Abstract
The purpose of this study is to stress the value of using intermittent pneumatic compression (IPC) in immobile patients. The use of IPC helps prevent limb oedema and the associated skin changes frequently seen on the legs of the immobile patient. Oedema formation is caused by an increase of fluid extravasation, while skin changes including leg ulcers are mainly because of a deficiency of the venous and lymphatic pumps. Conventional compression stockings and bandages impede leg swelling but are less efficient in supporting the deficient veno‐lymphatic pump when patients are unable to move. In this situation, actively compressing the limb using IPC is a very meaningful and effective treatment option. Because of a lack of literature on the specific indication of IPC in immobile patients, experimental studies and randomised controlled trials in similar situations are reviewed. IPC is a very effective although underused treatment modality, especially in immobile, wheelchair‐bound patients. By inflation and deflation of the air‐filled garments, IPC produces cycles of pressure waves on the leg, thus mimicking the working and resting pressures applied by compression bandages. IPC not only reduces leg swelling but also augments the veno‐lymphatic pump, which is essential for the restoration of the damaged microcirculation of the skin.
Keywords: Active compression therapy, Immobility, Intermittent pneumatic compression, Leg oedema, Leg ulcer
Introduction
In correlation with the steady increase in life expectancy in our western world, there is a growing population of patients with restricted or completely absent mobility. These patients are often bound to wheelchairs and are threatened by several immobility‐related medical problems.
Immobile patients sitting still for prolonged periods of time with their legs dependent will experience impairment of the venous and lymphatic return, resulting in swelling of the lower extremity and skin changes. In addition, lack of movement may also create problems for the general circulation, have a negative impact on the joints, promote demineralisation of the bones (1) and may even promote heterotopic ossification (2). Conventional compression therapy (bandaging/hosiery) applies a static force to the tissues of the limb and relies on a change in the patients muscle tone to create the correct sub‐bandage pressure to augment venous and lymphatic return and thus reduces swelling. However, in immobile patients, this change in muscle tone as a result of standing or ambulation is not possible because of the inability of the patient to perform these physical movements. Therefore, the deciding driving force of compression for venous and lymphatic return consisting of pressure waves in response to a change in the muscle tone during movement is missing. Especially in this situation in which the active venous calf muscle pump does not work, the assistance of venous return by intermittent pneumatic compression (IPC) is a rational approach.
Although experience has shown that IPC is a very effective and gratifying treatment modality in immobile patients, no specific references for this indication can be found in the literature. Therefore, the following review is mainly based on experimental studies and theoretical concepts that have shown a wide variety of beneficial effects when using this form of active compression therapy.
The venous and lymphatic return from the lower limbs: A delicate mechanism
The upright position of the human being and the physical force of gravity make the venous return from the leg difficult. In the leg, venous blood has to be pumped up against gravity towards the heart. This is achieved mainly by the action of the venous pump consisting of two major components: the (active) muscle contractions followed by movement of the joints and the (passively reacting) venous valves that prevent retrograde blood flow. Failure of the venous pumping mechanism can occur because of a lack of movement (immobility), an incompetence of the venous valves or a combination of the two (3).
Deficiency of the venous pump because of valvular incompetence
In mobile patients with incompetent venous valves, refluxes of blood in a retrograde direction will prevent the peripheral venous pressure falling with each step. The consequence of this is ambulatory venous hypertension, which is the deciding pathophysiological parameter characterising the severity of venous insufficiency. Blood will only be able to flow from the capillaries and venules into the larger veins if the transport in the larger vein segment is unimpeded. Little is known concerning the role of venous valves within the venules, but it may be assumed that they are of major importance for the development of regional skin changes on the leg. The target organ of all these changes in the venous macrocirculation is the microcirculation.
At this microcirculatory level, increased leakage of fluid containing large molecules like proteins and blood cells will occur and the lymphatics will be stimulated to compensate for this increase of what is called the ‘lymphatic load’. Consequent changes in the tissue can be described as a chronic inflammatory process that is responsible for skin changes including ulceration (4).
