Abstract
Rothmund‐Thomson syndrome (RTS) is a rare genodermatosis with characteristic skin changes such as atrophy, abnormal pigmentation and telengiectasias, skeletal abnormalities, short stature, juvenile cataract and predisposition to skin and bone malignancies. Data from the literature suggest that cutaneous findings of the syndrome include genetically programmed ageing changes and DNA repair abnormalities related to photosensitivity. Our patient is a 23‐year‐old male who presented with an unhealing ulcer for one and a half year on his left leg. Although he had received many various treatments, there had been no significant improvement during this period. We believe that this failure of healing might be to DNA repair abnormalities of fibroblasts. To our knowledge, this is the first case reported with coexistence of an unhealing ulcer without any findings of malignancy and RTS.
Keywords: Genodermatosis, Leg ulcer, Rothmund‐Thomson syndrome
INTRODUCTION
Rothmund‐Thomson syndrome (RTS) is an inherited autosomal recessive disorder characterized primarily by dermal and extradermal abnormalities such as poikilodermatous skin changes, juvenile cataract, hair and nail dystrophies, short stature, bone defects and hypogonadism 1, 2, 3, 4. Two hundred and sixty cases with variable clinical presentations and different associations have been reported and heterogeneity of the skin abnormalities has been highlighted in the literature. Leg ulcer because of the syndrome is extraordinarily rare. There is only one case reported in the literature, which had had shallow leg ulcers related to antiphospholipid syndrome. We present a case of RTS with chronic leg ulcer refractory to various therapies. No other disease or malignity was detected in this patient.
CASE REPORT
A 23‐year‐old male patient, diagnosed with RTS, attended to our outpatient clinic with complaint of unhealing wound on his left leg. In his medical story, the wound had arised following a trauma, and had been existed for over one and a half year. Over this period, he had received various local therapies such as creams, wound dressings (ComfeelR) and hyperbaric oxygen (55 sessions) suggested by a general surgeon, but no improvement could be obtained. The wound had gradually continued to enlarge.
The patient is an offspring of a consanguineous marriage. All findings had started since 6 months after birth. He had a medical history of sun intolerance and worsening episodes of erythema when he was exposed to sun. His physical examination showed saddle nose, maxillary hypoplasia, micrognathia, proptotic eyeballs, short stature, small hands and feet, pes planus, zygodactyly between second and third digits and anonychia. Some fingers and toes were tapered distally (Figure 1A and B). The muscles of the extremities were atrophic, especially on the distal regions. Bone X Ray showed osteopenia of all bone structures. Hypogonadism existed and secondary sex features were poorly developed. Neurological, cognitive assessments showed normal results and intelligence was normal as well. Femoral, popliteal and pedal pulses were palpable in both lower extremities. He had normal karyotypes in cytogenetic analysis.
Figure 1.
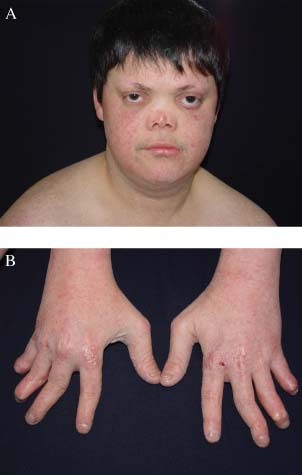
(A) Characteristic face appearance, poikilodermic skin changes on the cheeks, saddle nose, propitotic eyeballs, and lateral alopecia of the eyebrows. (B) Small hands, swan‐neck deformity, and dystrophic fingers and nails.
On dermatological examination, widespread poikiloderma (hypo/hyperpigmentations, atrophy, telengiectasias) was observed all over the skin. Mild erythema was apparent on sun‐exposed areas. An irregular shaped, sharp‐bordered, 7·5 × 9·5‐cm‐sized ulcer with yellow exudate was noted on the medial aspect of the left distal leg. Another 1 × 1‐cm‐sized ulcer was on the dorsum of the right foot and had crust. Both ulcers had red granulation tissue at their base and undermining of the wound edge was not present. The ulcers were surrounded with an erythematous, scaly, atrophic and stiff skin with palpation. Alterations related to varicose veins in the lower extremities such as oedema, skin pigmentation visible blood vessels or telengiectasias were not observed (Figure 2A). The patient did not suffer from pain or discomfort in rest or in walking.
Figure 2.
