Abstract
Burn injury is associated with a high incidence of death and disability; yet, its management remains problematic and costly. We conducted this clinical study to evaluate the efficacy of honey in the treatment of superficial and partial‐thickness burns covering less than 40% of body surface area and compared its results with those of silver sulphadiazine (SSD). In this randomised comparative clinical trial, carried out Burn Center of POF Hospital, Wah Cantt, Pakistan, from May 2007 to February 2008, 150 patients of all ages having similar types of superficial and partial‐thickness burns at two sites on different parts of body were included. Each patient had one burn site treated with honey and one treated with topical SSD, randomly. The rate of re‐epithelialization and healing of superficial and partial‐thickness burns was significantly faster in the sites treated with honey than in the sites treated with SSD (13·47 ± 4·06 versus 15·62 ± 4·40 days, respectively: P < 0·0001). The site treated with honey healed completely in less than 21 days versus 24 days for the site treated with SSD. Six patients had positive culture for Pseudomonas aeroginsa in honey‐treated site, whereas 27 patients had positive culture in SSD‐treated site. The results clearly showed greater efficacy of honey over SSD cream for treating superficial and partial‐thickness burns.
Keywords: Burns, Honey, Partial‐thickness burns, Silver sulphadiazine
INTRODUCTION
Burns are very common. Most burns occur in home, with young children being the most vulnerable group. The commonest type of injury is from scalding with hot liquids 1, 2, 3, 4. Despite the evolution of antiseptics, medications and advanced operative procedures, healing of burn injury is still difficult to achieve (5). Burn injury is associated with a significant incidence of death and disability, multiple operative procedures, prolonged hospitalisation and rehabilitation, and high health care costs. Many patients exposed to burn injury are hospitalised for prolonged treatment. More than 60% stay in hospitals for 8–30 days (6). The severity of a burn or scald depends on the depth of the wound and the proportion of the body affected. Burn wounds are very prone to infection, as the warm moist site of the wound presents an ideal environment for bacteria to multiply (7).
A variety of dressings and topical agents (such as creams) are used for burn wounds. The most common dressings used are paraffin‐impregnated gauze, polyurethane film, polyurethane foam and gel‐forming agents known as hydrocolloids. Creams containing silver are also commonly used in the first few days after a burn to reduce the risk of infection.
Honey has traditionally been used for wound healing, and there are biologically plausible reasons why it should be an effective treatment. Its high sugar content gives it the ability to absorb water from a wound (osmolarity), and this deprives bacteria of the moisture they need to thrive. Honey provides a non adherent interface between the dressing and the wound bed, which creates a moist healing environment and prevents the dressing from tearing away the newly formed tissue when removed. There is also evidence from laboratory studies to suggest that honey has antibacterial properties that are due partly to its acidity and partly to phytochemicals obtained from the nectar of particular plants. The antibacterial properties of honey vary according to its source and are often particularly high in the honey gathered from New Zealand's manuka (Leptospermum scoparium) (7).
Realising the potential use of honey in wound healing, we examined the effects of honey versus silver sulphadiazine (SSD) on the rate of burn wound healing, indicated by burn wound size and re‐epithelialization.