Deficiency of the venous pump because of immobility
Already, a stiff ankle is associated with a restriction of the venous pumping efficiency because of a reduction of the active calf muscle pump. It could be shown that a reduced range of ankle mobility is associated with ambulatory venous hypertension and with the clinical severity of venous disease (5).
Sitting still with dependent legs leads to venous stasis. Duplex ultrasonography has shown that leg dependency leads to a haemodynamic impairment of the venous return and reduces blood flow velocity in the deep veins by more than 50% in comparison to the supine position (6). IPC of the foot and calf during sitting simulates the venous calf muscle pump, activated by walking. It can be shown that active compression therapy (IPC) of the leg has a similar physiological impact to walking and leads to a reduction of the peripheral venous pressure (7).
Deficiency of the lymphatic pump because of immobility
Movement of the lower limbs is not only the driving force of the venous pump but also an important mechanism for the lymphatic transport, playing an important role not only for the rhythmic opening of initial lymphatics because of pressure changes in the tissue but also for the spontaneous contractions of the large lymph vessels that are stimulated by muscle contractions (8).
Long‐standing oedema will turn into lymphoedema when the compensatory mechanism of increased lymph drainage is exhausted and the propelling mechanism of rhythmic stimulation on the spontaneous contraction of the lymphatics fails. This will lead to induration of the skin and promote the development of ulceration.
Clinical consequences of venous stasis
Oedema
The physiological deficit of venous and lymphatic flow in the lower extremity because of immobility has been called ‘stasis’. A lack of venous transport in the large veins will lead to a stagnation of blood in the microcirculation with fluid penetrating from the small blood vessels into the tissue causing oedema. This form of swelling is a physiological phenomenon that can be observed in every individual after prolonged sitting. A typical example is the well‐known swelling of the legs after long flights (jet‐leg). Leg oedema in patients with restricted mobility (Figure 1) and especially in wheelchair patients may be extremely severe and may additionally impede already restricted mobility of the ankle joints. Sometimes, the weight of the lower legs increases to the point where the patient can no longer lift the extremity.
Figure 1.
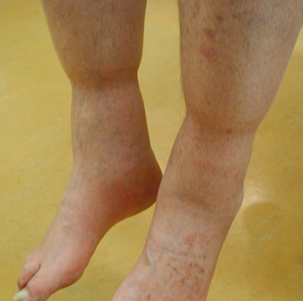
Oedema because of immobility. Swelling of both distal lower extremities in a patient with restricted walking ability because of Parkinson’s disease. As can be seen, the pressure of the upper edges of the socks (about 5 mmHg) is already effective to reduce swelling.
Skin changes
On the lower leg, skin changes include stasis dermatitis (eczema), pigmentation, lipodermatosclerosis and white atrophy, which often ulcerates. A failure of the calf pump owing to immobility causes a steady increase of the venous pressure in the depending parts of the leg. This sustained venous hypertension leads to changes in the microcirculation, which can be described as a chronic inflammation (4). An increased extravasation of fluid from the capillaries into the tissue that cannot anymore be compensated by an increased lymph drainage causes oedema, and the diffusion of large protein molecules stimulates fibrosis. Red blood cells penetrating into the skin are the basis for the developing pigmentation.
Ulcers
The above‐mentioned inflammatory process may be so severe that ultimately the skin ulcerates. In our outpatients, about 70% of leg ulcers are caused by venous insufficiency, 20% are seen in patients with arterial obstructions (frequently combined with venous refluxes) and in approximately 10%, other pathologies are of underlying importance. Gravity plays the deciding role for the prevalence of skin defects on the distal parts of the lower extremity in all sorts of leg ulcers. In immobile patients presenting with leg ulcers, oedema because of stasis will further impede the exchange of nutritional substances between the blood capillaries and the tissue so that the ulcers will not heal (9).
Therapeutic modalities used to reduce oedema and aid venous leg ulcer healing
Diuretics
As long as immobility persists, there will be no chance to eliminate the pathophysiology of stasis and all the clinical consequences that have been described above. In daily practice, diuretics are frequently prescribed to improve leg oedema. It should be considered that long‐term intake of diuretics is contraindicated because it will damage the physiological regulation of the renin–angiotensin mechanism with the consequence of ‘diuretic‐induced’ oedema (10).
