Abstract
This study examined if a series of epidermal growth factor (EGF) local infiltrations can enhance the healing process of complicated diabetic wounds. Twenty‐nine in‐hospital patients with diabetic neuropathic or ischaemic lesions with high risk of amputation were treated in a non controlled pilot study conducted at the National Institute of Angiology, Havana. Lesions, classified as Wagner’s grade 3 or 4, included ulcers ≥20 cm2 for ≥25 days or amputation residual bases ≥30 cm2 for ≥15 days, healing refractory despite comprehensive wound care. EGF (25 μg) intralesional infiltrations (≈250 μl of a 25 μg/ml solution/injection point) were performed thrice weekly up to the eighth week. Wound closure was monitored during the treatment and recurrence examined for a year following discharge from hospital. Eighty‐six per cent of the patients treated showed a productive granulation at infiltration session 8. Histological examination at this point indicated a substantial wound matrix transformation, granulation tissue cell repopulation and angiogenesis. Of the 29 patients treated, amputation was prevented in 17 (58·6%) of them who completed 24 infiltration sessions. They averaged 71·1 ± 18·3% of reepithelisation during a mean in‐hospital period of 66·5 ± 4·9 days. Wound recurrence after 1 year of follow‐up appeared in only one patient. Preliminary evidences suggest that EGF intralesional infiltrations may be effective in reducing diabetic lower limb amputation.
Keywords: Amputation, Diabetes, EGF, Ulcer, Wounds
Introduction
Diabetic foot ulcer (DFU) is a major and devastating complication for the population affected with diabetes. It is a major factor reducing the quality of life of the patient and a serious professional challenge for the medical community. DFU also represents a significant social and economic burden (1). Patients with diabetes pose a 5‐ to 50‐fold higher risk of non traumatic amputation compared with individuals without diabetes (2). Pathophysiology of diabetic complications is complex and multi‐factorial while converging to neuronal and vascular damages. Impairment in local infection control mechanisms due to diabetic immunopathy in association with weight bearing and mechanical stress make the lower extremities particularly vulnerable to the development of ulcers (3). Poor control of glycemia stands as a major contributing factor for all the diabetic complications (4). Increased protein glycation activates multiple pathways leading to biochemical dysfunctions, which have proved to hinder the normal course of the cutaneous healing process 5, 6.
Diabetic lower extremity ulcers are generally classified as neuropathic or ischaemic. Neuropathic ulcers are distinguished for their slow healing rate and resistance to traditional treatment methods. Ischaemic ulcers represent a particular form of recalcitrant wounds in which the events encompassed within the proliferation phase are mostly abolished and granulation hardly takes place (7). There is consensus in that healing of diabetic ischaemic wounds is an exceptional success due to the failure of arterial blood perfusion (8).
During the past years, there has been a deeper understanding of the pharmacology of growth factors aimed at the enhancement of healing process. Exciting therapeutic opportunities have also emerged with the clinical use of novel dressing systems and living human skin equivalents. These alternatives have positively affected small‐sized, uncomplicated neuropathic ulcers 9, 10, 11, 12. Nevertheless, effective therapeutic approaches targeted to reduce amputations in ischaemic and complicated forms of diabetic neuropathic wounds are still an unmet medical need.
The effect of epidermal growth factor (EGF) on the healing process has been studied. Topical application or subcutaneous injection of EGF produces skin keratinocytes and fibroblasts hyperplasia and hypertrophy, as well as corneous layer thickening 13, 14. Exogenous EGF can also play a significant role in stimulating peripheral nerve regeneration (15). Some clinical trials have been conducted to evaluate the efficacy and safety of topical application of EGF in different indications such as radiogenic (16) and venous ulcers (17), and burns 18, 19, 20. Besides, EGF has recently shown to be effective as an enema and as an oral formulation for healing of colitis or duodenal ulcers in randomised, placebo‐controlled clinical trials in the United Kingdom and Cuba, respectively 21, 22.