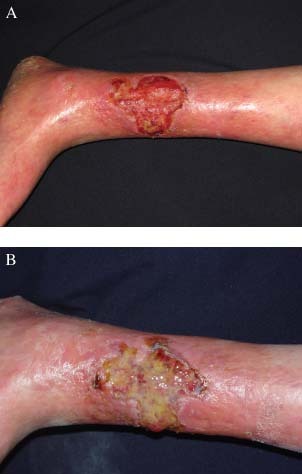
(A) Initial appearance of the leg ulcer. (B) The appearance of the leg ulcer with purulent material after 1 month’ treatment. No remission exists.
Routine haematological and biochemical examinations including some serological tests for vascular collagen disease (anticardiolipin antibodies) were all in the normal limits except mildly higher C reactive protein (CRP). Arterio‐venous doppler ultrasonography (USG) showed normal results in both arterial and venous system; any intraluminary pathology, narrowing in the blood vessel lumens or increased blood flow were not detected. Triphasic flow patters were seen in both vascular systems. Additionally, ankle‐brachial index was normal (1.16).
Bacterial investigations of the wound were performed twice during the treatment and follow‐up periods, and meticilline‐sensitive Staphylococcus aureus and pseudomonas were once cultured. Systemic antibiotherapy was started and also various wound dressings were applied It responded to the therapy initially and epithelization was observed with reduced size of the wound on the follow‐up. The patient was discharged. However, 1 month later, yellow–green purulent discharge, bad‐odour and pain re‐occurred and microbiological reinvestigation showed meticilline‐resistant Staphylococcus aureus and Haemophilus influenza this time (Figure 2B). Culture antibiogram was performed; ampicillin sulbactam and teicopianin were started and the patient was re‐hospitalized for close observation. Infection improved with additional wound care in a week. Later, wound dressing (EpigardR) was reapplied and the size of the ulceration decreased only very little in 1 month. Size of the ulcer has gradually decreased in months, although it has been a slow process. However, the patient is still in the follow‐up period for 8 months. Topical epithelizating creams and wound dressings have been used changeably. Although infection did not ever recur, complete closure has yet not occurred.
A 4 mm punch biopsy from the arm skin and incisional biopsies from the ulcer were obtained. Pathological examination of the ulcer showed hyperkeratosis, polymorphonuclear leucocytes in the keratin layer, irregular acanthosis and mild spongiosis of the epidermis, oedema in all dermis, proliferation of capillary vessels, mixed type inflammatory cell infiltration, fibrin deposits in vessels and among thickened collagen bundles. No neoplastic change was observed in this specimen (Figure 3A). The punch biopsy of poikilodermic and non ulcerous arm skin showed significant atrophy in the epidermis, occasionally hydropic alterations in stratum basale and rare lymphocytes, dilatation of the vessels and thick collagen bundles in the dermis (Figure 3B).
Figure 3.
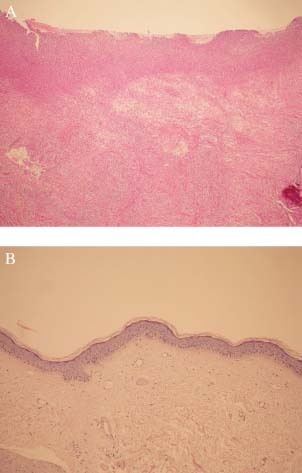
(A) Histopathological findings of the ulcer (HEx40). (B) Histopathological findings of the non ulcerous and atrophic skin of the arm; Atrophic epidermis and regionally sclerotic dermis are noticeable (HEx40).
DISCUSSION
The patient presented chronic leg ulcers associated with typical phenotypical characteristics of RTS. RTS is a highly heterogenous syndrome regarding clinical signs. Given its historical background, we can see that the first description of the syndrome made by an ophthalmologist, Auguste Rothmund, in 1868 was based on only two characteristics as juvenile cataract and a particular rash. After a half‐century, British dermatologist Sydney Thomson reported some patients with a similar rash, which he described as poikiloderma congenitale, and also with skeletal abnormalities without cataracts. The final status of the syndrome was described by William Taylor and these two disorder were united as ‘RTS’ in 1957 (2). Since then, 260 cases have been described, some of which had new features and also associations such as predisposition to skeletal and/or skin malignancies, Klippel‐Feil syndrome, late‐onset, etc. 2, 3, 4, 5, 6, 7, 8, 9. Among them, particularly, skin cancers and osteosarcomas are noteworthy 2, 3, 5, 6, 7, 8. Some cytogenetic studies from patients with RTS showed clonal/non clonal structural abnormalities of chromosome 8 and presence of DNA repair defect in lymphocytes and fibroblasts. Chromosomal or genomic instability and abnormal DNA repair have been blamed for both premature aging and increased risk of skin and skeletal cancers in these patients 2, 3, 4.
