Abstract
A group of international experts met in May 2006 to develop clinical guidelines on the practical application of vacuum assisted closure™ (V.A.C.®)† therapy in deep sternal wound infections. Group discussion and an anonymous interactive voting system were used to develop content. The recommendations are based on current evidence or, where this was not available, the majority consensus of the international group. The principles of treatment for deep sternal wound infections include early recognition and treatment of infection. V.A.C. therapy should be instigated early, following thorough wound irrigation and surgical debridement. V.A.C. therapy in deep sternal wound infections requires specialist surgical supervision and should only be undertaken by clinicians with adequate experience and training in the use of the technique.
Keywords: Consensus group, Deep sternal wounds, Infections, Mediastinitis, Treatment guideline, Vacuum assisted closure, Wound healing
Introduction
Median sternotomy is one of the most common incisions used in major operative procedures (1), carried out predominantly during cardiac surgery. Postoperative deep sternal wound infections following median sternotomy is a serious and potentially life‐threatening complication associated with significant morbidity and increased health care costs. Along with heart failure and stroke, it is one of the three most serious complications of open‐heart surgery (2) and can result in prolongation of hospital stay, increased intensive care unit (ICU) use and the need for multiple surgical procedures. While the reported incidence of deep sternal wound infections is relatively low, ranging between 0·16% and around 8% 3, 4, mortality rates associated with postoperative mediastinitis using conventional therapy range from 5% to 46% 5, 6, and it is estimated that the costs associated with postoperative deep sternal wound infections can be as high as two to three times those for uncomplicated surgery (7). These factors have informed the need for a rational approach to the management of deep sternal wound infections, with the goals of therapy being the resolution of infection, effective wound healing, sternal salvage and improved and/or reduced health care resource utilisation.
V.A.C. therapy is the controlled application of continuous or intermittent topical negative pressure across a wound surface (8). This therapy is used in a variety of clinical settings. Its adoption in deep sternal wound infections following coronary artery bypass grafting (CABG) has been associated with a reduction in both early and late mortality levels, similar to those seen in patients without a deep sternal infection (9), as well as with significantly improved overall survival compared with conventional treatment (10).
An international consensus group with representation from the United States and Europe in the fields of cardiothoracic surgery, plastic and reconstructive surgery, vascular surgery, paediatric cardiovascular surgery and general surgery convened in the Netherlands in May 2006 in order to debate the use of vacuum‐assisted closure (V.A.C.) therapy for the management of deep sternal wound infections and to develop guidelines for its application in this setting using the integrated V.A.C. (V.A.C.® Therapy™, KCI Licensing, Inc.) technique (11). This article reviews the historical development of this technique for deep sternal wound infections, considers the mechanisms of action and outlines key aspects of the consensus reached by the international expert group.
Defining deep sternal wound infections
Historically, terms including sternitis, mediastinitis, wound infection and wound complication have all been used to describe deep sternal wound infection following surgery. The US Centers for Disease Control and Prevention defines deep sternal wound infection as follows (12):
Infection involves fascia or deeper with at least one of the following: evidence of infection seen at re‐operation or spontaneous dehiscence, positive culture of mediastinal fluid and/or positive blood culture and/or chest pain with sternal instability and temperature higher than 38°C.
The definitions and classification for sternal wound complications (Table 1), as devised by El Oakley and Wright in 1996 (13), provide a more wide‐ranging scheme that does not rely on only a single defining clinical feature.
Table 1.
Definition of sternal wound complications (El Oakley and Wright, 1996) (13)
| Complication | Definition | Subtypes |
|---|---|---|
| Mediastinal dehiscence | Median sternotomy wound breakdown in the absence of clinical or microbiological evidence of infection | |
| Mediastinal wound infection | Clinical or microbiological evidence of infected presternal tissue and sternal osteomyelitis, with or without mediastinal sepsis and with or without unstable sternum | Superficial wound infection: infection confined to the subcutaneous tissue |
| Deep wound infection – mediastinitis: wound infection associated with sternal osteomyelitis with or without infected retrosternal space |
Mediastinitis is further categorised by El Oakley and Wright into subgroups (see Table 2). These are based on the time of first presentation, the presence or absence of risk factors and whether previous attempts at treatment have failed.
