Abstract
Patients with massive venous stasis ulcers that have very high bacterial burdens represent some of the most difficult wounds to manage. The vacuum‐assisted closure (VAC) device is known to optimise wound bed preparation; however, these patients have too high a bacterial burden for simple VAC application to facilitate this function. We present the application of the VAC with instillation of dilute Dakins solution as a way of bacterial eradication in these patients. Five patients with venous stasis ulcers greater than 200 cm2 that were colonised with greater than 105 bacteria were treated with the VAC instill for 10 days with 12·5% Dakins solution, instilled for 10 minutes every hour. Two patients had multi‐drug‐resistant pseudomonas, three with MRSA. All the five had negative quantitative cultures, prior to split thickness skin graft with 100% take and complete healing at 1 year. Adequate delivery of bactericidal agents to the infected tissue can be very difficult, especially while promoting tissue growth. By providing a single delivery system for a bactericidal agent for a short period of time followed by a growth stimulating therapy, the VAC instill provides a unique combination that appears to maximise wound bed preparation.
Keywords: Negative pressure wound therapy, Split thickness skin graft, Wound sterilisation
INTRODUCTION
Each year, approximately 5 million patients are treated for chronic wounds in the United States. This can cost more than $20 billion a year 1, 2. More than half of these wounds are chronic lower extremity ulcers (3). Infection can worsen both the clinical outcome and the economical burden of managing such wounds. Antibiotic treatment has its own impact on the costs and its associated side effects. The cost burden of unhealed ulcers can add up to $2·5 billion when considering the multiple essential physician visits, nursing care and the frequent hospital admissions for managing associated infections 4, 5.
On average, every day in the hospital costs Medicare approximately $2360 per patient. Therefore, an effective approach in managing wounds that involves less consumption of intravenous and oral antibiotics, lower length of stay and shorter time of healing would be considered clinically superior, and more cost‐effective (6). The vacuum‐assisted closure (VAC) device is known to optimise blood flow, decrease local tissue oedema and facilitate the removal of bacteria from the wound bed. It has been shown to have a better clinical and economical outcome in the management of venous stasis ulcers than conventional unna boot treatment (7).
However, patients with massive venous stasis ulcers that have very high bacterial load represent some of the most difficult wounds to manage and have a very high economical burden. We present the application of the VAC with instillation of dilute Dakin's solution as a way of bacterial eradication in these patients. Our primary objective was to provide a sterile environment for our split thickness skin graft (STSG) beds.
We believe that the VAC instillation device provides a promising therapy for the management of infected chronic venous stasis ulcers. Our primary interest is in how we may be able to deliver growth‐enhancing therapy such as growth factors in conjunction with negative pressure wound therapy, or as in this case where we can use the pro‐growth advantages of the VAC to offset the negative impact of caustic therapies, such as wound cleansing with sodium hypochlorite (Dakin's solution). Since in vitro sodium hypochlorite has demonstrated harmful effects to fibroblasts and growth factor modulation we believe that the clinical pro‐growth stimulation of NPWT, which in vitro has been in part attributed to microstrain, would act as rescue therapy to offset these negative effects (8).
METHODS
A retrospective review of an Institutional Review Board (IRB) approved prospective wound care database was carried out at a tertiary care, University affiliated wound practice. Over 2 years, five patients with venous stasis ulcers greater than 200 square cm that were colonised with greater than 105 bacteria were treated with the VAC instill. Two patients had multi‐drug‐resistant pseudomonas, three with MRSA. All five had coliforms present as well.
All patients were debrided in the operating room (OR) initially with epidural anaesthetic (Figure 1). All patients had the epidural left in place for 48 hours, whereas one had the epidural replaced two additional times and left in place for a total of 10 days. This was done for severe pain. After debridement, all patients had non stick layer placed on their wound (Adaptic, Johnson & Johnson, Somerville, NJ), and this was followed immediately with the black negative pressure wound therapy (NPWT) sponge (VAC, KCI, San Antonio, TX) being placed on the wound with the provided bio‐occlusive dressing. The absolute volume of instillate was determined by the size of the wound. Dressings were changed on Monday, Wednesday and Friday schedule.
Figure 1.
