ABSTRACT
The objective of this study was to use autologous plasma rich in growth factors (PRGF) on wound healing in the skin in New Zealand albino rabbits and to study reepithelialisation and inflammation at 7 and 28 days. A prospective study carried out on 20 adult rabbits. Two wounds were made on the in the skin on the back of each animal; one control, and the other in which PRGF was applied. The PRGF preparation was obtained from 10 ml of whole blood. The reepithelialisation and inflammation of wounds were measured at 7 and 28 days. Reepithelialisation improved in skin at 7 days (P = 0·007), with resolution of the inflammatory process (P = 0·005), having significant differences with respect to the control. Therefore, PRGF accelerates reepithelialisation and reduces inflammation at 7 days in skin.
Keywords: Plasma rich in growth factors, Skin, Experimental, New Zealand albino rabbits
INTRODUCTION
Platelets are a natural source of growth factors and cytokines that promote wound healing. A number of growth factors are sequestered in platelets including: Platelet derived growth factor (PDGF), transforming growth factor β1 (TGF‐ β1), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (BFGF) and insulin‐like growth factor 1 (IGF‐1) 1, 2, 3, 4, 5. While PDGF secreted from platelets plays an important role in initial wound healing, its subsequent secretion from macrophages continues the events of wound healing through up‐regulation of other growth factors and cells that ultimately promote fibroblastic and osteoblastic functions (2).
Plasma rich in growth factor (PRGF) is a mixture of autologous proteins prepared from a determined volume of platelet rich plasma (PRP). PRGF has been clinically studied for accelerating healing in various tissues such as bone and skin.
PRGF has a series of advantages; it facilitates the simultaneous joint action of multiple growth factors. It is an autologous product, and thus avoids the risk of transmitting disease. It increments tissue vascularisation through the promotion of angiogenesis. It provides an immediate safe and effective biocompatible agent. It is resorbed by the body within days, initiating local regeneration 1, 2, 3, 4. It is chemotactic for multiple cell lines. It aids in the compaction of grafts or biomaterials 1, 6, 7.
In cutaneous wounds, PRP has been used for split‐thickness skin graft donor sites expecting for acceleration of epithelialisation and better scar formation (6). However, there are few reports on the use of PRP for cutaneous incisional biopsy wounds. The objective is to study reepithelialisation and inflammation in the skin, at 7 and 28 days, using autologous PRGF, in New Zealand albino rabbits.
MATERIAL AND METHODS
The animals used in this study were obtained from the Animalary Medicine Faculty of the University of Murcia (Spain), and the experiment was approved on October 31, 2006, by the Bioethics Committee of the same university.
Animals
A total of 20 adult male New Zealand albino rabbits, with a mean weight of 3662 g (range 2700–5200 g) were used. Housing and care for the animals was in accordance with the Advice of the European Communities (8).
Surgical procedure
The animals were anaesthetised with a mixture of ketamine (60%) and xylazine (40%), administering 1 ml/kg of body weight by intramuscular injection. The animals were monitored for signs of infection and discomfort, pre‐and post‐surgery, until euthanasia.
PRGF preparation
To obtain PRGF a minimum of 10 ml of blood is required. The blood was obtained via cardiac aspiration. Immediately after collection the blood was placed in 2 to 5 ml sterile extraction tubes, with sodium citrate at 3.8% as anticoagulant. The tubes were placed in a centrifuge, BTI PRGF® System III (Biotechnology Institute® S.L. Álava, Spain) and centrifuged at 460 g for 8 minutes, thus separating the different phases of the blood. The plasma poor in growth factors was eliminated with pipettes as well as the upper 1000 μl of each of the 5 ml tubes. Once separated, the coagulant was activated just before application with 10% calcium chloride solution. The tubes were left for 10 minutes at room temperature, until a consistent easily‐handled gelatinous layer was formed. We have used a mean platelet concentration of 1 150 000/ mm3 (range 725 000–1 545 000/ mm3).
Two wounds were made in the dorsal skin. Nothing was applied to one wound (control wound) while to the other wound we applied the PRGF (study wound) (Figure 1). Incisions were made through the skin, using a 6‐mm diameter biopsy punch (Stiefel Laboratories®, Madrid, Spain), to ensure that all wounds were the same size. Finally, all wounds were the closed with two points simple with 3/0 absorbable suture (NORMON Laboratories® S.A., Madrid, Spain).
Figure 1.