Compression stockings
Medical compression stockings have been proved by evidence‐based medicine to be effective in several clinical situations (11). Main indications for light stockings are the prevention of leg oedema after prolonged sitting and standing and venous thromboembolism in bedridden patients. Higher compression classes are used for the maintenance treatment after deep vein thrombosis and lymphoedema to prevent a postthrombotic syndrome and after healing of venous ulcers to prevent recurrence. Especially, after a venous ulcer is healed, the consequent wearing of compression stockings is clearly cost‐effective (12).
However, patient’s concordance to compression hosiery is often less than optimal. In one study, only 52% of the patients reported wearing stockings every day for the first 6 months after their ulcers had healed (13). Two factors distinguished those patients who wore stockings from those who did not 75% of the time: the belief that wearing stockings was worthwhile and the belief that stockings were uncomfortable to wear. In a survey on the use of compression stockings in pregnant women, 18% of the non wearers found them uncomfortable (14). There are no data available that look at the use of stockings for immobile patients and the concordance of this patient group. It may be assumed that uncomfortable feelings will occur especially when the stockings do not fit exactly. This may be extrapolated from studies looking at the use of thromboprophylactic compression stockings in bedridden patients. A reversed pressure gradient has been described, which may exacerbate stasis and oedema (15). Elastic compression stockings with a pressure of more than 20 mmHg may not be tolerated over night because of the too high resting pressure caused by the recoiling force of the rubber‐like elastic fibres; so, they should therefore be removed before going to bed. Sometimes, nursing services are required to apply the stockings in the morning and remove them in the evening. Clearly, such a service is expensive and not cost‐effective.
The findings of several experimental studies have reported that the main action of compression stockings is on leg swelling and the microcirculation of the skin. The pressure of the stockings to effectively narrow the veins in the upright position is too low to produce strong haemodynamic effects (16). This is especially also true for the immobile patient sitting in a wheelchair with dependent legs.
Compression bandages
In contrary to stockings, skilfully applied bandages may stay on the leg for several days. This is especially true for multi‐component bandages with high stiffness. Single‐layer bandages using elastic material are similar to stockings and should be removed over night. There is ongoing debate whether immobile patients would benefit more from elastic than from inelastic material. Recent data showed a superiority of inelastic bandage systems concerning ulcer healing in patients with restricted mobility of the ankle joint (9). In the mobile patient, stiff (inelastic) bandages do not give way during walking. It can be shown that the high interface pressure peaks during walking are able to overcome the intravenous pressure during muscle systole, thereby intermittently occluding superficial and deep leg veins. This periodic compression acts like an artificial valve, leading to a reduction of venous reflux and of ambulatory venous hypertension in patients with venous insufficiency (16).
Compression bandages are the basic conservative treatment modality in patients with leg ulcers. Inelastic bandages and bandage systems are usually applied with considerable pressure and may stay on the leg for several days. However, good bandage application requires well‐trained staff, and the bandage material is not cheap, especially when it cannot be washed after use and has to be discarded. In real life, there is poor consistency concerning the use of correctly applied bandages mainly because of insufficient training, even in experienced centres. The variability of pressure among different bandagers is very high, and there is a considerable chance of too high or too low pressure bandages (17). Bandages applied with a too low pressure (or with the incorrect pressure gradient) may result in suboptimal outcomes for the patient (i.e. poor response to treatment, prolonged treatment times, additional cost, etc.). Applying bandages with too much force (and/or the incorrect pressure gradient) can result in pressure ulceration to the limb or obstruction of the arterial blood flow to the extremities, resulting in tissue necrosis. Hafner et al. showed that after the appropriate training, deviation from the target pressure of 35–45 mmHg reduced from 8·4 (95% CI 0–34·1) to 3·5 mmHg (95% CI 0–14) and that application of potentially dangerous pressures (up to 120 mmHg) could be avoided (17). Another important drawback of compression bandaging is the immediate drop in interface pressure, which can be as significant as a 25% reduction in pressure 2 hours after application. This is especially true of inelastic bandages (18). This drop in pressure is mainly because of a reduction of oedema and needs to be corrected by frequent renewals of the bandage, especially in the initial treatment phase of oedematous limbs. In the immobile patient, a compression bandage of high stiffness that is tolerated day and night will therefore reduce oedema after application as long as the interface pressure exceeds the tissue pressure. As soon as equilibrium is reached, oedema will refill again.