Table 2.
Categories of mediastinitis (El Oakley and Wright, 1996) (13)
| Class | Description |
|---|---|
| Type 1 | Mediastinitis presenting within 2 weeks after operation in the absence of risk factors (as identified in three or more major studies; diabetes, obesity, immunosuppressive therapy) |
| Type II | Mediastinitis presenting at 2–6 weeks after the operation in the absence of risk factors as above |
| Type IIIA | Mediastinitis Type I in the presence of one or more risk factors |
| Type IIIB | Mediastinitis Type II in the presence of one or more risk factors |
| Type IVA | Mediastinitis Type I, II or III after one failed therapeutic trial (including any surgical intervention with intention‐to‐treat mediastinitis) |
| Type IVB | Mediastinitis Type I, II or III after more than one failed therapeutic trial |
| Type V | Mediastinitis presenting for the first time more than 6 weeks after operation |
Approaches to sternal wound infection management
Before the development of V.A.C. therapy in the early 1990s, the standard approach to management of deep sternal wound infections in most centres consisted of thorough wound debridement, drainage and irrigation and delayed wound closure, with sternectomy and reconstructive surgery using omentum or pectoral muscle flaps 14, 15, 16. Treatment also involved removal of sternal wires, concomitant antibiotic therapy and frequent dressing changes. This approach was, however, associated with a number of limitations and problems, including unpredictable outcomes, significant risk of death and the likelihood of long‐term problems with pain or discomfort following sternectomy and muscle flap closure. Dressing changes were often time consuming, messy and painful for patients, required sedation and/or ventilation and carried the risk of CABG graft injury and bleeding. Furthermore, the labour‐intensive nature of care had implications for health care costs and staffing (7).
Since the advent of V.A.C. therapy in the early 1990s, a number of studies have shown that it is effective in managing poststernotomy deep sternal wound infections and sternal osteomyelitis 17, 18, 19, 20 and that it also reduces the number of dressing changes required, the number of final flap procedures needed and the length of hospital stay compared with conventional treatment (21). The advantages of V.A.C. therapy in the management of deep sternal wound infections may include the following: improved patient outcomes 9, 10, sternal stabilisation 22, 23, 24 and salvage, increased patient comfort and pain relief, early mobilisation of the patient and reduced ICU stay (19). Other possible mechanisms of action are effective wound drainage, increased angiogenesis and granulation tissue formation (25). In addition, compared with the use of traditional saline dressing changes, V.A.C. has been shown to decrease the costs of patient care by reducing the length of hospital stay (18) and by permitting earlier patient extubation (24). While the incidence of poststernotomy mediastinitis is likely to increase in future as cardiothoracic surgeons treat an ageing patient cohort with increasing comorbidity and attendant risk, the use of V.A.C. therapy with its benefits for patient compliance and user acceptability may help make dressing changes easier to achieve.
Pathophysiological effects of V.A.C. therapy
The effects of V.A.C. are complex and still not fully elucidated, but evidence from in vitro, animal and clinical studies has accumulated in recent years for a variety of beneficial physical and biochemical changes in the wound environment brought about by the application of negative pressure. While the relative contribution made by the various mechanisms remains to be clarified, it is clear that increased wound perfusion, reduction in inhibitory substances and lowering of bacterial load, reduced oedema and increased granulation tissue formation all contribute to the clinical efficacy of V.A.C. therapy (8).
Mechanical forces
The microdeformations and mechanical forces produced at the interface between the wound and the specialised foam dressing during V.A.C. therapy are believed to generate a number of clinically significant effects, including enhanced fluid flow through the underlying tissues, stretching of the extracellular matrix, activation of intracellular signalling pathways and alterations in gene expression, leading ultimately to changes in cell proliferation, differentiation and migration 26, 27, 28.