PATIENTS AND METHODS
After obtaining approval from the Local Ethical Committee, this clinical trial was carried out in Burn Center POF Hospital, Wah Cantt, from May 2007 to February 2008. The inclusion criteria for the patients with burn wounds in this study were as follows: the burn had to have occurred within 24 h before the initiation of treatment; the patient had to have two burns at the same site, such as on the feet or hands, right or left side of abdomen or chest etc.; the burns had to be of second degree with respect to depth and similar burned surface areas in two different parts of the body and the patients had to have less than 40% total burn surface area (TBSA) burns. Exclusion criteria included known diabetes, immunodeficiency, pregnancy and kidney diseases. Patients with electrical and chemical burns were also excluded. The patients and attendants were given information regarding the honey treatment and written informed consent was obtained from all the patients. Finally, 150 patients were enrolled in this study. All patients were treated with fluid resuscitation, daily dressings and other treatment protocols during their hospitalisation. The age and sex distribution pattern of patients is shown in Table 1. After admission, the wounds were cleaned with water or normal saline solution and the topical agent honey (Langnese – commercially available natural honey) or SSD cream was applied directly to the wound in different parts; for example, a left burned hand was treated with SSD and the right burned hand was treated with honey in the same patient. The dressing was changed and honey was applied twice daily. Treatment with the topical agents was continued until the burns were fully healed and epithelialized. All patients were given oral nutrition with occasional intravenous support in the form of amino acid infusion and blood products during their hospital stay. A wound swab culture was taken after 2 weeks. At the time of each dressing, the wound was observed clinically for signs of infection, size, and rate and nature of epithelialization by an expert surgeon. In this study, the ‘B’ part of the body was treated with SSD and the ‘A’ part was treated with honey. Patients and nursing staff were blinded to the procedure. The length and width of the wound were measured with a ruler and photographed, and these measurements were multiplied to calculate the area in square centimetres. The healing percentage of the wound and the healing time were recorded: the healing percentage of the wound = [(area of first day − area of second time)/(area at first time)] × 100.
Table 1.
Demographic characteristics
| Characteristic | Patients (n = 150) |
|---|---|
| Males/females | 67/83 |
| Mean ± SD age (years) | 28 ± 15·94 |
| %TBSA (range) | 10–38 |
| Mean ± SD TBSA | 22·7 ± 8·5 |
| Site of burn | |
| Right hand and left hand | 08 (5%) |
| Right foot and left foot | 12 (8%) |
| Right arm and left arm | 18 (12%) |
| Right side abdomen and left side abdomen | 09 (6%) |
| Right side chest and left side chest | 11 (7%) |
| Right forearm and left forearm | 31 (20%) |
| Right side back and left side back | 05 (3%) |
| Right leg and left leg | 56 (37%) |
TBSA, total burn surface area.
STATISTICAL ANALYSIS
Data were analysed by SPSS Win 10·0 (SPSS, Chicago, IL, USA) software. The Student t‐test and analysis of variance test were used to compare the study groups, wound size and healing time. Significance level was determined as less than 0·05.
RESULTS
A total of 150 patients, both males and females of different age groups, were included in the study.
The demographic characteristics of the 150 patients with burns at two sites are summarised in Table 1. Only patients with partial‐thickness burns of the symmetrical body sites such as hands or feet were selected, minimising the number of factors such as age, sex and biological systems. The extent of partial‐thickness burns and the size of the burn were the same at both sites. With this randomisation, one site was treated with SSD cream and the other site was treated with honey. The mean times for healing were 15·62 ± 4·40 and 13·47 ± 4·06 days for SSD and honey, respectively, being significantly shorter for honey (P < 0·0001; Table 2). The sites treated with honey healed approximately 2 days sooner than the sites treated with SSD in all the patients. In fact, wound healing took less than 20 days in 93% of the sites treated with honey, but more than 24 days in the sites treated with SSD (Table 2).
Table 2.
Time of healing after treatment with silver sulphadiazine (SSD) versus honey
| Time for complete healing | No. of patients | |
|---|---|---|
| Honey group (n = 150) | SSD group (n = 150) | |
| <10 days | 30 | 13 |
| <14 days | 92 | 67 |
| <19 days | 18 | 10 |
| <21 days | 2 | 21 |
| >24 days | 10 | |
| Healing (mean± SD: days) | 13·47 ± 4·06 | 15·62 ± 4·40 |
| P value | <0·0001 | |
In honey group eight patients failed to heal, six patients' wounds got infected, two patients' wounds did not infect, but failed to heal otherwise. In SSD group 29 patients failed to heal. All of them got infected and fail to heal.