However, the availability of the growth factor on the deeper layers of the wound is an important issue to achieve an adequate efficacy. This can be a limitation with topical formulations because diffusion of the active agent is affected by necrotic tissue, sepsis, inflammation and the action of wound proteases (23). Intralesional injection of the growth factor could bring the active agent into the desired region.
The data presented here stem from a pilot study conducted with a heterogeneous population of patients bearing severe DFU, which entailed an important risk of amputation with no further therapeutic choices. The intervention used herein is based on the direct infiltration of recombinant human EGF (rh‐EGF) in multiple points within the wound contours and bottom. The major goal of this intervention was to prevent amputation in patients, considering the severe condition of all the lesions.
Methods
Patients
Patients admitted at the Diabetic Angiopathy Service of the National Institute of Angiology and Vascular Surgery, Havana, received the treatment from April 2001 to September 2002. A written informed consent was obtained from the patients selected. The Ethics Committee of the Institute of Angiology and Vascular Surgery approved the intervention procedure according to the poor prognosis of lower extremity of the patients.
Ischaemic and critical neuropathic lesions were treated. The medical criteria to identify patients as candidates for the treatment were grade 3 or 4 ulcers or amputation residual bases, as defined by the Wagner’s classification (24). All the ulcers had to exceed 20 cm2 and 25 days of torpid evolution after hospital admission, with granulation failure and atonic contours aspect following repeated sharp debridements and conventional treatment. Amputation residual bases had to show clinical evidences of ischaemia and exceed 30 cm2 and 15 days without improvement after frequent debridements and other conventional approaches.
In addition, patients had to meet one of the following criteria:
-
1
Target lower extremity with an inadequate blood perfusion as shown by an ankle–brachial index pressure (ABI) <0·7 and a transcutaneous oxygen tension below 30 mmHg, absence of palpable posterior tibialis or dorsal pedis pulse.
-
2
Patients with ABI >0·7 but being considered difficult cases with a high risk of amputation due to extensive neuropathy, failing to granulate with standard wound care and with previous amputations.
No distinction was given to the topographic localisation of the wound on the lower extremity. Patients were included irrespective of their diabetes evolution time and type, specific medication for the diabetes control, gender or age. Patients with malignant diseases as well as pregnant or nursing women, patients with autoimmune diseases (except for type 1 diabetes) or psychiatric disorders or those receiving corticosteroids medication were excluded.
Treatment
Human EGF was produced by recombinant DNA technology using Saccharomyces cerevisiae at the Center for Genetic Engineering and Biotechnology, Havana. EGF was formulated in saline according to international quality standards for its parenteral administration (25). Vials containing 25 μg of EGF in 1 mL were diluted with 4 mL of normal saline before use. Before initiating administration of EGF, the lesions were sharply debrided in order to remove callus, fibrin and necrotic material and washed with normal saline. The EGF solution was injected using a standard disposable syringe with a 27G × 0·5″ insulin needle, first into the dermo‐epidermal junction at equidistant points all over the lesion contours and then deeply downward into the wound bottom in circles and centripetally to ensure a uniform distribution. Two hundred and fifty microlitres was instilled at each point. Then, the wounds were dressed with sterile gauze. Infiltrations were performed thrice weekly on alternate days up to the eighth week or less if complete granulation was achieved.
Concomitant therapy included bed rest and adequate control of infection, if present, using either topical or systemic antibiotics. Besides, all the patients received appropriate medical treatments according to their individual diabetes‐associated comorbidity.
Evaluation
At each therapy session, the lesions were examined and the outgrowth of the granulation tissue and the contours aspect was evaluated. The lesions were measured weekly, after debridements. Punch biopsies (2 mm) were collected prior to the first treatment and immediately before the eighth infiltration session. Samples were fixed in 10% buffered formalin, paraffin‐embedded and stained with hematoxylin/eosin and Masson’s trichrome staining for collagen.
Endpoint of primary efficacy was the outgrowth of a granulation tissue suitable to sustain either spontaneous reepithelialisation or a split‐thickness skin autograft, which at the end concurred to preserve the lower extremity. The patients’ out‐hospital evolution began 1 week after discharge from the hospital, and the patient was followed thereafter for 12 months in order to monitor in situ wound recurrence.
