Abstract
Debridement plays an essential role in the wound‐bed preparation of necrotic and sloughy ulcers, being a mandatory step to achieve a well‐debrided bed, proceeding towards healing. This study reports our experience with Versajet [Versajet Hydrosurgery System® (Smith & Nephew, Hull, UK)], a new device for the debridement of exudating ulcers, based on Fluidjet technology, which excises and aspirates the unwanted tissue by using the Venturi effect. In a 10‐month time period, a total of 68 patients, out of a setting of 167 patients, hospitalised as affected by chronic, hard‐to‐heal leg ulcers, stuck in the inflammatory phase, were treated with Versajet. Based on ulcer characteristics and clinical conditions, the remaining 99 patients underwent traditional debridement with moist dressings (controls). In the majority of Versajet‐treated cases (46), an adequately debrided wound bed was achieved with one operative procedure; two and three procedures were required in 17 and 5 patients, respectively. Almost all the procedures were performed in the ward at the patient's bedside. This procedure is quick (mean time per treatment is about 5 minutes); when compared with a traditional treatment with moist dressings, Versajet considerably shortens the in‐hospital stay and promotes a quicker healing process. When used by an experienced surgeon, Versajet allows a selective debridement, as it makes it possible to remove only the tissue centred in the working end and spare the healthy tissue. Debriding with Versajet is highly effective in reducing the bacterial load of the ulcer bed. The pain caused by Versajet is well tolerated, especially when set for gentle debridement. If multiple treatments are required, the combined use with moist dressings is synergistic, as the dressings soften the necrotic tissue, thus facilitating the following Versajet debridement. The results indicate that Versajet offers more precision than standard mechanical debridement and, at high settings, offers an alternative to surgical debridement.
Keywords: Hydrosurgery, Leg ulcer, Necrotic tissue, Skin graft, Surgical debridement, Wounds
Introduction
Wound healing represents a complex process that involves several events: from chemotaxis to synthesis of extracellular matrix and scar formation; in acute wounds, the healing process can be schematically divided into four phases: haemostasis, inflammation, proliferation and remodelling (1).
In chronic, non healing wounds, this process is completely disrupted because of many general and local causes: the underlying vascular disease, infections, diabetes and other metabolism disorders, anaemia, tissue senescence, drugs and hypoxia 2, 3, 4, 5, 6, 7).
Chronic wounds can be stuck in the inflammatory or proliferative phase 8, 9, 10, 11, 12), but ‘usually all chronic wounds tend to be characterised by non resolving inflammation. This inability to resolve inflammation is believed to be the most significant delaying factor in the healing of chronic wounds’ (13).
In this case, a relevant tissue loss with tendons or bone exposition is possible, the wound being covered by necrotic tissue or fibrin slough and other contaminants, infected, undermined.
When we face wounds at this stage, ulcer bed debridement is mandatory if we want to promote healing process: necrotic tissue, fibrin slough, contaminants are, actually, a breeding‐ground for bacteria, prolong the inflammatory reaction, mechanically prevent the ulcer contraction and block the re‐epithelisation (14).
Wound‐bed preparation can be achieved with several methods: autolytic, osmotic, enzymatic, biological, mechanical and surgical. The first ones permit a selective ulcer‐bed debridement allowing the non viable tissue removal and sparing the healthy one, but they can require several days to have their action carried out; the mechanical and surgical methods act in a fast way, but they are non selective, as they also remove healthy tissue; moreover they can be very painful 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26).
Aim
We report our experience with Versajet Hydrosurgery System, a new tool for debridement of exudating ulcers.
Materials and methods
Versajet is based on Fluidjet technology that uses the Venturi effect: high‐pressure waterjet is pushed through a suitable hose to the tip of a procedure‐specific handpiece; here is placed the working end between a jet nozzle and a collector 8 or 14 mm in length (Figure 1).
Figure 1.
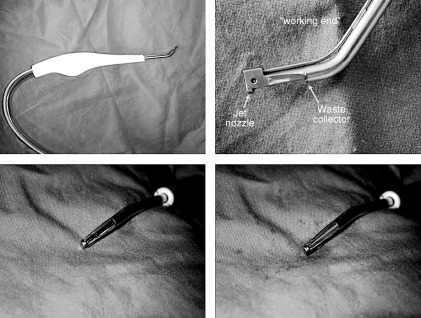
In clockwise direction: handpiece, working end, working end in front view, working end with waterjet running.