The mechanical forces generated by the vacuum applied across the wound surface have direct effects on wound contraction (29), possibly via remodelling of collagen fibrils, and are also believed to promote increased local perfusion and hence tissue oxygenation, possibly by decompressing small blood vessels (8). Mechanical stress is also known to have direct effects on angiogenesis 30, 31, 32 and formation of granulation tissue 25, 33, 34, which has been shown in animal models to be increased by between 63% and 103% in response to negative pressure (34). In addition, it has been observed that V.A.C. induces alterations in cytokine and growth factor expression, with a threefold to fourfold increase in transforming growth factor β‐1 and vascular endothelial growth factor expression, and a 2·5‐fold increase in platelet‐derived growth factor expression (35). V.A.C. may also assist wound healing by bringing about a reduction in levels of inflammatory cytokines 36, 37.
Effects on tissue fluid dynamics
V.A.C. removes fluid from the wound environment (29), and it is believed that this results in a shift in the interstitial fluid gradient (34), which in turn may reduce local tissue oedema, as well as bring about a secondary increase in dermal perfusion and removal of wound fluid. It is recognised that changes in interstitial fluid flow can alter the components and organisation of the extracellular matrix and can also influence cell division and growth factor expression (38). Furthermore, the removal of wound fluid facilitated by V.A.C. therapy helps clear bacteria from the wound environment, as demonstrated in both experimental animal models and in humans in the clinical setting 34, 39, 40. V.A.C. therapy also removes toxic compounds such as inhibitory mediators and matrix metalloproteinases 39, 41, 42, thereby enhancing wound healing. Finally, the alterations in tissue fluid dynamics and wound perfusion facilitated by V.A.C. therapy may also lead to enhanced absorption of antibiotics (43) and other adjuncts instilled into the local wound environment.
Maintenance of a closed wound environment
The use of an impermeable drape, which covers the wound in V.A.C. therapy, helps maintain a moist wound environment. This may facilitate wound healing by promoting angiogenesis and enhanced cellular metabolism, as well as by physically preventing the wound from drying out (29). The drape also acts as a barrier to reduce the risk of wound contamination.
V.A.C. therapy in deep sternal wound infections
Using V.A.C. therapy
Diagnosing deep sternal wound infection can be complex and requires a combination of clinical judgement, a high index of suspicion based on observation of the patient (e.g. presence of fever, wound pain, tenderness or cellulitis, sternal instability or dehiscence) and laboratory parameters. Definitive diagnosis relies on positive wound biopsy and/or swab cultures, or blood cultures. Treatment usually involves prompt surgical debridement of all infected tissues. Figure 1 shows that V.A.C. therapy has a major role and should be undertaken against a background of best practice with regard to early recognition of infection and antibiotic therapy, thorough irrigation and surgical debridement of the wound. V.A.C. therapy in deep sternal wound infections requires specialist surgical supervision and should be undertaken only by clinicians with adequate experience and training in the use of the technique.
Figure 1.
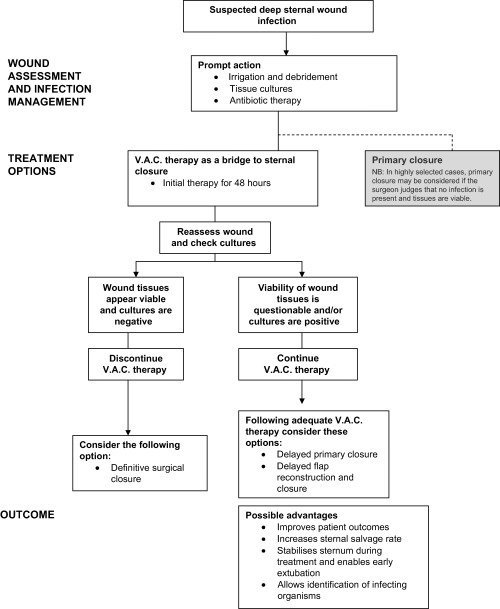
Overview of treatment options (adapted from ref 11).
V.A.C. therapy should be used following adequate surgical investigation, irrigation and wound debridement. It is contraindicated where the patient has untreated or undebrided osteomyelitis, excessive or uncontrolled bleeding or chest or pulmonary malignancy. Special care should be used in the circumstances described in Box 1, (11).
Table Box 1 .