Local adverse events were monitored after each infiltration session. Systemic events and vital signs were monitored daily. Clinical laboratory measurements (haemoglobin, leukocyte and platelet counts, total and fractioned proteins, creatinine, cholesterol, transaminases) were done before treatment and after the last infiltration session. Glycemia was monitored weekly. Recurrences and other long‐term adverse events were checked during the patients’ 1‐year external follow‐up.
Results
Efficacy
Twenty‐nine patients were included. Their demographic and baseline characteristics are shown in Table 1. Most of the patients were type 2 diabetics with a long disease history. All suffered from different diabetes‐associated comorbidities. The median size of the lesions was 36 cm2, largely predominating the ischaemic form and Wagner’s grade 4.
Table 1.
Characteristics of patients and lesions
| Parameter | Description | ||
|---|---|---|---|
| Age (years) | 63·5 (median) | 46 (minimum) | 85 (maximum) |
| Gender (female/male) | 18/11 | ||
| Diabetes type 1/2 | 1/28 | ||
| Diabetes history (years) | 20·5 (median) | 5 (minimum) | 35 (maximum) |
| No. of ulcers/amputation residual bases treated | 12/17 | ||
| Lesion history (days) | 37 (median) | 15 (minimum) | 62 (maximum) |
| Lesions size (cm2) | 36·1(median) | 21 (minimum) | 78·8 (maximum) |
| No. of ischaemic/neuropathic lesions | 23/6 | ||
| No. of Wagner grade 3/4 | 10/19 | ||
| Most frequent comorbidities (%) | Ischaemic cardiopathy (63·6) | ||
| Arterial hypertension (63·6) | |||
| Diabetic nephropathy (45·4) | |||
Figure 1 shows the course of the study. One patient withdrew before the eighth infiltration session due to an acute severe adverse event (chest pain). Three individuals did not show development of granulation tissue at the eighth infiltration session; eight other patients did not complete the 8‐week treatment schedule (five abandoners and three withdrawn due to sepsis). All these patients had an unfavourable progress and were amputated. The rest (n= 17) who completed 24 EGF infiltrations healed.
Figure 1.
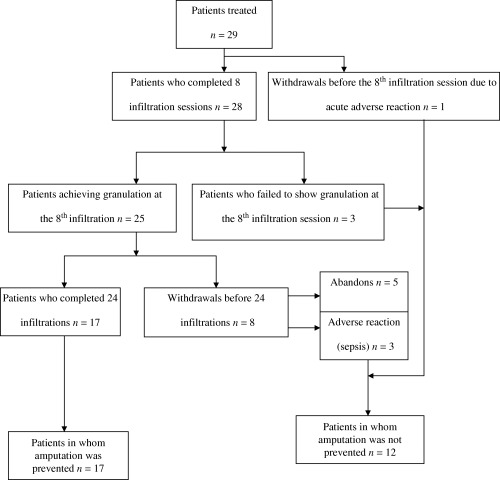
Schematic of patients’ outcome.
A steady granulative response was achieved in 25 of the 29 patients at infiltration session 8, including those with confirmed lower limb hypoperfusion and with a history of insufficient healing process. Seven of them had undergone a contra‐lateral amputation during the previous 3 years. Besides the patient who withdrew because of the adverse event, the other three who did not show response at this point were ischaemic.
Two main clinical changes were noted proportional to treatment progression. For ischaemic lesions, the neo‐formed granulation tissue turned to bleed normally, whereas for neuropathics, granulation was accelerated while appearing progressively consolidated and not dehiscent.