The advantage of bandages that produce high pressure peaks during walking thereby exerting a massaging effect does not come into play in immobile patients. Iglesias et al. reported that ankle mobility is directly correlated to the likelihood of ulcer healing and that people with greater ankle mobility had a higher healing rate regardless of whether a four‐layer or short‐stretch bandage system was used (19). Therefore, the less mobile the patient, the greater the reduction in ankle mobility and the less benefit the patient will receive from these traditional bandage systems. In these patients, an alternative method of limb compression should be considered as a first‐line intervention in patient treatment.
Intermittent pneumatic compression
When dealing with immobile patients or those with impaired mobility, it is imperative to consider which type of compression will help reduce tissue oedema and improve venous return. The use of an intelligent active compression therapy system (IPC) may have benefits above and beyond the use of a conventional (static) compression bandage wrapped around a limb where the calf muscle pump is unable to activate.
Active compression therapy by IPC does not only exert the required pressure to the leg at the correct gradient but also mimics rhythmic muscle contractions in a situation in which such active movements are restricted or entirely absent. IPC can be used either alone on the limb or over compression hosiery or compression bandages. The haemodynamic consequences that can be measured are comparable to those in a walking individual 7, 20. The beneficial effects of IPC have been shown in multiple experiments (21), and examples of the data demonstrating changes of physiological parameters are summarised in Table 1. The experimental set‐ups including the IPC systems used showed a wide variation so that only approximate main results are given.
Table 1.
Effects of IPC on several physiological parameters
| Effects of IPC | Main results of the quoted studies | |
|---|---|---|
| Venous pressure | Decrease (7) | IPC (foot + calf) 120–140 mmHg, frequency of three to four impulses/minute and 1‐second delay provided the most pronounced pressure decrease in healthy limbs |
| Venous velocity | Increase (37) | Significant increase in venous velocity (26 normal individuals, femoral vein) |
| Foot/calf venous volume | Expulsion (38) | Venous volume expelled with IPC (calf) and IPC (foot + calf) was 2·25 and 3–3·5 times than that with IPC (foot) (20 normals, 25 claudicants, femoral and popliteal vein) |
| Skin blood flux | Increase (39) | Increase of laser Doppler flux on the big toe +57–66% (15 patients with arterial occlusive disease and 15 normals) |
| Partial oxygen tension | Increase (39) | Increase of tcPO2 +8% in 15 patients with arterial occlusive disease, +10% in 15 normals |
| Nitric oxide | Increased release (40) | Upregulation of eNOS mRNA expression by a factor of 2·08 ± 0·25 and 2·11 ± 0·21, respectively at 6 hours in cultured human umbilical vein endothelial cells |
| Oedema | Decrease (24) | Highest mean reduction in limb volumes with pressure of 40 mmHg, 10‐second deflation, 15‐second inflation time |
| Fibrinolysis | Increase 41, 42 | tPA increase in normals: 3·8 ± 1·9% |
| Decrease in plasma tPA‐Ag: −12·4 ± 3·8% (normals) | ||
| −17·2 ± 3·1% (patients with postthrombotic syndrome) | ||
| Decrease of PAI‐1‐Ag: −13·4 ± 3·8% (normals) | ||
| −12·0 ± 3·1% (patients with postthrombotic syndrome) | ||
| Suppression of pro‐coagulant activity (TFPI) and enhancement of fibrinolytic activity (tPA) (20 healthy male volunteers) |
IPC, intermittent pneumatic compression; eNOS, endothelial nitric oxide synthase; PAI‐1, plasminogen activator inhibitor 1; TFPI, tissue factor pathway inhibitor; tPA, tissue plasminogen.