The surfaces of both sites were swabbed to test for microbial contamination, and found to be positive for Pseudomonas in six patients in the sites treated with honey, whereas SSD sites showed positive culture for Pseudomonas in 27 patients, while two had Escherichia coli infection (data not shown). Typical examples of partial‐thickness burns treated with honey and SSD dressings are shown in Figure 1. Eight patients in honey group required split thickness skin graft, whereas 29 patients in SSD group were grafted.
Figure 1.
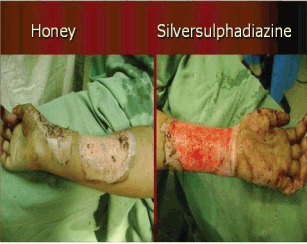
Burn in 30‐year‐old women treated with silver sulphadiazine (SSD) (right) and honey (left).
DISCUSSION
The use of natural products to enhance wound healing is a common practice in many parts of the world. A survey conducted by Hermans (8) in 1998 to review worldwide use of different treatment options for burns found that honey was used in 5·5% of instances, whereas 1% silver sulphadiazine was the preferred treatment for partial‐thickness and mixed burns. Since that time, a number of scientific research papers have testified to the beneficial effects of honey in controlling infection and promoting burn wound healing.
The findings of this clinical study showed that honey promoted burn wound healing more effectively than did SSD. The wounds treated with honey were smaller and took less time to heal. In our study honey in comparison with SSD cream produced excellent results as a burn dressing. It produced wound healing in 3 weeks as compared with 4 weeks in patients treated with SSD. These results are much similar to the previous studies. In a study conducted in 1991 in India, 91% of the burn wounds treated with honey got sterile within 7 days compared with only 7% of the wounds treated with SSD (9). In another study conducted in India in 1998, 100% epithelialization occurred in burns treated with honey by the 21st day compared with 84% healing with 1% SSD. It was further concluded in the study that in honey‐dressed wounds there was early subsidence of inflammation, better control of infection and quicker wound healing compared with the other group (10).
Honey provides a moist healing environment yet prevents bacterial growth even when wounds are heavily infected. It is a very effective means of quickly rendering heavily infected wounds sterile, without the side effects of antibiotics, and it is effective against antibiotic‐resistant strains of bacteria (11). Its antibacterial properties and its viscosity also provide a barrier to cross‐infection of wounds. It also provides a supply of glucose for leucocytes, essential for the ‘respiratory burst' that produces hydrogen peroxide, the dominant component of the antibacterial activity of macrophages (12).
Furthermore it provides substrates for glycolysis, which is the major mechanism for energy production in the macrophages, and thus allows them to function in damaged tissues and exudates where the oxygen supply is often poor (12). The acidity of honey (typically below pH 4) (13) may also assist in the antibacterial action of macrophages, as an acid pH inside the vacuole is involved in killing ingested bacteria (12). In addition, the high osmolarity of honey causes an outflow of lymph which serves to provide nutrition for regenerating tissue which otherwise can only grow around points of angiogenesis (seen as granulation): healing is delayed if the circulation to an area is poor, or if a patient is poorly nourished. It has also been suggested that the decreased turgor resulting from the application of honey may increase oxygenation of tissues (14).
The antimicrobial effect of SSD is the only mechanism justifying its continued use in burn injury. The silver ion binds with the DNA of an organism and releases the sulphonamide, which kills microbes (15). Hepatic or renal toxicity and leukopenia may be caused by the topical application of SSD. In fact, these side effects have been observed in the treatment of large wounds 16, 17.
One of the main limitations of clinical trials is matching patients in case and control groups, with a minimum number of factors. The results of most such studies are not creditable as a result of the poor matching of the patients. The findings of our study are supported by the fact that it selected one patient with two burns of similar size and type; one of each was treated with honey and SSD, thus eliminating the many parameters that would cause alteration.
