Abstract
The aim of this article was to present results of warm immersion recovery test in the diabetic foot with neuropathy using a liquid crystal‐based contact thermography system. It is intended to provide a ‘proof of concept’ for promoting the role of supplementary thermal assessment techniques and evidence‐based diagnosis of diabetic neuropathy. A total of 81 subjects from the outpatient department of MV Hospital for Diabetes, India, were assessed using a liquid crystal thermography system. Each subject was assigned to one of three study groups, that is diabetic neuropathy, diabetic non neuropathy and non diabetic healthy. The room temperature and humidity were consistently maintained at 24°C and less than 50%, respectively, with air conditioning. The right foot for each subject was located on the measurement platform after warm immersion in water at 37°C. Whole‐field thermal images of the plantar foot were recorded for 10 minutes. Local measurements at the most prevalent sites of ulceration, that is metatarsal heads, great toe and heel, show highest temperature deficit after recovery for diabetic neuropathy group. The findings of the current study support the ones of a previous study by the authors, which used cold immersion recovery test for the neuropathic assessment of the diabetic foot. A temperature deficit between the recovery and the baseline temperature for the neuropathic group suggests degeneration of thermoreceptors. Thermal stimulus tests can be useful to validate the nutritional deficits’ (during plantar loading and thermal stimulus) contribution in foot ulceration.
Keywords: Diabetic foot, Liquid crystal thermography, Thermometry, Ulcer, Warm immersion
Introduction
Research during several decades has shown temperature as an important factor in assessing the diabetic foot disease 1, 2, 3, 4, 5, 6, 7. Relevant thermographic assessment techniques and parameters are reviewed in literature (8). It is suggested that temperature measurements may have higher sensitivity than radiographic assessment of the foot (1). Thermography and scintigraphy (to detect infection in the bone) are commonly used, particularly to test for Charcot’s foot, that is a destructive arthropathy in a single or multiple joints (9). Both techniques are used independent of each other, the former shows better sensitivity for foot infections (10) and the latter benefits from high specificity (11).
Careful investigation of the current techniques for assessing the neuropathic diabetic foot suggests a bias in measuring the response of mechanoreceptors. This bias is inappropriate in the opinion of the authors, and therefore, a clear requirement exists to assess the response of thermoreceptors. There is a need for standardised guidelines for thermal assessment of the diabetic foot.
There has been a growing interest in home monitoring and ambulatory measurements of the foot temperatures in diabetic neuropathic foot to prevent foot ulceration using simple digital thermometers 5, 7, liquid crystal thermography (LCT) technology (Visual Footcare Technologies LLC, Pound Ridge, NY, USA) and smart thermometry insoles (Zephyr Technology Ltd., Auckland, New Zealand). Armstrong et al. suggest that one‐time thermal screening of the plantar foot is not useful and emphasise the importance of home monitoring of temperatures under the feet to record trends and inflammatory responses (12). However, a clinical thermometry system with a standardised assessment protocol can further current knowledge about diabetic foot complications and provide evidence‐based diagnosis of symptomatic or asymptomatic neuropathic condition. The assessment protocol for the clinical system may include both static and dynamic assessments. The use of home monitoring devices is further strengthened by the clinical evidence that neuropathic thermal patterns are not constant from day to day (13). Using LCT over infrared thermography to assess diabetic neuropathic foot may yield useful diagnostic information and facilitate clinical use of thermography in developing nations. This will, however, be achieved at the expense of thermal accuracy (14).
A collaborative research initiative within the authors’ research group has led to the development of a contact thermography system using thermochromic liquid crystals (TLCs) 15, 16. The system comprises a robust measurement platform, TLC polyester sheet, instrumentation and analysis software. The aim of this article was to present results of warm up recovery test in the diabetic foot with neuropathy using an LCT system. Using the results from this investigation, the authors intend to provide a ‘proof of concept’ for promoting the role of supplementary thermal assessment techniques and evidence‐based diagnosis of diabetic neuropathy. The current work is consistent with the requirement of developing useful thermal assessment tools to study thermal patterns at the lower extremities in diabetic patients.