Although IPC and compression bandaging are markedly different medical devices, the physiological impact of IPC upon a limb (Table 1) will be very similar to that of conventional compression devices for many of the parameters listed in Table 1, (22). It must be stressed that the majority of research studies concerning the microcirculation and its associated mediators have been undertaken using IPC rather than conventional compression devices. Although it may be assumed that short‐stretch bandages during walking will exert similar effects, clear results are missing.
Numerous IPC systems with various cycle times, inflation pressures and garment design have been developed whose primary aim is to assist venous return. One example of such systems can be seen in Figure 2. In addition to the beneficial effects on venous macrocirculation, several important consequences of IPC in the microcirculation are reported by laboratory‐based measurements and verified by clinical studies (Table 1). These effects result in a wide recognition of this treatment modality in guidelines in which the use of IPC is recommended for several indications (Table 2) (23).
Figure 2.
An example of two current active compression therapy systems that are suitable for the immobile patient.
Table 2.
Indications for active compression therapy (intermittent pneumatic compression) (41)
| Indications | Quality of recommendation |
|---|---|
| Prevention of venous thromboembolism | A |
| Faster healing of venous ulcers | A |
| Subjective symptoms* in postthrombotic syndrome | B |
| Oedema reduction in lymphoedema | A |
| Reduction of posttraumatic and post surgical oedema of the leg | A |
| Improved ulcer healing in diabetic foot | A |
| Reduction of sensory impairment after stroke | B |
| Improvement of arterial circulation in patients with arterial occlusive disease | A |
Typical subjective symptoms assigned to venous pathology are heaviness, swelling and itching, pronounced after long standing or sitting and in a warm environment.
Indications for IPC proved by evidence‐based medicine
Table 2 summarises the Guidelines of the German Society of Phlebology, which are based on a review of randomised controlled trials and meta‐analyses (23). The listed recommendations to use IPC were judged to be of high (A) or medium (B) quality.
No specific randomised controlled trials have been performed in the situation of swollen legs because of prolonged sitting with or without ulceration. The vast majority of clinical trials were performed in the field of thromboprophylaxis after surgery, demonstrating impressive effects by tackling the pathomechanism of stasis. As we know, because of the important work of Rudolf Virchow, the reduction of blood flow velocity (stasis) is one of the three deciding underlying factors triggering thrombosis (the other two being increased blood coagulation and damage of the endothelium). The thromboprophylactic efficacy of IPC in the immobilised patient after surgery may therefore be taken as a model for the haemodynamic effects in patients who are immobilised because of other reasons.
IPC in the home environment
While most of the clinical trials have been performed in hospitals where IPC is used primarily for thromboprophylaxis, the long‐term use of pneumatic compression devices in home care medicine has not become routine up to now. Especially, for patients who are unable to apply or unable to tolerate compression bandaging (or hosiery) and who are sitting in wheelchairs, daily application of IPC for several hours would stimulate blood flow and counteract oedema. Active compression therapy (IPC) can be effectively used either in addition to or instead of compression bandaging/hosiery.
Immobile patients often develop refractory leg oedema, which occurs because of the lymphatic system being unable to cope with the increased lymphatic load resulting in a progressive induration of the tissue. This condition responds poorly to the usual treatment of compression bandaging/hosiery and is a gratifying indication for IPC.
An ideal indication for active compression therapy is the wheelchair‐bound patient with swollen legs. Where additional ulcers present, these will not heal as long as the oedema is present. In this situation, IPC will not only enhance venous blood return but also decrease oedema, both factors that will promote ulcer healing.
Which IPC system for which indication?
There is currently no ‘gold standard’ IPC treatment advocated for any of the medical conditions that can be treated with IPC, but numerous compression profiles (cycle times and inflation pressures) work for a wide range of patients and for a wide range of conditions.