The five patients who refused to continue with the treatment group were ischaemic and complained of spontaneous pain at rest. They abandoned after the tenth infiltration session having a productive granulation and finally concurred to lower extremity amputation. Of the 29 patients included, potentially minor or major amputation was prevented in 17 (58·6%) of them who completed the 24‐session treatment. From this group, 2 patients received autologous split‐thickness skin graft due to their wound dimension, whereas the other 15 patients reepithelialised spontaneously. The mean in‐hospital time for the 17 non amputated patients was 66·5 ± 4·9 days, with an average 71·1 ± 18·3% repithelialisation of the wounded area at discharge. During the 12‐month follow‐up as outpatients, only one subject showed in situ ulcer recurrence, 10 months after full reepithelisation.
Granulation tissue appeared with a consolidated aspect at the eighth infiltration session (third week of treatment) for both ischaemic and neuropathic patients. These changes are supported by histological findings in both ischaemic and neuropathic lesions (Figure 2). For the former, abundant functional capillaries started to emerge and vascular endothelial nuclei were clearly less hypertrophic. For neuropathics, a substantial qualitative transformation in collagen bundles thickness and compactness was noted as compared to the histological aspect predominating in ischaemic and neuropathic lesions before treatment (Figure 2). For both type of lesions, inflammatory infiltration appeared far much attenuated.
Figure 2.
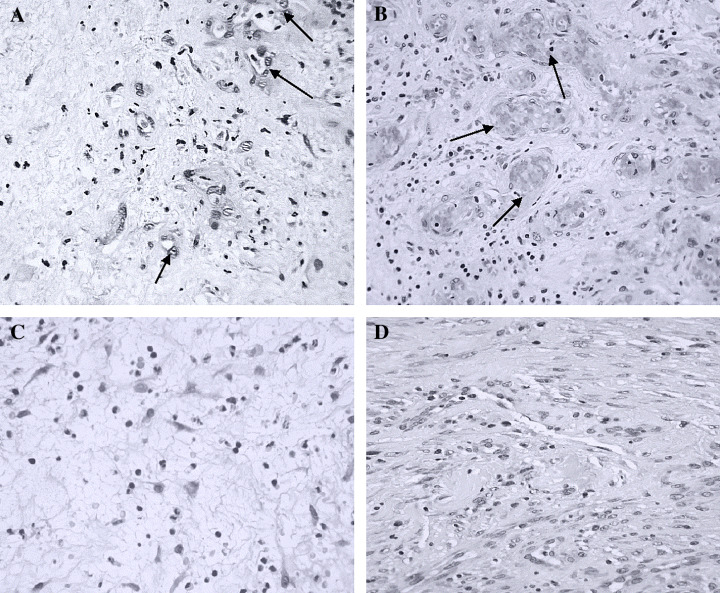
Histological aspects representative of ischaemic and neuropathic lesions before and after epidermal growth factor (EGF) local infiltrations. Representative images of the normal appearance of ischaemic (A, B) and neuropathic (C, D) lesions. Samples were collected just before the first EGF infiltration (A, C) and during the third week of treatment, eighth infiltration session (B, D). (Hematoxylin/eosin staining, magnification ×10.) (A) Ischaemic lesion before treatment. Wound matrix and its cellularity appear disorganised, with unfunctional small vessels exhibiting prominent endothelial nuclei (indicated by arrows). Irreversible nuclear changes (pyknosis) are abundant. (B) Ischaemic lesion at the eighth infiltration session. The wound matrix appears consolidated and organised, and with a number of well‐shaped vessels showing luminal blood (indicated by arrows). (C) Neuropathic lesion before treatment. It is characteristic of the lesion the absence of collagen bundles and the spider web image of the matrix. The reduced number of fibroblastic cells is remarkable. (D) Neuropathic lesion at the eighth infiltration session. The matrix appears dense, consolidated and indurated by compact collagen material. The presence of fibroblastic cells appears largely increased.
Safety
Except for neuropathic patients, all others complained of pain during the infiltrations and discomfort thereafter for 1 to 2 hours. Sepsis led to withdrawal of three patients after the 11th infiltration session despite advanced granulation. Major debridements (two patients) or trans‐metatarsal amputation (one patient) had to be practiced. A serious adverse syndrome was observed in one patient at the second infiltration session, characterised by chest pain, transient fever, muscular tremor, dizziness and vomits. Treatment was discontinued as well. No distinguishable changes were detected in haematological and blood chemistry parameters comparing pre‐treatment to end‐of‐treatment levels (data not shown).