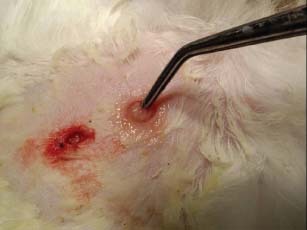
Two wounds were made in the dorsal skin. Nothing was applied to one wound (control wound) while to the other wound we applied the PRGF (study wound).
The animals were euthanised at 7 and 28 days of the surgical procedure by CO2 inhalation. For the biopsy, we used an 8‐mm diameter biopsy punch (Stiefel Laboratories®).
Histopathological study
The 40 specimens were immediately introduced in a wide‐mouthed container and fixed in abundant 10% formalin‐buffered saline. The specimens were embedded in paraffin. Samples were cut into 4 μm sections and stained with hematoxylin and eosin. The pathologist was blind to the wound repair method used.
To measure the grade of reepithelialisation, the criteria established by Sinha and Gallagher (9) were used; grade 0: reepithelialisation at the edge of the wound, grade 1: reepithelialisation covering less than half of the wound, grade 2: reepithelialisation covering more than half of the wound, grade 3: reepithelialisation covering the entire wound, with irregular thickness, grade 4: reepithelialisation covering the entire wound, normal thickness.
The grade of inflammation was measured using the resolution phases of inflammatory processes described by Cotran et al. (10); grade 1: acute inflammation (pyogenic membrane is formed), grade 2: predominance of diffuse acute inflammation (predominance of granulation tissue), grade 3: predominance of chronic inflammation (fibroblasts beginning to proliferate), grade 4: resolution and healing (reduction or disappearance of chronic inflammation, although occasional round cells may persist).
Statistical analysis
The data were processed using the SPSS version 12·0 (SPSS® Inc, Chicago, USA). A descriptive study was made of each variable. The associations between the different qualitative variables were studied using Pearson's chi squared test. Statistical significance was accepted for P value≤ 0·05.
RESULTS
Seven days after provoking the wounds in the skin, the reepithelialisation was greater in those in which PRGF had been applied, with statistically significant differences with respect to the controls (P = 0·007). At 28 days, we found no statistically significant differences with respect to the controls (P = 0·687) (Table 1) (Figure 2).
Table 1.
Grade of wound reepithelialisation at 7 and 28 days after surgical intervention (total values) (Pearson's chi squared test)
| Histopathologic scale to evaluate reepithelialisation * | ||||||||
|---|---|---|---|---|---|---|---|---|
| Day | Groups | Skin Total | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | P value |
| 7 | Wound + PRGF | 10 | 0 | 0 | 1 | 1 | 8 | 0·007 |
| Control | 10 | 0 | 0 | 3 | 6 | 1 | ||
| 28 | Wound + PRGF | 7 | 0 | 0 | 0 | 1 | 6 | 0·687 |
| Control | 9 | 0 | 0 | 0 | 2 | 7 | ||
PRGF, plasma rich in growth factors.
*Grade 0: reepithelialisation at the edge of the wound; grade 1: reepithelialisation covering less than half of the wound; grade 2: reepithelialisation covering more than half of the wound; grade 3: reepithelialisation covering the entire wound, with irregular thickness; grade 4: reepithelialisation covering the entire wound, normal thickness.
Note: Three samples of study group and one of control group were lost during the processing phase in the laboratory at 28 days.
Figure 2.
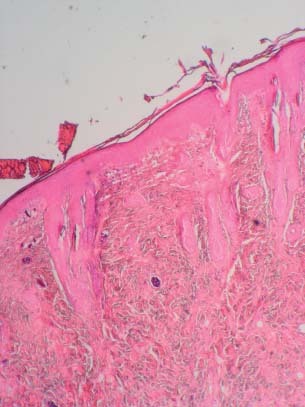
Histopathologic image showing a wound 7 days after being caused by biopsy and with PRGF. Note the reepithelialisation covering the entire wound, with irregular thickness (Hematoxylin and eosin stain ×40).
Regarding resolution of the inflammatory process, at 7 days of provoking the wounds, 40% of those in which PRGF was applied had resolved the inflammatory process, while none of the control wounds presented full resolution, with statistically significant differences (P = 0·005). At 28 days, there were no significant differences between the PRGF and the controls (P = 0·849) (Table 2).
Table 2.