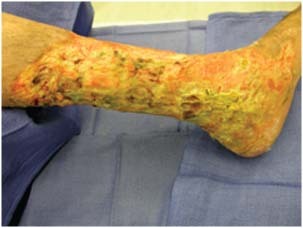
Immediately prior to debridement, heavily infected with pseudomonas.
At 10 days, all the five patients underwent quantitative culture prior to undergoing STSG (Figure 2) which was matured under a VAC for 4 days (Figure 3). Once skin grafted, the patients had immediate placement of a non stick layer (Adaptic, Johnson & Johnson) and immediately covered black foam and attached to the NPWT device (VAC, KCI). After skin grafting, the patients were kept in the hospital on an average of 5 days with their NPWT dressing being removed on postoperative day 4. After discharge, the patients were seen at 1 week, 4 week, 2 month and 3‐month intervals. They were then followed up on a 6‐month basis.
Figure 2.
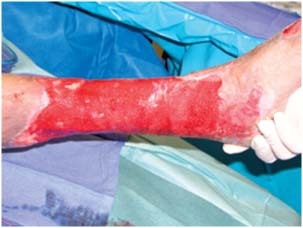
After 10 days of VAC instill and sodium hypochlorite.
Figure 3.

Immediaely after split thickness skin graft.
Upon discharge, all patients were maintained in a multilayer wrap (Profore, Smith and Nephew, Largo, FL) and a non stick sustained release topical silver dressing (Acticoat, Smith and Nephew) which was changed weekly.
RESULTS
The patients were of the age 48–58, with four being male and one female. None of the patients were diabetic, and all had ankle brachial indices greater than 1·0. Two of five were active smokers, 100% were Hepatitis C positive, 80% were Hepatitis B positive and 1/5 was HIV positive. All patients were post‐phlebitic with 80% having their deep venous thrombosis (DVT) etiology secondary to intravenous drug abuse and 1/5 having suffered their DVT secondary to a motor vehicle accident. We had 5/5 patients with complete 3‐year follow‐up.
All patients were treated with 10 days of VAC instill therapy with 12·5% Dakins instilled for 10 minutes every hour. As previously noted, after 10 days of therapy, all five patients appeared to have clean wounds. Quantitative biopsies were taken from two locations in each wound. All 16 samples showed no growth. Once these results were returned, the patients underwent STSG as outlined above. By one month post‐operatively all five patients exhibited 100% closed wounds Figure 4).
Figure 4.
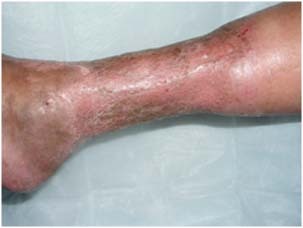
Four weeks after skin grafting.
At 1 and 2 years, all patients remained healed. At 2·5 years, one patient re‐opened secondary to non compliance with graded compression stockings.
DISCUSSION
With the mounting costs of the long‐term care of the lower extremity wound in the United States and the costs and morbidity associated with long‐term antibiotic care, we set out to determine whether a highly aggressive inpatient treatment for massive infected venous stasis ulcers could be used to close these very problematic wounds. We sought to develop an effective approach in managing wounds that involves less consumption of intravenous and oral antibiotics, lower length of stay and shorter time to heal, which would be considered clinically superior, and more cost‐effective in the long run (6).
The VAC device is known to optimise blood flow, decrease local tissue oedema and facilitate the removal of bacteria from the wound bed. It has been shown to have a better clinical and economical outcome in the management of venous stasis ulcers than conventional unna boot treatment (8). Although the device is not FDA approved for the treatment of venous leg ulcers, many of us use it to at least help prepare the wound bed.
By providing a stressful environment for the cells, VAC forms oxygen tension and promote tissue development and healing 9, 10. However, the accumulation of bacterial products and chronic wound oedema restrains healing 11, 12, 13. Therefore, we postulate that by providing a single delivery system for a bactericidal agent for a short period of time followed by a growth‐stimulating therapy, the VAC instill provides a unique combination that appears to maximise wound bed preparation. In addition, we were successfully able to reduce the bacterial counts to maximise STSG take.