Few studies have compared single‐chamber and multiple‐chamber devices. Also, comparisons between different variables of IPC devices like inflation time, exerted pressure and pressure‐free intervals are sparse 21, 24, 25. Besides thromboprophylaxis in the postoperative phase (26), beneficial effects of IPC have been found in arterial occlusive disease 27, 28, 29, rheumatoid flexion deformities of the knees (30), fractures 31, 32, reduced bone density (33) and venous ulcer healing 34, 35.
The use of active compression therapy (IPC) to simulate the haemodynamic action of normal ambulation and to enable non ambulatory patients to maintain a pulsatile venous blood flow may be achieved by different IPC products that are on the market.
Practical conclusions
Swollen legs in immobilised patients frequently associated with non healing ulcers are a common and costly problem in hospitals, nursing homes and especially in the home environment of elderly patients. The average cost of treating an ulcer has been estimated between EURO 1332 and EURO 2585 in Sweden and from EURO 814 to EURO 1994 in the UK (36). Adequate compression treatment is the therapy of choice. The use of conventional bandages or stockings is often limited by patient mobility, patient concordance and the inability of the patient to apply such devices so that daily nursing services may be required. A very effective alternative that should be considered as a key front‐line treatment is the use of IPC, which applies an active compression to the limb that will not only reduce oedema but also promote venous blood flow and help expedite venous ulcer healing. Evidence‐based medicine data are available showing beneficial effects concerning oedema reduction and ulcer healing when using IPC.
For the situation of the immobilised patient, specific trials are to be recommended.
Principle differences of compression devices in immobile patients
-
•
Compression stockings exert a sustained pressure and are mainly used for maintenance therapy to prevent refilling of the leg with oedema.
-
•
Compression bandages can be applied with a higher pressure and are mainly used for the initial therapy to treat dependency oedema. The main action of bandages to produce high pressure peaks with ankle movement does not work in immobile patients.
-
•
IPC is an intelligent solution in this situation by producing cycles of pressure waves mimicking working and resting pressure of bandages by inflation and deflation of the pump.
References
- 1. Ringe RD. Generalized osteoporosis in chronic polyarthritis – pathomechanisms and treatment approaches. Z Rheumatol 1996;55:149–57. [PubMed] [Google Scholar]
- 2. Hudson SJ, Brett SJ. Heterotopic ossification – a long‐term consequence of prolonged immobility. Crit Care 2006;10:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gardner AMN, Fox RH. The return of blood to the heart: venous pumps in health and disease. London: John Libbey, 1989. [Google Scholar]
- 4. Bergan JJ, Schmid‐Schönbein GW, Coleridge Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med 2006;355:488–98. [DOI] [PubMed] [Google Scholar]
- 5. Dix FP, Brooke R, McCollum CN. Venous disease is associated with an impaired range of ankle movement. Eur J Vasc Endovasc Surg 2003;25:556–61. [DOI] [PubMed] [Google Scholar]
- 6. Delis KT, Knaggs AL, Sonecha TN, Zervas V, Jenkins MP, Wolfe JH. Lower limb venous hemodynamics impairment of dependency: quantification and implications for the economy class position. Thromb Haemost 2004;91:941–50. [DOI] [PubMed] [Google Scholar]
- 7. Delis KT, Azizi ZA, Stevens RJ, Wolfe JH, Nicolaides AN. Optimum intermittent pneumatic compression stimulus for lower‐limb venous emptying. Eur J Vasc Endovasc Surg 2000;19:261–9. [DOI] [PubMed] [Google Scholar]