Discussion
To our knowledge, this is the first report on the clinical assessment of an intralesional infiltration of a growth factor in critical and extensive diabetic lower extremity lesions. Although the data presented are not derived from a placebo‐controlled clinical trial, it is worthy to communicate the efficacy obtained with a series of EGF intralesional infiltrations in a small number of patients affected with diabetes, prone to amputation due to the lesions of the lower extremity being extensive and recalcitrant to standard wound care resources lesions.
Despite the limitations of the data, the fact that 86% of the patients, most of them ischaemic, showed a productive granulation at the eighth infiltration session and that amputation was prevented in 58% of the study population remarks the efficacy of the infiltrative intervention. Its relevance is more significant when considering that it was a heterogeneous population of patients with diabetes, with complex wounds, most of them affected by a historic healing deficit, sustaining long evolving diabetic comorbidities. It seems that the eighth infiltration time point evaluation can be useful to indicate responsiveness or refractoriness to treatment because the presence of granulation tissue at this point correlated with the final outcome.
The fact that the treatment proved to be tolerable and safe at long term, and that ulcer recurrence was an exceptional finding after 1 year of outpatient evolution supports the clinical reliability of the EGF infiltrations. Adverse reactions were mild or moderate. Only in one case a severe event, probably related to treatment, occurred and the patient was withdrawn.
Comparisons with other recent studies on diabetic wound healing appear limited because in those where the efficacy of platelet‐derived growth factor (PDGF) (9), EGF (10), living human skin equivalent (11) and dermal substitute (12) was shown, the target ulcers were not ischaemic, all were milder than Wagner’s grade 3 and the size of the lesions was far smaller than those treated in this study.
The infiltrations seemed to transform the diabetic wound matrix, resulting in a salutary clinical effect. Although the basic mechanisms whereby the EGF infiltrations stimulated the healing process were beyond our scope, its mechanistic support may be within what has been already described. It is likely that substantial differences exist between the pharmacodynamic profile of topical and locally injected EGF. Previous studies did not show a significant healing enhancement effect for topically administered EGF in controlled acute wounds in healthy volunteers (26). The infiltration modality efficacy may theoretically rely on the assumption that the growth factor was made readily available in a more direct, integral and active form to target cells in deeper strata, circumventing its degradation by local wound surface proteases (27). Additional hypothetical explanations are that (i) EGF receptor may be more functionally active in non superficial zones of the chronic wound, (ii) the in situ infiltrated EGF may down‐regulate the expression of pro‐inflammatory cytokines (28) that exert a catabolic effect against the wound matrix (29), and/or (iii) EGF triggered cyto‐protective mechanisms (30) on the wound‐resident cells, particularly in ischaemic lesions, which would allow for resuming proliferation, migration and a neo‐matrix secretion. The later arguments are known attributes of most growth factors and appear justified according to the histopathological and clinical findings. The cellularity of the granulation tissue definitively increased in deep biopsy layers, with abundant fibroblastic cells exhibiting a secretor phenotype, yielding an appropriate extracellular matrix that expanded upwards facilitating detachment of fibrin and tissue detritus and turning the debridements manoeuvre less frequent and more conservative.
In summary, the finding that the EGF intralesional infiltrations enhance the healing of recalcitrant ischaemic and of complicated and extensive neuropathic diabetic lower extremity lesions offers new hopes for the diabetic wound healing armamentarium. It confirms the role of EGF as potent tissue repair agent under particular delivery circumstances. In order to determine optimal doses and make the EGF infiltration procedure less discomforting further studies appear to be warranted.
Acknowledgements
Authors express their gratitude to Prof. I. Kelman Cohen and Prof. Gregory S. Schultz for their assistance and encouragement.