A chronic leg ulcer in a patient with RTS was striking regarding a skin cancer in the beginning. The wound had been highly resistant to various therapies in a very long treatment period. Hyperbaric oxygen had been suggested by a general surgeon and he had received this therapy in a hyperbaric oxygen therapy (HOT) center. The complete closure of the wound could have never been achieved despite HOT, wound dressings, and topical epithelizating creams which had been tried along with HOT therapy. The possibility of squamous epithelial skin cancer or basal cell cancer for his unhealing ulcer, we considered initially, was excluded following hospitalisation of the patient in our clinic, because no microscobic finding about cancer existed on punch and incisional biopsies.
The wound healing is a complex and dynamic biological process that consists of inflammatory, fibroblastic and scar maturation phases. Collagen is the principal protein providing structure, strength and stiffness to dermal tissue. It is synthesized by mostly dermal fibroblasts and plays a role in proliferative and remodelling phases of wound healing as an essential protein. Wound healing is affected by numerous factors such as blood supply, available proteins, minerals, aminoacids and enzymes, circulating hormones, mechanical stress and infection as well (10). Generally, age‐related physiological changes of skin morphology such as dryness and atrophy may lead to modified cellular responses to injury and make older skin more sensitive to damage and traumas. Wound closure is achieved less frequently in elderly patients compared with younger patients because of numerous factors consisting of delayed epithelization, reduced collagen synthesis and angiogenic activity, etc. (11).
In this case, the infection was controlled by antibiotics and topical care. Although infections can be considered a reason for delayed healing of the wound, we saw infection only two times in 8 months. The first one was when he was admitted to our clinic and the second one was in the follow‐up period after his first discharge. The patient was not on our close observation in these periods, thus we are not sure of appropriate wound care. We used wound dressings for given periods, changeably with conventional therapy including creams, gauzes, etc., after the control of infections in both conditions. More accelerated healing of the wound has occurred after application of the wound dressing, although they were not enough to close the wound completely.
No abnormality of vascular system was established as confirmed by clinical examination, doppler ultrasonography and ankle‐brachial test. Neither findings of venous insufficiency such as pitting oedema, stasis dermatitis and/or skin discolouration, aching nor signs implying arterial insufficiency such as cold limb, pain with the leg elevation, etc. were found. Thus, we excluded the diagnosis peripheric arterial disease. Moreover, electromyography (EMG) and neurological examinations helped us to rule out ulcers because of neuropathic disorders.
Although we did not make fibroblast culture, it may be proposed that chronic leg ulcer is related to reduced DNA repair capacity, chromosomal instability of fibroblasts and lymphoblasts and reduced restoration of skin cell structures and layers, particularly faulty collagen. There are some evidence supporting this idea. Kerr et al. (4) indicated that there is some phenotypic overlap between RTS and some syndromes showing chromosomal instability and premature aging. They suggested that RTS patients may have macroscobic and microscobic alterations of both photodamaged skin and the intrinsically aged skin possibly associated with faulty DNA repair. Some clinical manifestations of their patients with RTS such as atrophy, erythema, poikilodermia, and photosensitivity were well‐matched with microscobic features occurring with acellular, avascular skin, reduced collagen and/or thick collagen bundles throughout the dermis, mild perivascular inflammation and irregular proliferations of capillary vessels. Miozzo et al. (12) considered RTS as a chromosomal instability syndrome characterized by senescence of cultured fibroblast, like Werner syndrome, Bloom syndrome and Cockayne syndrome. They concluded that some abnormalities of mesenchimal tissues such as poikiloderma, bone cysts and neoplasms are derived from this chromosomal instability.
There is only one case of RTS reported with leg ulcer in the literature. Nevertheless, this case was different from ours in clinic and aetiology. The shallow ulcerations were associated with atrophy blanche and raised anticardiolipin antibodies. The authors suggested that these associations were coincidental (1). However, Werner syndrome, one of premature aging syndromes, has been reported as a rare cause of chronic leg ulcers and possible pathogenetic mechanisms have been based on defective DNA metabolism and fibroblast senescence(13).