Special care should be taken in the following circumstances (adapted from ref 11)
| 1. In the presence of exposed blood vessels, nerves or organs. These structures must be protected using overlying fascia, tissue or other protective barrier, such as one or more layers of a non adherent, vapour‐permeable interposed dressing. The use of a non adherent interposed dressing layer is particularly important in the sternal wound setting to prevent right ventricular rupture 23, 44 during therapy. |
| 2. Where the patient has vascular anastomoses, including coronary artery bypass grafts, or weakened, irradiated or sutured blood vessels or organs. |
| 3. In the presence of existing or potential bleeding problems, e.g. uncontrolled bleeding, coagulopathy or concomitant therapy with anticoagulant medication. |
| 4. Where the wound contains bone fragments or sharp edges that could puncture protective barriers, vessels or organs. This can be managed by debriding sharp edges and using one or more layers of a non adherent interposed dressing. |
| 5. In paediatric patients and neonates, in whom the potential for haemodynamic effects of V.A.C. therapy are more significant than in adults. |
| 6. In the presence of postcardiotomy syndrome. V.A.C. therapy can be used as a temporary closure technique, usually after 24 hours, when bleeding is controlled and the patient is haemodynamically stable. |
| 7. In fluid overload and systemic inflammatory response syndrome. V.A.C. therapy may be helpful in decreasing tissue fluid overload and increasing the microcirculation. |
The following advice is based on the recommendations of the international consensus panel (11).
Initiating therapy
The first application of V.A.C. therapy should take place in the operating room under general anaesthesia and should be performed or supervised by a cardiothoracic surgeon experienced in the technique. Following removal of foreign bodies from the wound, including free sternal wires, meticulous debridement and culturing of tissue samples from appropriate sites for definitive diagnosis of infection (tissue biopsy is preferable to swab cultures), the wound should be checked for irregular or sharp sternal edges in contact with the heart. If appropriate, and judged safe to do so, the heart and lungs should be freed from the sternal edges.
The heart and any exposed blood vessels/anastomoses, nerves or other organs must be protected using one or more layers of a non‐adherent, vapour‐permeable dressing. If possible, the interposed dressing should be tunnelled under the sternal edges by at least 1–2 cm, and especially under the left sternal portion. Protection of underlying structures is possibly the most important component of V.A.C. therapy in deep sternal wound infection. Structures may shift on application of the vacuum, which may cause the heart to come into contact with the sternal edges. The non‐adherent interposed layer and correct positioning of the foam dressing is intended to protect the heart and prevent adhesions and injury (e.g. organ rupture) during therapy.
Evaluating treatment
Treatment should be accompanied by regular wound assessment, carried out by an experienced clinician as indicated by the clinical situation and preferably by the same member of staff for continuity. The wound should be assessed at each dressing change. The manufacturers currently recommend that dressings should be repeated every 48 hours or more often (i.e. every 12–24 hours) in the presence of infection. In patients with deep sternal wound infections, it may be difficult to change the dressing more frequently than every 3 days. Further work is required to define the optimum dressing change interval.
Photographic documentation may be useful in providing a record of wound progress, especially where more than one member of staff is involved in monitoring wound healing. The wound should begin to change colour and become a deeper red as perfusion increases and there is evidence of granulation tissue formation. As the wound forms new granulation tissue, new epithelial growth should be seen at the wound edges. In addition, the volume of wound exudate should decrease, and it may change in appearance from serosanguinous to serous or there may be no secretion at all. The time scale for observed wound changes varies depending on the clinical circumstances, but visible evidence of healing can be expected within 2–7 days.
In some centres, serum C‐reactive protein levels may be checked daily as an adjunct to monitor wound healing (45). The wound may be considered to be free of infection when the serum C‐reactive protein level is 50–70 mg/l and decreasing, without other tissue injury or infection elsewhere (45). In centres where this is not practised, or in patients who are non responders, sternal tissue cultures may be used to inform the timing of sternal closure.
V.A.C. therapy should be discontinued when the desired outcome has been achieved for the patient as defined by the lead physician, such as resolution of infection with good granulation tissue in the wound bed (11).
