management of partial‐thickness burns: results of a clinical trial. J Burn Care Rehabil 2004; 25:89–97. [DOI] [PubMed] [Google Scholar]
- 2. Muangman P, Chuntrasakul C, Silthram S, Suvanchote S, Benjathanung R, Kittidacha S, Rueksomtawin S. Comparison of efficacy of 1% silver sulfadiazine and Acticoat® for treatment of partial thickness burn wounds. J Med Assoc Thai 2006;89:953–8. [PubMed] [Google Scholar]
- 3. Twomey JA, Caruso D, Silverstein P, Antimarino J, Bauer G, Blome‐Eberwein SA, Herndon D, Luterman A. Randomized controlled study of silver dressing effects on partial‐thickness burn outcomes [abstract P58]. Plast Reconstr Surg 2005;116(Suppl 3):194. 15988267 [Google Scholar]
- 4. Barnea Y, Amir A, Leshem D, Zaretski A, Weiss J, Shafir R, Gur E. Clinical comparative study of Aquacel® and paraffin gauze dressing for split‐skin donor site treatment. Ann Plast Surg 2004;53:132–6. [DOI] [PubMed] [Google Scholar]
- 5. Mooney E, Lippitt C, Friedman J. Silver dressings. Plast Reconstr Surg 2006;117:666–9. [DOI] [PubMed] [Google Scholar]
- 6. Jones SA, Bowler PG, Walker M, Parsons D. Controlling wound bioburden with a novel silver‐containing Hydrofiber® dressing. Wound Repair Regen 2004;12:288–94. [DOI] [PubMed] [Google Scholar]
- 7. Bowler PG, Jones SA, Walker M. Microbicidal properties of a silver‐containing hydrofiber® dressing against a variety of burn wound pathogens. J Burn Care Rehabil 2004;25:192–6. [DOI] [PubMed] [Google Scholar]
- 8. Jones SA, Bowler PG, Walker M. Antimicrobial activity of silver‐containing dressing is influenced by dressing conformability with a wound surface. Wounds 2005;17: 263–70. [Google Scholar]
- 9. O’Neill M, Vine G, Beezer A. Antimicrobial properties of silver‐containing wound dressings: a microcalorimetric study. Int J Pharm 2003;263: 61–8. [DOI] [PubMed] [Google Scholar]
- 10. Percival SL, Bowler PG, Russell D. Bacterial resistance to silver in wound care. J Hosp Infect 2005;60:1–7. [DOI] [PubMed] [Google Scholar]
- 11. Jones SA, Bowler PG, Walker M. Antimicrobial activity of silver‐containing dressing is influenced by dressing conformability with a wound surface. Wounds 2005;17:263–70. [Google Scholar]
- 12. Caruso DM, Foster KN, Blome‐Eberwein SA, Twomey JA, Herndon DN, Luterman A, Silverstein P, Antimarino JR, Bauer GJ. Randomized clinical study of hydrofiber dressing with silver or silver sulfadiazine in the management of partial‐thickness burns. J Burn Care Res 2006;27:298–309. [DOI] [PubMed] [Google Scholar]
- 13. Chuntrasakul, Gaerunpong V, Chumsang P. The use of a new topical burn agent silver zinc sulfadiazine cream in the treatment of burn wounds: preliminary report in 236 burned Patients. Siriraj Hosp Gaz 1984;36:377–87. [Google Scholar]