Research design and methods
This ‘proof of concept’ study was completed with 81 subjects assigned to three independent study groups. All subjects were selected from the outpatient department of MV Hospital for Diabetes and Diabetes Research Centre at Chennai (India). Ethical approval was obtained from the local ethics committee at the centre. A diverse type 2 diabetic patient group (minimum duration 12 months) was assessed, excluding patients with active foot ulceration, peripheral vascular disease, Charcot’s foot deformity or any physical disability. Patients with active foot ulcers, foot infection (toe nail infection and fungal infection) or wounds were excluded.
The study groups included (a) diabetic patients with neuropathy (n = 28), (b) diabetic patients without neuropathy (n = 23) and (c) healthy controls (n = 30). For the neuropathic diabetic group, mean age was 58 years (range 41–71 years), whereas for the non neuropathic diabetic group, mean age was 50 years (range 33–63 years). For healthy controls, mean age was 32 years (range 20–51 years). This group was not well matched in terms of age to the other two groups because of difficulty in recruiting age‐matched subjects. A summary of the composition of study groups and parameters such as age, sex, duration of diabetes, % glycosylated haemoglobin (HbA1c) and body mass index is listed in Table 1.
Table 1.
Summary of the composition of study groups for the clinical study
| Patient group/parameters | Diabetic patients with neuropathy | Diabetic patients without neuropathy | Healthy normals |
|---|---|---|---|
| Number of subjects | 28* | 23* | 30 |
| Male/female | 24/4 | 15/8 | 8/22 |
| Age (years), mean ± SD | 57·92 ± 7·08 | 50·35 ± 9·79 | 32·43 ± 7·3 |
| Duration of diabetes (years), mean ± SD | 14·75 ± 6·8 | 9·45 ± 5·8 | n/a |
| Glycosylated haemoglobin (%), mean ± SD | 9·01 ± 1·81 | 8·79 ± 1·82 | n/a |
| Body mass index (kg/m2), mean ± SD | 25·24 ± 3·77 | 25·31 ± 3·48 | 25·07 ± 4·16 |
A total of 30 subjects per group were included in the clinical study. Two (male) neuropathic diabetic subjects and seven (three male/four female) non neuropathic diabetic subjects were excluded from the final analysis, as they were either recently diagnosed with diabetes or had duration of diabetes less than 12 months.
n/a = Not applicable.
All subjects were given a prior verbal and written description of the test objectives and test procedure. Informed written consent was obtained from all patients before the thermographic examination.
Assessment protocol
A comprehensive evaluation of the patient’s foot was performed, typical of the routine foot care programme at the hospital. Visual inspection of the foot followed by sensory neuropathy tests using 10‐g Semmes–Weinstein monofilament and biothesiometer was performed by trained nurses. Both tests have been shown clinically useful in previous studies 17, 18. Both tests were made at five sites on the foot, and a vibration perception threshold for neuropathy was taken as 30 V. Insensitivity to a graded 10‐g nylon monofilament at three or more sites was considered as clinical neuropathy. Furthermore, peripheral vascular disease was assessed by determination of ankle brachial perfusion index, with values at or above 0·9 considered as normal 9, 19. Data for mean HbA1c that indicate glycaemic control over previous 3 months were also recorded and are documented in Table 1.
The testing procedure commenced with a 20‐minute rest period in order for the plantar temperature to equilibrate with the room temperature. The room temperature and humidity were consistently maintained at 24°C and less than 50%, respectively, with air conditioning. During the equilibration period, the patient was seated on a chair with the feet flat on ground. Subsequently, the plantar foot temperature was measured using a digital thermometer at the first metatarsal head and the heel before each test. These were used as a reference measurement for each site to compare against the LCT temperature measurements. The patient’s feet were then located on the measurement platform using consistent alignment with reference markers on the platform. All subjects were advised to avoid movement during the duration of the test and use support from the handrail along the adjacent wall.