Historically, antibiotic wound instillation has been considered an acceptable method to deliver significant amounts of antibiotic into wounds (14). More than two and a half decades ago, Westaby et al. described a set up for wound irrigation used to treat purulent wounds on 100 patients in a multicentre trial. This method was considered as effective as a twice‐daily dressing change (15). In 2002, Vranckx et al. used antibiotics in a fluid‐filled chamber to assist in wound healing (16). In addition, it has been shown in animal models that epithelialisation in moist wounds is superior to wounds exposed to air (17). Therefore, moist wound healing is considered the standard of care (18). Our laboratory has shown that infected wounds that are not appropriately debrided prior to placement of negative pressure wound therapy (NPWT) exhibit increasing bioburdens. (19). Therefore, adequate mechanical debridement, usually sharp, is a mandatory first step.
Our choice to use Dakin's solution was based on the fact that it is readily available, cheap and cost‐effective. While we recognize that Dakin's appears unacceptable to many advanced wound care practioners it remains the most widely cited wound treatment fluid in the United States next to normal saline. Several other solutions described in the literature are considered satisfactory such as benzalkonium chloride and bacitracin. Surfactant has been tested on rat models with orthopaedic implant contamination 19, 21, 22. In addition, Iodine, which has been diluted to a concentration less than 0·9%, is not harmful to proliferating fibroblasts and could be used for irrigation with the VAC. A new approach may be to use the antimicrobial agent polyhexamethylene biguanide (PHMB) as an instillate, this is currently primarily available in a pre‐moistened gauze product, which has shown some promise in small case series (23). Other strategies, which can be considered to help sterilise an adequately debrided wound while enhancing granulation tissue, include using a NPWT device with a silver containing sponge or a nano‐crystalline silver eluting layer under the NPWT contact layer. We were more comfortable with using an antimicrobial agent versus an antibiotic secondary to efficacy and toxicity concerns about large volume of antibiotic instillation.
Adequate delivery of bactericidal agents to the infected tissue can be very difficult, especially while promoting tissue growth. The VAC instill therapy with 12·5% Dakins instilled for 10 minutes every hour in our case series proves to be effective and sufficient to prepare the wound bed for skin grafting. STSG in our patient population was very effective with 100% graft take and complete healing still evident at 1 year. This is in comparison to venous stasis ulcers treated with unna boot followed by STSG described almost 50% recurrence of the ulcer within 3 months of discharge (24).
Although this small non controlled case series only offers one way to close these very difficult wounds, it appears that the VAC instillation device provides an effective therapy for the management of infected chronic venous stasis ulcers. Although we did not assess the absolute costs, it would appear that the high long‐term closure rates in this very difficult to heal patient population would provide a socio‐economic benefit compared with conventional therapeutic modalities. Therefore, while this device is not suitable or necessary for every dirty wound, it provided significant benefit to this select patient population.
REFERENCES
- 1. Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, Wagner EH. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999;22:382–7. [DOI] [PubMed] [Google Scholar]
- 2. Edmonds ME, Blundell MP, Morris ME, Thomas EM, Cotton LT, Watkins PJ. Improved survival of the diabetic foot: the role of a specialized foot clinic. Q J Med 1986;60:763–71. [PubMed] [Google Scholar]
- 3. Bungen V. Evaluating the impact on patients of living with a leg ulcer. NurseTimes 2004;100:30–1. [PubMed] [Google Scholar]
- 4. Walshe C. Living with a venous leg ulcer: a descriptive study of patients' experiences. J Adv Nurs 1995;22:1092–100. [DOI] [PubMed] [Google Scholar]