Grade of wound inflammation at 7 and 28 days after surgical intervention (total values) (Pearson's chi squared test)
| Histopathologic scale to evaluate inflammation * | |||||||
|---|---|---|---|---|---|---|---|
| Day | Groups | Skin Total | Grade 1 | Grade 2 | Grade 3 | Grade 4 | P value |
| 7 | Wound + PRGF | 10 | 0 | 1 | 5 | 4 | 0·005 |
| Control | 10 | 0 | 6 | 4 | 0 | ||
| 28 | Wound + PRGF | 7 | 0 | 0 | 6 | 1 | 0·849 |
| Control | 9 | 0 | 0 | 18 | |||
PRGF, plasma rich in growth factors.
*Grade 1: acute inflammation (pyogenic membrane is formed); grade 2: predominance of diffuse acute inflammation (predominance of granulation tissue); grade 3: predominance of chronic inflammation (fibroblasts beginning to proliferate); grade 4: resolution and healing (reduction or disappearance of chronic inflammation, although occasional round cells may persist).
Note: Three samples of study group and one of control group were lost during the processing phase in the laboratory at 28 days.
DISCUSSION
The results of this study show that the application of autologous PRGF accelerates reepithelialisation and reduces inflammation at 7 days in wounds produced in the skin. Similar results were found by other authors 1, 6.
In the literature, the controversy surrounding this procedure is clear, the discrepancies are probably related to the lack of standardisation of the different PRP preparations, protocols and surgical techniques 2, 7. Once the PRP is obtained, the complete and efficient release of the growth factors and the remaining platelet proteins is fundamental, for which it is necessary to reproduce the natural coagulation process, known as ‘degranulation’, of the platelets (2).
To obtain PRGF, we have followed the protocol described by Anitua (2) in 1999; PRGF was chosen because the activator is calcium chloride, this eliminates the risk of immunological reactions and the transmission of diseases associated with the use of exogenous bovine thrombin. The time period used to study healing was 7 and 28 days as they are standard periods (9). We used the same criteria for reepithelialisation as Sinha and Gallagher (9) did in 2003 on oral mucosa of Guinea pig.
Schmitz and Hollinger (5) questioned the actual isoforms and biological actions of the various growth factors concentrated in PRP, and stated their opinion that at this time, basic research does not strongly endorse the ability of PRP to promote healing.
CONCLUSION
PRGF accelerates reepithelialisation and reduces inflammation at 7 days in skin. However, the regenerative effects of PRGF in soft tissue are unclear, and in this respect we should continue investigating.
REFERENCES
- 1. Anitua E, Sanchez M, Orive G, Andia I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials 2007;28:4551–60. [DOI] [PubMed] [Google Scholar]
- 2. Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants 1999;14:529–35. [PubMed] [Google Scholar]
- 3. Steed DL. Clinical evaluation of recombinant human platelet‐derived growth factor for the treatment of lower extremity ulcers. Plast Reconstr Surg. 2006;117:143S–9S. [DOI] [PubMed] [Google Scholar]
- 4. Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet‐rich plasma: implication for wound healing. Plast Reconstr Surg 2004;114:1502–8. [DOI] [PubMed] [Google Scholar]
- 5. Schmitz JP, Hollinger JO. The biology of platelet‐rich plasma. J Oral Maxillofac Surg 2001;59:1120. [DOI] [PubMed] [Google Scholar]
- 6. Kimura A, Ogata H, Yazawa M, Watanabe N, Mori T, Nakajima T. The effects of platelet‐rich plasma on cutaneous incisional wound healing in rats. J Dermatol Sci 2005;40:205–8. [DOI] [PubMed] [Google Scholar]
- 7. Marx RE. Platelet‐rich plasma: evidence to support its use. J Oral Maxillofac Surg 2004;62:489–96. [DOI] [PubMed] [Google Scholar]
- 8.Directive 86/609/CEE, of 24 of November by that the experimentation animal protection settles down, European Union 1986, Brussels.
- 9. Sinha UK, Gallagher LA. Effects of steel scalpel, ultrasonic scalpel, CO2 laser, and monopolar and bipolar electrosurgery on wound healing in guinea pig oral mucosa. Laryngoscope 2003;113:228–36. [DOI] [PubMed] [Google Scholar]
- 10. Cotran RS, Kumar V, Collins T. Reparación de los tejidos: regeneración celular y fibrosis. In: Robbins C, editor. Patologí a estructural y functional, 6th edn. Madrid: McGraw‐Hill Interamericana, 2000;112–7. [Google Scholar]