The LCT system is capable of storing RGB colour images using a digital camera and laptop. For the baseline measurement, 60 static images of the right foot were recorded over a continuous 5‐minute period. The sampling rate was one image every 5 seconds. For the warm up recovery test, the patient’s foot was placed in a water bath at 37°C for 3 minutes. The justification for using 3 minutes is discussed in another study. To summarise, 3 minutes is the optimal duration to record measurable changes without potential errors because of change in elastic properties of the skin 20, 21. The temperature of the non insulated water bath was maintained at 37°C by a ceramic water heating rod with a thermostat. In order to ensure patient safety, the ceramic rod was removed from the bath prior to placing the subject’s foot inside. Thermal changes during the test were recorded for 10 minutes, capturing static images every 5 seconds, giving a total of 120 images. The foot was dried thoroughly using a pre‐sterilised towel prior to placing on the measurement platform for the warming and recovery protocol.
Results
The results for evaluation of plantar temperatures following warm immersion in all study groups are presented. All temperature values were recorded during the 10‐minute recovery period following warming. Table 2 provides a detailed summary of the mean temperatures measured by the LCT system for baseline and warm immersion tests. Mean temperatures (°C) and standard deviation among the study group are listed. Table 3 lists the ‘delta temperatures’, that is difference in mean skin temperature between final temperatures after 10‐minute recovery and final temperatures after 5 minutes of baseline (before immersion control period) test at the three regions of interest for all study groups.
Table 2.
Summary of the mean temperature measurement at all regions of interest for three study groups*
| Study group | Mean temperature in °C (SD) | |
|---|---|---|
| Baseline | Warm immersion recovery | |
| Neuropathic | ||
| First MTH | 29·65 (1·84) | 28·36 (1·93) |
| Second MTH | 29·68 (1·82) | 28·49 (1·97) |
| Heel | 29·01 (1·43) | 27·68 (1·21) |
| Non neuropathic | ||
| First MTH | 30·26 (2·36) | 30·04 (2·93) |
| Second MTH | 30·2 (2·21) | 29·93 (2·64) |
| Heel | 28·66 (1·73) | 28·39 (1·85) |
| Non diabetic healthy | ||
| First MTH | 28·64 (1·77) | 28·27 (1·83) |
| Second MTH | 28·78 (1·8) | 28·314 (1·82) |
| Heel | 28·01 (1·39) | 27·11 (1·46) |
MTH, metatarsal head.
Mean temperatures (°C) and standard deviation at baseline and warm immersion recovery tests are listed.
Table 3.
Differences between mean temperature after 10 minutes for warm up recovery test and baseline temperatures after 5 minutes for all study groups
| Healthy (°C) | Non neuropathic (°C) | Neuropathic (°C) | |
|---|---|---|---|
| First MTH | 0·37 | 0·22 | 1·29 |
| Second MTH | 0·47 | 0·22 | 1·19 |
| Heel | 0·9 | 0·27 | 1·33 |
MTH, metatarsal head.
Neuropathic patients show highest delta temperatures, indicated in bold.
Diabetic patients with neuropathy show the highest differences at all the three sites. This is consistent with the findings of the cold immersion test published by the authors, providing supplementary evidence of impaired response of the thermoreceptors. Consider the histogram analysis at the first metatarsal head for all study groups as shown in Figure 1. Approximately 30% (n = 7) of the non neuropathic diabetic subjects show the final recovery temperatures ranging from 32°C to 35°C.
Figure 1.
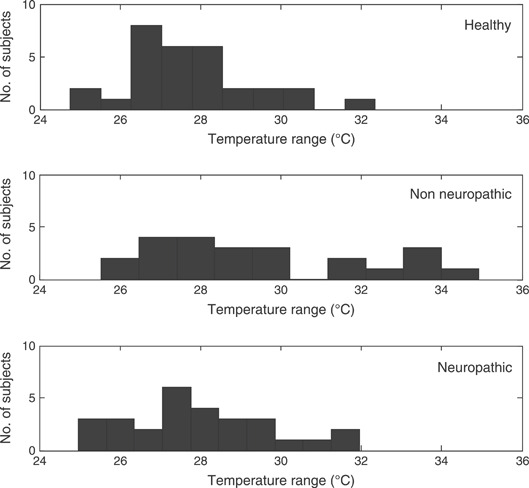
Histogram representation of the final temperatures after 10 minutes warm up recovery test for all study groups at first metatarsal head.