Although histopathological findings of the non ulcerous skin in our patient were compatible with poikilodermia, those of ulcerous skin were actually reminder of lipodermatosclerosis seen in venous insufficiency, but clinical picture was completely unrelated to venous insufficiency. It may be considered that the histopathological changes resembling lipodermatosclerosis can be derived from disorganized microcirculation, chronic inflammation and sclerotic alterations of collagen fibres in actinically damaged poikilodermic skin.
In conclusion, chronic ulcer or delayed wound healing in this patient has occurred because of photodamaged skin changes and/or intrinsically aged skin changes developed on the genetic background. It is important to prevent these patients from traumas and close follow‐up of wounds for poor healing.
REFERENCES
- 1. Mak RKH, Griffiths WAD. Mellerio. An unusual patient with Rothmond‐Thomson syndrome, porokeratosis and bilateral iris dysgenesis. Clin Exp Dermatol 2006;31:401–3. [DOI] [PubMed] [Google Scholar]
- 2. Wang LL, Levy ML, Lewis RA, Chintagumpala MM, Lev D, Rogers M, Plon SE. Clinical manifestations in a cohort of 41 Rothmond‐Thomson syndrome patients. Am J Med Genet 2001;102:11–17. [DOI] [PubMed] [Google Scholar]
- 3. Stinco G, Governatori G, Mattighello P, Patrone P. Multiple cutaneous neoplasms in a patient with Rothmund‐Thomson syndrome: case report and published work review. J Dermatol 2008;35:154–61. [DOI] [PubMed] [Google Scholar]
- 4. Kerr B, Ashcroft GS, Scott D, Horan MA, Ferguson MW, Donnai D. Rothmund‐Thomson syndrome: two case reports show heterogeneous cutaneous abnormalities, an association with genetically programmed ageing changes, and increased chromosomal radiosensitivity. J Med Genet 1996;33:928–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anbari KK, Ierardi‐Curto LA, Silber JS, Asada N, Spinner N, Zackai EH, Belasco J, Morrissette DJ, Dormans JP. Two primary osteosarcomas in a patient with Rothmund‐Thomson syndrome. Clin Orthop Relat Res 2000;378:213–23. [DOI] [PubMed] [Google Scholar]
- 6. Marín‐Bertolín S, Amorrortu‐Velayos J, Aliaga Boniche A. Squamous cell carcinoma of the tongue in a patient with Rothmund‐Thomson syndrome. Br J Plast Surg 1998;51:646–8. [DOI] [PubMed] [Google Scholar]
- 7. Piquero‐Casals J, Okubo AY, Nico MM. Rothmund‐thomson syndrome in three siblings and development of cutaneous squamous cell carcinoma. Pediatr Dermatol 2002;19:312–6. [DOI] [PubMed] [Google Scholar]
- 8. Gelaw B, Ali S, Becker J. Rothmund‐Thomson syndrome, Klippel‐Feil syndrome, and osteosacoma. Skeletal Radiol 2004;33:613–5. [DOI] [PubMed] [Google Scholar]
- 9. Kumar P, Sharma KP, Gautam RK, Jain RK, Kar HK. Late‐onset Rothmund‐Thomson syndrome. Int J Dermatol 2007;46:492–3. [DOI] [PubMed] [Google Scholar]
- 10. Tuderman‐Bruckner L. Biology of the extracellular matrix. In: Bolognia JL, Jorizzo JL, Rapini RP, editors. Dermatology, Vol. 1, 2nd edn. Edinburgh: Mosby, 2008:1447–1450. [Google Scholar]
- 11. Wicke C, Bachinger A, Coerper S, Beckert S, Witte MB, Königsrainer A. Aging influences wound healing in patients with chronic lower extremity wounds treated in a specialized wound care center. Wound Rep Reg 2009;17:25–33. [DOI] [PubMed] [Google Scholar]
- 12. Miozzo M, Castorina P, Riva OP, Dalpra L, Conti AMF, Volpi L, Hoe TS, Khoo A, Wiegant J, Rosenberg C, Larizza L. Chromosomal instability in fibroblasts and mesenchymal tumors from 2 sibs with Rothmund‐Thomson syndrome. Int J Cancer 1998;77:504–10 [DOI] [PubMed] [Google Scholar]