- 5. Nemeth KA, Harrison MB, Graham ID, Burke S. Understanding venous leg ulcer pain: results of a longitudinal study. Ost Wound Manage 2004;50:34–46. [PubMed] [Google Scholar]
- 6. Wagner A, Reike H, Angelkort B. Highly resistant pathogens in patients with diabetic foot syndrome with special reference to methicillin‐resistant Staphylococcus aureus infections. Dtsch Med Worchenschr 2001;126:1353–6. [DOI] [PubMed] [Google Scholar]
- 7. Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum‐assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114:1086–96. [DOI] [PubMed] [Google Scholar]
- 8. Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State‐of‐the‐art treatment of chronic leg ulcers: A randomized controlled trial comparing vacuum‐assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg 2006;44:1029–37. [DOI] [PubMed] [Google Scholar]
- 9. Sumpio BE, Banes AJ, Levin LG, Johnson G. Mechanical stress stimulates aortic endothelial cells to proliferate. J Vasc Surg 1987;6:252–6. [PubMed] [Google Scholar]
- 10. Ilizarov GA. The tension‐stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft‐tissue preservation. Clin Orthop 1989;238:249–81. [PubMed] [Google Scholar]
- 11. Ovington LG. Bacterial toxins and wound healing. Ost Wound Manage 2003;49(Suppl 7A):8–12. [PubMed] [Google Scholar]
- 12. Ladwig GP, Robson MC, Leu R, Kuhn MA, Muir DF, Schultz GS. Ratios of activated matrix metalloproteinase‐9 to tissue inhibitor of matrix metalloproteinase‐1 in wound fluid are inversely correlated with healing of pressure ulcers. Wound Repair Regen 2002;10:26–37. [DOI] [PubMed] [Google Scholar]
- 13. Kirsner RS, Katz MH, Eaglstein WH, Falanga V. The biology of wound fluid. Wounds 1993;5: 122–8. [Google Scholar]
- 14. Golightly L, Branigan T. Surgical antibiotic irrigations. Hosp Pharm 1989;24:116–9. [PubMed] [Google Scholar]
- 15. Westaby S, McPherson S, Everett W. Treatment of purulent wounds and fistulae with an adhesive wound irrigation device. A multicentre trial of 100 patients. Ann R Coll Surg Engl 1981;63: 353–6. [PMC free article] [PubMed] [Google Scholar]
- 16. Vranckx JJ, Slama J, Preuss S, Perez N, Svensjö T, Visovatti S, Breuing K, Bartlett R, Pribaz J, Weiss D, Eriksson E. Wet wound healing. Plast Reconstr Surg 2002;110:1680–7. [DOI] [PubMed] [Google Scholar]
- 17. Winter GD. Formation of the scab and the rate of epithelialization of superficial wounds in the skin of young domestic pig. Nature 1962;193:293–4. [DOI] [PubMed] [Google Scholar]
- 18. Change H, Wind S, Kerstein MD. Moist wound healing. Dermatol Nurs 1996;8:174–6. [PubMed] [Google Scholar]
- 19. Boone D, Braitman E, Gendics C, Afthinos J, Latif J, Sordillo E, Todd G, Lantis J. Bacterial burden and wound outcomes as influenced by negative pressure wound therapy. Wounds 2010;22:32–7. [PubMed] [Google Scholar]
- 20. Conroy B, Anglen J, Simpson W, Christensen G, Phaup G, Yeager R, Gainor BJ. Comparison of castile soap, benzalkonium chloride and Bacitracin as irrigation solutions for complex contaminated orthopaedic wounds. J Orthop Trauma 1999;13:332–7. [DOI] [PubMed] [Google Scholar]
- 21. Tarbox B, Conroy B, Malicky E, Moussa FW, Hockman DE, Anglen JO, Simpson WA, Adelstein EH, Christensen G, Gainor BJ. Benzalkonium chloride. A potential disinfecting irrigation solution for orthopaedic wounds. Clin Orthop Relat Res 1998;346:255–61. [PubMed] [Google Scholar]
- 22. Marberry K, Kazmier P, Simpson W, Christensen GD, Phaup JG, Hendricks KJ, Anglen JO, Gainor BJ. Surfactant wound irrigation for the treatment of Staphylococcal clinical isolates. Clin Orthop Rel Res 2002;403:73–9. [DOI] [PubMed] [Google Scholar]
- 23. Motta GJ, Milne CT, Corbett LQ. Impact of antimicrobila gauze on bacterial colonies in wounds that require packing. Ost Wound Manage 2004;50:48–62. [PubMed] [Google Scholar]
- 24. Jull AB, Mitchell N, Arroll J, Jones M, Waters J, Latta A, Walker J, Arroll B. Factors influencing concordance with compression stockings after venous leg ulcer healing. J Wound Care 2004;13:90–2. [DOI] [PubMed] [Google Scholar]