Discussion
The results of this study allowed an evaluation of warm up recovery responses for plantar tissue under loading using a low‐cost LCT system. Previous studies of diabetic neuropathy 17, 18, 22 have utilised several instruments to detect lack of protective sensation in the foot. However, there has not been any instrument designed specifically for evaluation of thermal patterns (and other temporal parameters, such as rate of change of vascularity and thermal hyperaemia) under the effect of load. Ideally, there should be an instrument to evaluate neuropathy independently and objectively, which is easy to use, readily available, sensitive and specific. Despite evidence from the past studies suggesting that local changes in the response to thermal variations may be significant in ulceration, there is no conclusive link, and evaluation of thermal sensitivity remains a research topic that has seen little progress towards adoption as a routine clinical measure. A key development has been the relatively recent availability of pressure‐independent temperature measurements using liquid crystal films (16).
The foot was immersed in warm water at 37°C for 3 minutes to evaluate its response following the induced thermal hyperaemia. Warming of the plantar tissue (to temperatures up to 44°C) is used as vasodilatory stimuli for TcPO2 assessment (23). The justification for 10‐minute recovery is discussed in a separate study (21). To summarise, this was an optimal trade‐off between the system capabilities and the physiologically relevant information when measuring temperature in weight‐bearing mode. The temperature after immersion in warm water at 37°C indicates values much lower than the temperature of the water. This may be because of two reasons: (a) heat exchange with the surroundings at 24°C when the foot is taken out from water and wiped using a pre‐sterilised towel and (b) withdrawal of nutritive blood supply following thermal vasodilation. The first reason results in hypothalamus‐mediated activity that counteracts the preceding thermal stimulus, that is changes in ambient temperature could affect thermoregulatory blood flow independent of the temperature of water. The time difference between removal from water bath and LCT measurement was less than 60 seconds. The second reason can be physiologically justified with the findings of Flynn and Tooke (24) and Meinders et al. (25). It is suggested that blood flow remained uninfluenced by heating when measured in a dependent position. The importance of these physiological effects on interpreting results from current study is affected by the extent of underlying neuropathy and impairment of microvascular system.
For the recovery test, the neuropathic diabetic group has highest delta temperature at all the three sites, that is first metatarsal head, second metatarsal head and heel. This may be indicative of sub‐clinical neuropathy and failure of the thermoreceptors to regulate perfusion to the foot following an event of warm immersion. This is consistent with the findings from cold immersion recovery test, published as a separate study (21).
From the histogram analysis of the non neuropathic group during the warm up recovery test, 30% of the subjects reached maximum temperatures above 32°C. This response of non neuropathic diabetic group leads to the overall high mean temperatures for this group.
The recovery response at the heel is comparatively slower, which is likely to be because of the presence of adipose tissue that provides thermal insulation. Besides, the geometrical condition of the skin surface may itself modify the processes of emission and absorption of heat (26).
Use of an insulated water bath for thermal recovery tests is appropriate for standardisation of the bath temperatures. If the foot is immersed in warm water, there is often creep of the dermis. This was not investigated in the current study and therefore must be considered for future clinical studies as it may affect the thermal coupling to the TLC sheet. For the thermal recovery tests in general, it is recommended to use a plastic bag before immersion. This prevents any water retention. Water retention by the skin is likely to affect the temperature measurements as heat exchange in water is eight times faster than in air. This is a standard procedure in cold stress tests for Raynaud’s phenomenon and prevents water retention by the skin 27, 28. In the current study, the patient’s foot was towel dried to prevent any physical damage to the TLC. This is a serious limitation of the current study.
Conclusions
Contact thermography is suitable for assessing the plantar temperatures in the neuropathic diabetic foot and considers the effect of neuropathy on regulation of blood flow. The findings of such an evaluation provide a whole‐field analysis of the plantar foot, identifying any localised event of neuropathic complication. The most important benefit of such an evaluation is the assessment of the foot in weight‐bearing mode.