Preventing and dealing with complications of V.A.C. therapy
Complications of V.A.C. therapy often reflect inadequate debridement or inadequate protection of exposed vital structures. To reduce the risk of bleeding complications of V.A.C. therapy, surgical debridement and foam dressing changes should be avoided in anticoagulated patients with an international normalised ratio (INR) value >2·0. In patients who experience pain during V.A.C. therapy, the therapy pressure can be lowered (e.g. from −125 to −100 or −75 mmHg). If there is a loss of the dressing seal, more drape should be applied to prevent the wound from drying out.
Conclusions
The involvement of a cardiothoracic surgeon with appropriate expertise is essential in the management of deep sternal wound infections. These are complex wounds to manage and involve major organs, and complications can be life threatening.
Treatment of sternal wound infections using V.A.C. therapy provides important improvements over traditional approaches to management, and the approach should be regarded as an ‘opt out’ rather than an ‘opt in’ choice.
Vacuum assisted closure™ and V.A.C. ® are trademarks of KCI International, Inc.
References
- 1. Pezella T. Mediastinitis following open heart surgery: introduction. Semin Thorac Cardiovasc Surg 2004;16:51–2. [DOI] [PubMed] [Google Scholar]
- 2. Gustafsson R. Vacuum‐assisted closure therapy: a new treatment modality in post sternotomy mediastinitis. Doctoral thesis, Lund University, 2004. [Google Scholar]
- 3. Baskett RJ, MacDougall CE, Ross DB. Is mediastinitis a preventable complication? A 10‐year review. Ann Thorac Surg 1999;67:462–5. [DOI] [PubMed] [Google Scholar]
- 4. Brown IW, Moor GF, Hummel BW, Marshall WG Jr, Collins JP. Toward further reducing wound infections in cardiac operations. Ann Thorac Surg 1996;62:1783–89. [DOI] [PubMed] [Google Scholar]
- 5. Milano CA, Georgiade G, Muhlbaier LH, Smith PK, Wolfe WG. Comparison of omental and pectoralis flaps for poststernotomy mediastinitis. Ann Thorac Surg 1999;67:377–80. [DOI] [PubMed] [Google Scholar]
- 6. Sutherland RD, Martinez HE, Guynes WA, Miller L. Postoperative chest wound infections in patients requiring coronary bypass: a controlled study evaluating prophylactic antibiotics. J Thorac Cardiovasc Surg 1977;73:947. [PubMed] [Google Scholar]
- 7. Loop FD, Lytle BW, Cosgrove DM, Mahfood S, McHenry MC, Goormastic M, Stewart RW, Golding LA, Taylor PC. Sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity, and cost of care. Ann Thorac Surg 1990;49:179–86. [DOI] [PubMed] [Google Scholar]
- 8. Banwell PE. Topical negative pressure therapy: mechanisms and indications. Int Wound J 2004;1: 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sjogren J, Nilsson J, Gustafsson R, Malmsjo M, Ingemansson R. The impact of vacuum‐assisted closure on long‐term survival after poststernotomy mediastinitis. Ann Thorac Surg 2005;80:1270–75. [DOI] [PubMed] [Google Scholar]
- 10. Sjogren J, Gustafsson R, Nilsson J, Malmsjo M, Ingemansson R. Clinical outcome after poststernotomy mediastinitis: vacuum assisted closure versus conventional treatment. Ann Thorac Surg 2005;79:2049–55. [DOI] [PubMed] [Google Scholar]
- 11. V.A.C.® Therapy™ . Clinical guidelines for deep sternal wound infections. A reference source for clinicians. The Netherlands: KCI Licensing Inc. 2006. [Google Scholar]