In this area, the waterjet runs at a very high speed and pressure (from 265 to 670 mph and from 103 to 827 bar depending on the 10‐step speed setting on the console) and parallel to the axis of the device, and it is collected by the collector device, thus creating a localised vacuum.
This results in capture and excision of unwanted tissue centred in the working end and in its aspiration at the collection point; this operating method prevents vapourisation and allows a good visibility; moreover, the risk of the operators to inhale contaminated particles is absent.
The operator can regulate the excising effect of the waterjet by adjusting its pressure and velocity (10 power settings) and modifying the handpiece direction and pressure. The more waterjet speed, handpiece orthogonal direction (respect to the ulcer bed) and handpiece pressure (on the treating surface), the more the excising effect on the unwanted tissue so that it is also possible to excise hard, contaminated tissue and even bone (Figure 2); while reducing the power setting, steering the handpiece and exerting on it a light pressure, the main action of the device is suction, irrigation and scrubbing of the target tissue.
Figure 2.
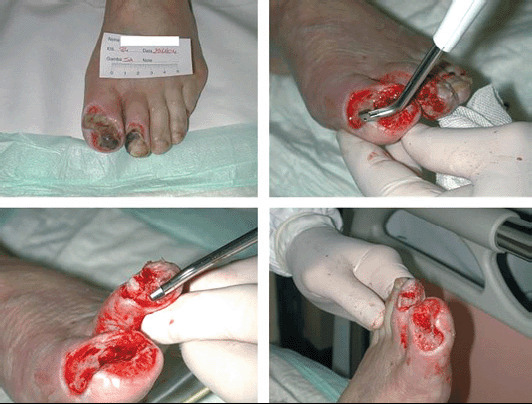
Necrotic lesion of toe and second finger; it is possible to observe the bone cutting action of Versajet.
From November 2003 to September 2004, we treated 68 patients with Versajet (21 men and 47 women; mean age 69·0 ± 13·4; from 29 to 88; 118 ulcers) out of the 167 (57 men and 109 women; mean age 71·8 ± 14·3; from 22 to 89; 277 ulcers) hospitalised for hard‐to‐heal vascular leg ulcers stuck at the inflammatory stage and candidates to skin grafting.
The patients were considered eligible for hydrosurgical debridement according to the presence of wide ulcer surface, with abundant and/or malodorous exudate, with heavy bacterial colonisation (in case with greenish material), covered by necrotic tissue or thick fibrin slough.
The other 99 patients were treated only with traditional moist dressings and considered the control group.
In the group treated with Versajet, the ulcers had an arteriopathic aetiology in 19 cases (31 in the control group), a venous disease in 21 cases (38 in the control group), a mixed aetiology in one case (two in the control group), a vasculitis in 19 cases (19 in the control group), a microangiopathy in six cases (nine in the control group), a trauma in one case and iatrogenic in one case.
Thirteen patients in the Versajet group (20 in the control group) were smokers, 15 (23) were affected by diabetes mellitus, 26 (40) were affected by arterial hypertension, ten (15) were affected by hypercholesterolaemia, six (ten) were affected by hepatitis C and three (five) were affected by cryoglobulinaemia.
The mean surface of the ulcers was 186·4 ± 163·0 cm2 (range 20–600), 133·6 ± 203·5 cm2 (range 1–800) in the control group.
The mean duration of the ulcers was 51·3 ± 92·3 months (range 1–480), 33·3 ± 69·8 months (range 4–444) in the control group (Table 1).
Table 1.
Case report general data
| Case report | Versajet | Control |
|---|---|---|
| Number of patients | 68 | 99 |
| Age (mean, SD, range) | 69·0 ± 13·4; 29–88 | 71·8 ± 14·3; 22–89 |
| Sex | 21 M; 47 F | 36 M; 62 F |
| Number of ulcers | 118 | 159 |
| Arterial disease | 19 | 31 |
| Venous disease | 21 | 38 |
| Mixed aetiology | 1 | 2 |
| Vasculitis | 20 | 19 |
| Microangiopathy | 6 | 9 |
| Trauma | 1 | |
| Smoke | 13 | 20 |
| Diabetes mellitus | 15 | 23 |
| Arterial hypertension | 26 | 40 |
| Hypercholesterolaemia | 10 | 15 |
| HCV | 6 | 10 |
| Cryoglobulinaemia | 3 | 5 |
| Ulcer surface (mean, SD, range) | 186·4 ± 163·0 cm2; 20–600 | 133·6 ± 203·5 cm2; 1–800 |
| Ulcer duration (mean, SD, range) | 51·3 ± 92·3 months; 1–480 | 33·3 ± 69·8 months; 4–444 |
In the Versajet group, no patient was on anticoagulant treatment.