In conclusion, honey promoted wound healing in burned patients better than SSD cream, with smaller lesions and shorter healing times. The re‐epithelialization process was faster in the skin of patients treated with honey than in those treated with SSD. The mechanism of the remarkable efficacy of honey in the healing of burn injuries may be explained by its antimicrobial, cell proliferation and antiinflammatory effects.
REFERENCES
- 1. Campbell F, Seers K. Dressing and topical agents for burns. Cochrane Database Syst Rev 2000; Issue 2. [Google Scholar]
- 2. Cooper R, Molan P. The use of honey as an antiseptic in managing Pseudomonas infection. J Wound Care 1999a;8:161–4. [DOI] [PubMed] [Google Scholar]
- 3. Cooper R, Molan P, Harding KG. Antibacterial activity of honey against strains of Staphylococcus aureus from infected wounds. J Roy Soc Med 1999;92:283–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edwards‐Jones V, Greenwood JE. What's new in burn microbiology? James Laing Memorial Prize Essay 2000. Burns 2003;29:15–24. [DOI] [PubMed] [Google Scholar]
- 5. Atiyeh BS, Costagliola M, Hayek SN, Dibo S. Effect of silver on burn wound infection control and healing: review of the literature. Burns 2007;33:139–48. [DOI] [PubMed] [Google Scholar]
- 6. Klasen HJ. A historical review of the use of silver in the treatment of burns. II. renewed interest for silver. Burns 2000;26:131–8. [DOI] [PubMed] [Google Scholar]
- 7. Karayil S, Deshpande SD, Koppikar GV. Effect of honey on multidrug resistant organisms and its synergistic action with three common antibiotics. J Postgrad Med 1998;44:93–6. [PubMed] [Google Scholar]
- 8. Hermans MHE. Results of a survey on the use of different treatment options for partial and full‐thickness burns. Burns 1998;24:539–51. [DOI] [PubMed] [Google Scholar]
- 9. Subrahmanyam M. Topical application of honey in treatment of burns. Br J Surg 1991;78:497–8. [DOI] [PubMed] [Google Scholar]
- 10. Subrahmanyam M. A prospective randomized clinical and histological study of superficial burn wound healing with honey and silver sulphadiazine. Burns 1998;24:157–61. [DOI] [PubMed] [Google Scholar]
- 11. Molan PC, Betts JA. Clinical usage of honey as a wound dressing: an update. J Wound Care 2004;13:353–6. [DOI] [PubMed] [Google Scholar]
- 12. Moore OA, Smith LA, Campbell F, Seers K, McQuay HJ, et al. Systematic review of the use of honey as a wound dressing. BMC Complement Altern Med 2001;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Burn Centre. 2005: available at: www.burns.org.nz [accessed on 15 February 2010].
- 14. Kaufman T, Eichenlaub EH, Angel MF, Levin M, Futrell JW. Topical acidification promotes healing of experimental deep partial thickness skin burns: a randomized double‐blind preliminary study. Burns 1985;12:84–90. [DOI] [PubMed] [Google Scholar]
- 15. Ulkür E, Oncül O, Karagöz H, Celiköz B. Cavuşlu S. Comparison of silver‐coated dressing (acticoat), chlorhexidin acetate 0.5% (bactigrass), and silver sulfadiazine 1% (silverdin) for topical antibacterial effect in pseudomonas aeruginus‐contaminated, full‐skin thickness burn wounds in rats. J Burn Care Rehabil 2005;26:430–3. [DOI] [PubMed] [Google Scholar]
- 16. Chaby G, Viseux V, Poulain JF, De Cagny B, Denoeux JP, Lok C. Topical silver sulfadiazine‐induced acute renal failure. Ann Dermatol Vener 2005;132:891–3. [DOI] [PubMed] [Google Scholar]
- 17. Fraser JF, Cuttle L, Kempf M, Kimble R. Cytotoxicity of topical antimicrobial agents used in burn wounds in Australia. Aust NZ J Surg 2004;74:139–42. [DOI] [PubMed] [Google Scholar]