- 8. Olszewski WL. Lymph stasis: pathophysiology, diagnosis and treatment. Boca Raton: CRC Press, 1991. [Google Scholar]
- 9. Morison MJ, Moffatt CJ, Franks PJ, editors. Leg ulcers. A problem‐based learning approach. Edinburgh: Mosby Elsevier, 2007. [Google Scholar]
- 10. Braunwald E. Edema. In: Isselbacher KJ, Braunwald E, Wilson JD, Martin JB, Fauci AS, Kasper DL, editors. Harrison’s principles of internal medicine, 13th edn. New York: McGraw Hill, 1994:183–7. [Google Scholar]
- 11. Partsch H, editors. Evidence based compression therapy. VASA 2003;32,(Suppl 63):1–39. [Google Scholar]
- 12. Korn P, Patel ST, Heller JA, Deitch JS, Krishnasastry KV, Bush HL, Kent KC. Why insurers should reimburse for compression stockings in patients with chronic venous stasis. J Vasc Surg 2002;35;950–7. [DOI] [PubMed] [Google Scholar]
- 13. Jull AB, Mitchell N, Arroll J, Jones M, Waters J, Latta A, Walker N, Arroll B. Factors influencing concordance with compression stockings after venous leg ulcer healing. J Wound Care 2004;13:90–2. [DOI] [PubMed] [Google Scholar]
- 14. Gray G, Ash AK. A survey of pregnant women on the use of graduated elastic compression stockings on the antenatal ward. J Obstet Gynaecol 2006;26:424–8. [DOI] [PubMed] [Google Scholar]
- 15. Best AJ, Williams S, Crozier A, Bhatt R, Gregg PJ, Hui AC. Graded compression stockings in elective orthopaedic surgery. An assessment of the in vivo performance of commercially available stockings in patients having hip and knee arthroplasty. J Bone Joint Surg Br 2000;82:116–8. [DOI] [PubMed] [Google Scholar]
- 16. Partsch B, Partsch H. Calf compression pressure required to achieve venous closure from supine to standing positions. J Vasc Surg 2005;42:734–8. [DOI] [PubMed] [Google Scholar]
- 17. Hafner J, Luthi W, Hanssle H, Kammerlander G, Burg G. Instruction of compression therapy by means of interface pressure measurement. Dermatol Surg 2000;26:481–6. [DOI] [PubMed] [Google Scholar]
- 18. Larsen AM, Futtrup I. Watch the pressure‐it drops. EWMA J 2004;4:8–12. [Google Scholar]
- 19. Iglesias C, Nelson EA, Cullum NA, Torgerson DJ. VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers. Health Technol Assess 2004;8:1–105. [DOI] [PubMed] [Google Scholar]
- 20. Chen AH, Frangos SG, Kilaru S, Sumpio BE. Intermittent pneumatic compression devices – physiological mechanisms of action. Eur J Vasc Endovasc Surg 2001;21:383–92. [DOI] [PubMed] [Google Scholar]
- 21. Berliner E, Ozbilgin B, Zarin DA. A systematic review of pneumatic compression for treatment of chronic venous insufficiency and venous ulcers. J Vasc Surg 2003;37:539–44. [DOI] [PubMed] [Google Scholar]
- 22. Partsch H. Mechanism and effects of compression therapy. In: Bergan JJ, editor. The vein book. Boston: Elsevier, 2007:103–9. [Google Scholar]
- 23. Wienert V, Partsch H, Gallenkemper H, Gerlach H, Jünger M, Marshall M, Rabe E. Intermittierende pneumatische Kompression. Phlebologie 2005;34:176–80 [WWW document]. URL www.uni‐duesseldorf.de/awmf/II/037‐007.htm [accessed on 8 April 2008]. [Google Scholar]
- 24. Grieveson S. Intermittent pneumatic compression pump settings for the optimum reduction of oedema. J Tissue Viability 2003;13:98–100, 102, 104. [DOI] [PubMed] [Google Scholar]
- 25. Kumar S, Walker MA. The effects of intermittent pneumatic compression on the arterial and venous system of the lower limb: a review. J Tissue Viability 2002;12: 58–66. [DOI] [PubMed] [Google Scholar]
- 26. Nicolaides AN, Fareed J, Kakkar AK, Breddin HK, Goldhaber SZ, Hull R, Kakkar VV, Michiels JJ, Myers K, Samama M, Sasahara A, Kalodiki E. Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence). Int Angiol 2006;25:101–61. [PubMed] [Google Scholar]