The findings of the current study support the ones of a previous study by the authors, which used cold immersion recovery test for the neuropathic assessment of the diabetic foot. A temperature deficit between the recovery and the baseline temperature for the neuropathic group suggests degeneration of thermoreceptors. Thermal stimulus tests can be useful to validate the nutritional deficits’ (during plantar loading and thermal stimulus) contribution in foot ulceration.
Thermal changes at the plantar surface reflect vascular status, skeletal changes and inflammation at the site under consideration, all of which can be attributed to diabetic neuropathy. Considering clinical evidence suggesting dynamic loading leading to foot ulceration in diabetes, it is useful to measure foot temperature under load. The authors have shown two independent thermal recovery tests, that is cold immersion (21) and warm immersion, to be useful in assessment of the diabetic neuropathy. Both test results provide ‘proof of concept’ and merit further detailed investigation to determine sensitivity of each test.
Acknowledgements
The authors are grateful to the Engineering & Physical Sciences Research Council (GR/S0732201) and to the European Foundation for Study on Diabetes for the Albert Renold Travel fellowship (European Foundation for study of Diabetes) for providing funding to carry out this work. The authors also acknowledge Prof. Ann Anderson at Union College (Schenectady, NY, USA) for providing access to the LCT laboratory and technical support.
References
- 1. Sandrow R, Torg J, Lapayoker M, Resnick E. The use of thermography in the early diagnosis of neuropathic arthropathy in the feet of diabetics. Clin Orthop Relat Res 1972;88:31–3. [DOI] [PubMed] [Google Scholar]
- 2. Bergtholdt H. Temperature assessment of the insensitive foot. Phys Ther 1979;59:18–22. [DOI] [PubMed] [Google Scholar]
- 3. Benbow S, Chan A, Bowsher D, Williams G, Macfarlane I. The prediction of diabetic neuropathic plantar foot ulceration by liquid‐crystal contact thermography. Diabetes Care 1994;17:835–9. [DOI] [PubMed] [Google Scholar]
- 4. Stess RM, Sisney PC, Moss KM, Graf PM, Louie KS, Gooding GA, Grunfeld C. Use of liquid crystal thermography in the evaluation of the diabetic foot. Diabetes Care 1986;9:267–72. [DOI] [PubMed] [Google Scholar]
- 5. Lavery LA, Higgins KR, Lanctot DR, Constantinides GP, Zamorano RG, Armstrong DG, Athanasiou KA, Agrawal CM. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care 2004;27:2642–7. [DOI] [PubMed] [Google Scholar]
- 6. Armstrong DG, Lipsky B, Polis A, Abramson M. Does dermal thermometry predict clinical outcome in diabetic foot infection? Analysis of data from the SIDESTEP trial. Int Wound J 2006;3:302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lavery LA, Higgins KR, Lanctot DR, Constantinides GP, Zamorano RG, Armstrong DG, Athanasiou KA, Agrawal CM. Preventing diabetic foot ulcer recurrence in high‐risk patients: use of temperature monitoring as a self‐assessment tool. Diabetes Care 2007;30:14–20. [DOI] [PubMed] [Google Scholar]
- 8. Bharara M, Cobb J, Claremont D. Thermography and thermometry in the assessment of diabetic neuropathic foot: a case for furthering the role of thermal techniques. Int J Low Extrem Wounds 2006;5:250–60. [DOI] [PubMed] [Google Scholar]
- 9. Pendsey S. Diabetic foot: a clinical atlas, 1st edn. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd, 2003. [Google Scholar]
- 10. Harding J, Banerjee D, Wertheim D, Williams R, Melhuish J, Harding K. Infrared imaging in the long‐term follow‐up of osteomyelitis complicating diabetic foot ulceration. Proceedings of 21st Annual International Conference of the IEEE, Atlanta (GA): Engineering in Medicine and Biology; 1999. IEEE, 1999:1104. [Google Scholar]