References
- 1. Apelqvist J, Ragnarson‐Tenvall G, Larsson J, Person U. Long‐term costs for foot ulcers in diabetic patients in a multidisciplinary setting. Foot Ankle Int 1995;6:388–94. [DOI] [PubMed] [Google Scholar]
- 2. Most RS, Sinnock P. The epidemiology of lower extremity amputations in diabetic individuals. Diabetes Care 1983;6:87–91. [DOI] [PubMed] [Google Scholar]
- 3. Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care 1990;13:513–21. [DOI] [PubMed] [Google Scholar]
- 4. The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. New Engl J Med 1993;327:977–86. [DOI] [PubMed] [Google Scholar]
- 5. Goova MT, Li J, Kislinger T, Qu W, Lu Y, Bucciarelli LG, Nowygrod S, Wolf BM, Caliste X, Yan SF, Stern DM, Schmidt AM. Blockade of receptor for advanced glycation end‐products restores effective wound healing in diabetic mice. Am J Pathol 2001;159:513–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thornalley PJ. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch Biochem Biophys 2003;419:31–40. [DOI] [PubMed] [Google Scholar]
- 7. Weiman TJ, Griffiths GD, Polk HC Jr, Ashmore JD, Ryan B, Das SK, Classen J, Johnson C. Management of diabetic midfoot ulcers. Ann Srug 1992;15:627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palumbo PJ, Melton LJ III. Peripheral vascular disease and diabetes. In Harris MI, Hamman RF., editors. Diabetes in America. Washington, DC: Government Printing Office, 1985;1–21. [Google Scholar]
- 9. Weiman JT, Smiell JM, Su Y. Efficacy and safety of topical gel formulation of recombinant human platelet‐derived growth factor BB (Becaplermin) in patients with chronic neuropathic diabetic ulcers. Diabetes Care 1998;21:822–7. [DOI] [PubMed] [Google Scholar]
- 10. Tsang MW, Wong WKR, Hung CS, Lai K‐M, Tang W, Cheun EYN, Kam G, Leung L, Chan CW, Chu CM, Lam EKH. Human epidermal growth factor enhances healing of diabetic foot ulcers. Diabetes Care 2003;26:1856–61. [DOI] [PubMed] [Google Scholar]
- 11. Veves A, Falanga V, Armstrong DG, Sabolinski ML. Is: a prospective randomized multicenter trial. Apligraf Diabetic Foot Ulcer Study. Graftskin, a human skin equivalent, is effective in the management of non‐infected neuropathic diabetic foot ulcer. Is: a prospective randomized multicenter trial. Diabetes Care 2001;24:290–5. [DOI] [PubMed] [Google Scholar]
- 12. Narston WA, Hanft J, Norwood P, Pollak R., for the Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetes foot ulcers. Diabetes Care 2003;26:1701–5. [DOI] [PubMed] [Google Scholar]
- 13. Berlanga J, Moreira E, Perez L, Boix E, Gonzalez T, Lopez‐Saura P. Wound healing promotion in rats treated with EGF is dose dependent. Biotecnologia Aplicada 1996;13:181–5. [Google Scholar]
- 14. Berlanga J, Lodos J, Labarta V, Merino N, Gonzalez T, Hayes O, Puentes P, Lopez‐Saura P. The effect of the epidermal growth factor treatment schedule on the healing of full‐thickness wounds in pigs. Biotecnologia Aplicada 1997;14:163–8. [Google Scholar]
- 15. Prats PA, Castañeda LO, Falcón V, Ortega R, De La Rosa MC, Menéndez I, Labarta V, Gómez R. Efecto del factor de crecimiento epidérmico sobre la regeneración del nervio ciático transectado en ratas. Biotecnologia Aplicada 1998;15:237–41. [Google Scholar]
- 16. Barroso MC, Díaz C, Alsina S, Areces F, Vázquez E. Human recombinant epidermal growth factor in the treatment of radiogenic ulcers. Biotecnología Aplicada 1993;10:12–7. [Google Scholar]