- 27. Labropoulos N, Leon LR Jr, Bhatti A, Melton S, Kang SS, Mansour AM, Borge M. Hemodynamic effects of intermittent pneumatic compression in patients with critical limb ischemia. J Vasc Surg 2005;42:710–6. [DOI] [PubMed] [Google Scholar]
- 28. Delis KT, Knaggs AL. Duration and amplitude decay of acute arterial leg inflow enhancement with intermittent pneumatic leg compression: an insight into the implicated physiologic mechanisms. J Vasc Surg 2005;42:717–25. [DOI] [PubMed] [Google Scholar]
- 29. Ramaswami G, D’Ayala M, Hollier LH, Deutsch R. McElhinney AJ. Rapid foot and calf compression increases walking distance in patients with intermittent claudication: results of a randomized study. J Vasc Surg 2005;41:794–801. [DOI] [PubMed] [Google Scholar]
- 30. Majkowski RS, Atkins RM. The treatment of fixed flexion deformities of the knee in rheumatoid arthritis using the Flowtron intermittent compression stocking. Br J Rheumatol 1992;31:41–3. [DOI] [PubMed] [Google Scholar]
- 31. Juliano PJ, Myerson MS, Koman JD. The use of a pneumatic intermittent impulse compression device in the treatment of calcaneus fractures. Mil Med 2000;165:721–5. [PubMed] [Google Scholar]
- 32. Park SH, Silva M. Effect of intermittent pneumatic soft‐tissue compression on fracture‐healing in an animal model. J Bone Joint Surg Am 2003;85:1446–53. [DOI] [PubMed] [Google Scholar]
- 33. Albertazzi P, Steel SA, Bottazzi M. Effect of intermittent compression therapy on bone mineral density in women with low bone mass. Bone 2005;37:662–8. [DOI] [PubMed] [Google Scholar]
- 34. Mani R, Vowden K, Nelson EA. Intermittent pneumatic compression for treating venous leg ulcers. Cochrane Database Syst. Rev. 2008; Apr. 16(2) CD001899. [DOI] [PubMed] [Google Scholar]
- 35. Vowden K. The use of intermittent pneumatic compression in venous ulceration. Br J Nurs 2001;10:491–509. [DOI] [PubMed] [Google Scholar]
- 36. Ragnarson Tennvall G, Hjelmgren J. Annual costs of treatment for venous leg ulcers in Sweden and in the United Kingdom. Wound Repair Regen 2005;13:13–8. [DOI] [PubMed] [Google Scholar]
- 37. Flam E, Berry S, Coyle A, Dardik H, Raab L. Blood‐flow augmentation of intermittent pneumatic compression systems used for prevention of deep vein thrombosis prior to surgery. Am J Surg 1996;171:312–5. [DOI] [PubMed] [Google Scholar]
- 38. Delis KT, Slimani G, Hafez HM, Nicolaides AN. Enhancing venous outflow in the lower limb with intermittent pneumatic compression. A comparative haemodynamic analysis on the effect of foot vs. calf vs. foot and calf compression. Eur J Vasc Endovasc Surg 2000;19:250–60 8. [DOI] [PubMed] [Google Scholar]
- 39. Abu‐Own A, Cheatle T, Scurr JH, Coleridge Smith PD. Effects of intermittent pneumatic compression of the foot on the microcirculatory function in arterial disease. Eur J Vasc Surg 1993;7:488–92. [DOI] [PubMed] [Google Scholar]
- 40. Dai G, Tsukurov O, Chen M, Gertler JP, Kamm RD. Endothelial nitric oxide production during in vitro simulation of external limb compression. Am J Physiol Heart Circ Physiol 2002;282:2066–75. [DOI] [PubMed] [Google Scholar]
- 41. Comerota AJ, Chouhan V, Harada RN, Sun L, Hoisking J, Veermansunemi R, Comerota A Jr, Schlappy D, Rao AK. The fibrinolytic effects of intermittent pneumatic compression: mechanism of enhanced fibrinolysis. Ann Surg 1997;226:306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morris RJ, Giddings JC, Ralis HM, Jennings GM, Davies DA, Woodcock JP, Dunstan FD. The influence of inflation rate on the hematologic and hemodynamic effects of intermittent pneumatic calf compression for deep vein thrombosis prophylaxis. J Vasc Surg 2006;44:1039–45. [DOI] [PubMed] [Google Scholar]