- 12. Bruce J, Russell EM, Mollison J, Krukowski ZH. The measurement and monitoring of surgical adverse events. Health Technol Assess 2001;5:1–194. [DOI] [PubMed] [Google Scholar]
- 13. El Oakley RM, Wright JE. Postoperative mediastinitis: classification and management. Ann Thorac Surg 1996;61:1030–36. [DOI] [PubMed] [Google Scholar]
- 14. Rand RP, Cochran RP, Aziz S, Hofer BO, Allen MD, Verrier ED, Kunzelman KS. Prospective trial of catheter irrigation and muscle flaps for sternal wound infection. Ann Thorac Surg 1998;65:1046–49. [DOI] [PubMed] [Google Scholar]
- 15. Jones G, Jurkiewicz MJ, Bostwick J, Wood R, Bried JT, Culbertson J, Howell R, Eaves F, Carlson G, Nahai F. Management of the infected median sternotomy wound with muscle flaps. The Emory 20‐year experience. Ann Surg 1997;25:766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El Gamel A, Yonan NA, Hassan R, Jones MT, Campbell CS, Deiraniya AK, Lawson RA. Treatment of mediastinitis: early modified Robicsek closure and pectoralis major advancement flaps. Ann Thorac Surg 1998;65:41–6. [DOI] [PubMed] [Google Scholar]
- 17. Domkowski PW, Smith ML, Gonyon DL, Drye C, Wooten MK, Levin LS, Wolfe WG. Evaluation of vacuum assisted closure in the treatment of post‐sternotomy mediastinitis. J Thorac Cardiovasc Surg 2003;126:386–90. [DOI] [PubMed] [Google Scholar]
- 18. Luckraz H, Murphy F, Bryant S, Charman SC, Ritchie AJ. Vacuum‐assisted closure as a treatment modality for infections after cardiac surgery. J Thorac Cardiovasc Surg 2003;125:301–5. [DOI] [PubMed] [Google Scholar]
- 19. Fleck TM, Fleck M, Moidl R, Czerny M, Koller R, Giovanoli P, Hiesmayer MJ, Zimpfer D, Wolner E, Grabenwoger M. The vacuum‐assisted closure system for the treatment of deep sternal wound infections after cardiac surgery. Ann Thorac Surg 2002;74:1596–600. [DOI] [PubMed] [Google Scholar]
- 20. Doss M, Martens S, Wood JP, Wolff JD, Baier C, Moritz A. Vacuum‐assisted suction drainage versus conventional treatment in the management of post‐sternotomy osteomyelitis. Eur J Cardiothorac Surg 2002;22:934–8. [DOI] [PubMed] [Google Scholar]
- 21. Song DH, Wu LC, Lohman RF, Gottleib LJ, Franczyk M. Vacuum‐assisted closure for the treatment of sternal wounds: the bridge between debridement and definitive closure. Plast Reconstr Surg 2003;111:92–7. [DOI] [PubMed] [Google Scholar]
- 22. Kutschka I, Frauendorfer P, Harringer W. Vaccum‐assisted closure therapy improves early postoperative lung function in patients with large sternal wounds. Zentralbl Chir 2004;129 Suppl. 1:S33–4. [DOI] [PubMed] [Google Scholar]
- 23. Gustafsson R, Sjogren J, Ingemansson R. Deep sternal wound infection: a sternal‐sparing technique with vacuum‐assisted closure therapy. Ann Thorac Surg 2003;76:2048–53. [DOI] [PubMed] [Google Scholar]
- 24. Hersh RE, Jack JM, Dahman MI, Morgan RF, Drake DB. The vacuum‐assisted closure device as a bridge to sternal wound closure. Ann Plast Surg 2001;46:250–4. [DOI] [PubMed] [Google Scholar]
- 25. Morykwas MJ, Faler BJ, Pearce DJ, Argenta LC. Effects of varying levels of sub‐atmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg 2001;47:547–51. [DOI] [PubMed] [Google Scholar]
- 26. Vogt P. The foam wound interface: present and future. ETRS TNP focus group meeting; Dec 2003; London, UK.