- 11. Poirier J, Garin E, Derrien C, Devillers A, Moisan A, Bourguet P, Maugendre D. Diagnosis of osteomyelitis in the diabetic foot with a 99mTc‐HMPAO leucocyte scintigraphy combined with a 99mTc‐MDP bone scintigraphy. Diabetes Metab 2002;28:485–90. [PubMed] [Google Scholar]
- 12. Armstrong D, Lavery L, Wunderlich R, Boulton A. Skin temperatures as a one‐time screening tool do not predict future diabetic foot complications. J Am Podiatr Med Assoc 2003;93:443–7. [DOI] [PubMed] [Google Scholar]
- 13. Clark R, Goff M, Hughes J, Klenerman L. Thermography and pedobarography in the assessment of tissue damage in neuropathic and atherosclerotic feet. Thermology 1988;3:15–20. [Google Scholar]
- 14. Cavanagh P, Ulbrecht J, Caputo G. The biomechanics of the foot in diabetes mellitus. In: Bowker J, Pfeifer M, editors. The diabetic foot. St. Louis: Mosby Inc., 2001: 125–96. [Google Scholar]
- 15. Bharara M, Cobb J, Anderson A, Claremont D. Characterisation and calibration of three physical forms of thermochromic liquid crystals. Imaging Sci J 2007;55:232–41. [Google Scholar]
- 16. Bharara M. Liquid crystal thermography for neuropathic assessment of the diabetic foot. PhD Thesis. Bournemouth: Bournemouth University, 2007. [Google Scholar]
- 17. Miranda‐Palma B, Sosenko J, Bowker J, Mizel M, Boulton A. A comparison of the monofilament with other testing modalities for foot ulcer susceptibility. Diabetes Res Clin Pract 2005;70:8–12. [DOI] [PubMed] [Google Scholar]
- 18. Viswanathan V, Snehalatha C, Seena R, Ramachandran A. Early recognition of diabetic neuropathy: evaluation of a simple outpatient procedure using thermal perception. Postgrad Med J 2002;78:541–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. NHS . Type 2 diabetes – prevention and management of foot problems [WWW document]. http://www.guideline.gov [accessed on 10 June 2006]
- 20. Cobb JE. An in‐shoe laser Doppler sensor for assessing plantar blood flow in the diabetic foot. PhD Thesis. Bournemouth University, Bournemouth, 2000. [DOI] [PubMed] [Google Scholar]
- 21. Bharara M, Viswanathan V, Cobb J. Cold immersion recovery responses in diabetic foot with neuropathy. Int Wound J 2008. doi: 10.1111/j.1742‐481x.2008.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang P, Hoffman S, Krimitsos E, Rutkove S. Ambulatory foot temperature measurement: a new technique in polyneuropathy evaluation. Muscle Nerve 2003;27:737–42. [DOI] [PubMed] [Google Scholar]
- 23. Gaylarde P, Fonseca V, Llewellyn G, Sarkany I, Thomas P. Transcutaneous oxygen tension in legs and feet of diabetic patients. Diabetes 1988;37:714–6. [DOI] [PubMed] [Google Scholar]
- 24. Flynn M, Tooke J. Diabetic neuropathy and the microcirculation. Diabetes Medicine 1995;12:298–301. [DOI] [PubMed] [Google Scholar]
- 25. Meinders MJ, De Lange A, Netten PM, Wollesheim H, Lutterman JA. Microcirculation in the foot sole as a function of mechanical pressure. Clinical Biomechanics 1996;11:410–417. [DOI] [PubMed] [Google Scholar]
- 26. Jung A, Zuber J. Thermographic methods in medical diagnostics. Warsaw: MedPress, 1998. [Google Scholar]
- 27. Howell K, Kennedy L, Smith R, Black C. Temperature of the toes in Raynaud’s phenomenon measured using infra‐red thermography. Thermology 1997;7:132–7. [Google Scholar]
- 28. Cherkas L, Howell K, Carter L, Black C, MacGregor A. The use of portable radiometry to assess Raynaud’s phenomenon: a practical alternative to thermal imaging. Rheumatology 2001;40:1384–7. [DOI] [PubMed] [Google Scholar]