- 17. Quiñones M, Mc Cook J, Zacca E, Martínez MA. Efecto del Factor de Crecimiento Epidérmico humano recombinante sobre las úlceras de miembros inferiores causadas por insuficiencia venosa crónica. Progresos en Ciencias Médicas 1991;5:11–4. [Google Scholar]
- 18. Brown GL, Nanney LB, Griffith J, Schultz GS. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med 1989;321:76–9. [DOI] [PubMed] [Google Scholar]
- 19. Borges HA, Martínez A, López LD, Ung EV, Wade MM, Mella CM, Hernández F, González T, López‐Saura PA. El Factor de Crecimiento Epidérmico humano recombinante acelera la cicatrización de quemaduras en niños. Estudio a doble ciegas. Biotecnología Aplicada 1994;3:22–8. [Google Scholar]
- 20. Martínez A, López LD, Pérez R, Antón J, Agüero MM, Peón O, Taquechel L, López‐Saura PA. Uso del Factor de Crecimiento Epidérmico humano recombinante en crema de Sulfadiacina de Plata en el tratamiento de pacientes quemados. Biotecnología Aplicada 1994;11:124–30. [Google Scholar]
- 21. Sinha A, Nightingal J, West K, Berlanga‐Acosta J, Playford RJ. Epidermal growth factor enemas with oral mesalamine for mild‐to‐moderate left‐sided ulcerative colitis or proctitis. N Engl J Med 2003;349:350–7. [DOI] [PubMed] [Google Scholar]
- 22. Palomino A, Hernandez‐Bernal F, Haedo W, Franco S, Mas JA, Fernandez JA, Soto G, Alonso A, Gonzalez T, Lopez‐Saura P. A multicenter, randomized, double‐blind clinical trial examining the effect of oral human recombinant epidermal growth factor on the healing of duodenal ulcers. Scand J Gastroenterol 2000;35:1016–22. [DOI] [PubMed] [Google Scholar]
- 23. Berlanga J, Lodos J, Reyes O, Infante JF, Caballero E, López‐Saura P. Epidermal growth factor stimulated re‐epithelialization in pigs. The possible role of acute‐wound proteases. Biotecnología Aplicada 1998;15:83–7. [Google Scholar]
- 24. Wagner FW. Algorithms of diabetic foot care. In Levin ME, O’Neal FW, editors. The Diabetic Foot. St Louis, MO: Mosby, 1983;290–5. [Google Scholar]
- 25. Gonzalez G, Crombet T, Torres F, Catala M, Alfonso L, Osorio M, Neninger E, Garcia B, Mulet A, Perez R, Lage R. Epidermal growth factor‐based cancer vaccine for non‐small‐cell lung cancer therapy. Ann Oncol 2003;14:461–6. [DOI] [PubMed] [Google Scholar]
- 26. Cohen IK, Crossland MC, Garrett A, Diegelmann RF. Topical application of epidermal growth factor onto partial‐thickness wounds in human volunteers does not enhance reepithelialization. Plast Reconstr Surg 1995;96:251–4. [DOI] [PubMed] [Google Scholar]
- 27. Bennett NF, Schultz GS. Growth factors and wound healing. Part II: role in normal and chroci wound healing. Am J Surg 1993;166:74–81. [DOI] [PubMed] [Google Scholar]
- 28. Berlanga J, Prats P, Remirez D, Gonzalez R, Lopez‐Saura P, Aguiar J, Ojeda M, Boyle JJ, Fitzgerald AJ, Playford RJ. Prophylactic use of epidermal growth factor reduces ischemia/reperfusion intestinal damage. Am J of Pathol 2002;161:373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maish GO, Shumate ML, Ehrlich HP, Vary TC, Cooney RN. Interleukin‐1 receptor antagonist attenuates tumor necrosis factor‐induced alterations in wound breaking strength. J Trauma-Injury Infect Crit Care 1999;47:533–7. [DOI] [PubMed] [Google Scholar]
- 30. Berlanga J, Caballero E, Prats P, López‐Saura P, Playford RJ. The role of epidermal growth factor in cell and tissue protection. Med Clin (Barcelona) 1999;113:222–9. [PubMed] [Google Scholar]