- 27. Morykwas MJ. External application of subatmospheric pressure and healing: mechanisms of action. Wound Heal Soc News 1998;8:4–5. [Google Scholar]
- 28. Banwell PE, Teot L. Topical negative pressure (TNP): the evolution of a novel wound therapy. J Wound Care 2003;12:22–8. [DOI] [PubMed] [Google Scholar]
- 29. Banwell PE. Topical negative pressure therapy in wound care. J Wound Care 1999;8:79–84. [DOI] [PubMed] [Google Scholar]
- 30. Ryan TJ, Barnhill RL. Physical factors and angiogenesis. In: development of the vascular system. Ciba Foundation Symposium 100. Nugent J, O’Connor M (eds). London: Pitman, 1983: 80–94. [DOI] [PubMed] [Google Scholar]
- 31. Ichioka S, Shibata M, Kosaki K, Sato Y, Harii K, Kamiya A. Effects of shear stress on wound healing angiogenesis in the rabbit ear chamber. J Surg Res 1997;72:29–35. [DOI] [PubMed] [Google Scholar]
- 32. Sumpio BE, Banes AJ, Levin LG, Johnson G Jr. Mechanical stress stimulates aortic endothelial cells to proliferate. J Vasc Surg 1987;6:2552–56. [PubMed] [Google Scholar]
- 33. Fabian TS, Kaufman HJ, Lett ED, Thomas JB, Rawl DK, Lewis PL, Summitt JB, Merryman JI, Schaeffer TD, Sargent LA, Burns RP. The evaluation of subatomospheric pressure and hyperbaric oxygen in ischaemic full‐thickness wound healing. Am Surg 2000;66:1136–43. [PubMed] [Google Scholar]
- 34. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment – animal studies and basic foundation. Ann Plast Surg 1997;38:553–62. [DOI] [PubMed] [Google Scholar]
- 35. Kopp J, Hoff C, Rosenberg B, Von Beulow S, Kilpadi DV, Schroeder W, Pallua N, Horch R. Application of V.A.C. therapy upregulates growth factor levels in neuropathic diabetic foot ulcers. ETRS, 13th Annual Meeting; 2003 Sept; Amsterdam. [Google Scholar]
- 36. Banwell PE. The biochemical wound environment and topical negative pressure therapy. ETRS TNP Focus Group Meeting; 2003 Dec; London, UK. [Google Scholar]
- 37. Bennett K, Komorowska‐Timek E, Gabriel A, Gupta S. Characterising the changing gene statement of chronic wounds treated with subatmospheric pressure therapy. Plastic Surgery Research Council Annual Meeting; 2002 April; Boston, MA.
- 38. Swartz M, Tschumperlin D, Kamm R, Drazen J. Mechanical stress is communicated between different cell types to elicit matrix remodelling. Proc Nat Acad Sci USA 2001;98:6180–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van Wicjck R, Manicourt D. Feruzi‐Lukina G. Biological monitoring of wounds treated by negative pressure. ETRS New Technologies Focus Group Meeting; 2002 September; Nice, France.
- 40. Fleischmann W, Becker U, Bischoff M, Hoekstra H. Vacuum sealing: indication technique and results. Eur J Orthop Surg Traumatol 1995;5:37–40. [DOI] [PubMed] [Google Scholar]
- 41. Shi B, Chen SZ, Zhang P, Li JQ. Effects of vacuum‐assisted closure (VAC) on the expressions of MMP‐1, 2,13 in human granulation wound. Zhonghua Zheng Xing Wai Ke Za Zhi 2003;19:279–81. [PubMed] [Google Scholar]
- 42. Stechmiller J, Paddock H, Gowda S, Mozingo D, Chin G, Porter T, Schultz G. Biochemical analysis of wound fluid from chronic wounds treated with VAC. Wound Healing Society –European Tissue Repair Society Combined meeting; 2002 May; Baltimore.
- 43. Fleischmann W, Russ M, Westhauser A, Stampehl M. Vacuum sealing as a drug release system for controlled local drug administration in wound infection. Unfallchirurg 1998;101:649–54. [DOI] [PubMed] [Google Scholar]
- 44. Francel TJ, Kouchoukos NT. A rational approach to wound difficulties after sternotomy: the problem. Ann Thorac Surg 2001;72:1411–8. [DOI] [PubMed] [Google Scholar]
- 45. Gustafsson R, Johnsson P, Algotsson L, Blomquist S, Ingemansson R. Vacuum assisted closure therapy guided by C‐reactive protein level in patients with deep sternal wound infection. J Thorac Cardiovasc Surg 2002;123:895–900. [DOI] [PubMed] [Google Scholar]