All the patients were informed in detail of the potential risks and benefits of the procedure, and a signed consent was obtained from each of them.
The procedure was performed in the ward at the patient's bedside; in case of painful ulcers, a local anaesthesia was given: lidocaine/ prilocaine ointment in 19 cases or local xilocaine infiltration in four cases; four patients affected by large, necrotic, painful ulcer, in which a sharp debridement was considered, were moved to the surgical theatre and submitted to a short intravenous anaesthesia.
In 21 patients, we measured the wound bacterial burden before and after the Versajet procedure, with quantitative swab culture obtained by firmly twirling the end of a cotton‐tipped applicator on a 1‐cm2 area of the wound for 5 seconds (27, 28).
Analysing the effects of this new equipment, we focused on (i) time for complete debridement, (ii) effect on bacterial burden, (iii) effect on pain evaluated with the visual‐analogic scale during the procedure and (iv) bleeding effect.
Results
In 46 patients, an adequately debrided wound bed was achieved with one operative procedure (3, 4), in 17 cases, two steps were required and, in five patients, three steps were required; when several treatments were required, they were performed daily or every other day (depending on the type of dressing), and between treatments, the ulcers were dressed with moist dressings to make the spontaneous non viable tissue autolysis easier.
Figure 3.
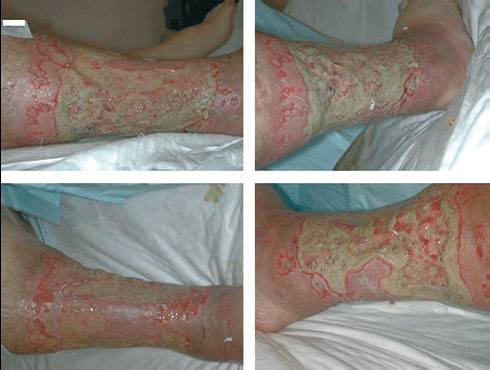
Large ulcer surrounding all the leg in a 59‐year‐old woman affected by deep venous insufficiency; in clockwise order: lateral, posterior‐lateral, anterior and medial views.
Figure 4.
Sharp one‐step ulcer debridement with Versajet; complete removal of all fibrin slough; in this case, the patient received a short‐term intravenous anaesthesia.
The mean time per hydrosurgical procedures was 5·8 ± 3·6 minutes (range 2–20).
The average time to obtain the wound bed clean decreased from 6·1 ± 5·2 days in the control group treated only with moist dressings to 1·4 ± 0·6 days in the patients treated with Versajet allowing an equivalent reduction in the hospitalisation time.
The bacterial burden decreased from 106 (range from 25 000 to 10 000 000) to 103[range from 0, in nine patients (42·8%), to 30 000] CFU/cm2. The most frequently isolated bacteria were Staphylococcus aureus, S. epidermidis, Pseudomonas aeruginosa, P. putida, Streptococcus faecalis, Group D Streptococcus, Escherichia coli, Proteus mirabilis, Serratia marcescens, and none of these was Versajet resistant.
The pain caused by Versajet was acceptable to the majority of patients by adjusting the power level according to the patient's tolerance, although some form of anaesthesia was given to patients complaining of very painful ulcers: a local anaesthetic lidocaine‐prilocaine ointment was used in 18, a local anaesthetic infiltration in three and an intravenous short‐term anaesthesia in four.
The pain, evaluated with the visuo‐analogic scale in all the patients (except, of course, in those who were submitted to short‐term, general anaesthesia), was 4·3 ± 1·6; in five patients, the procedure was painful (level 8 in the visuo‐analogic scale), despite local anaesthetic infiltration in two of them.
No patient reported a thermal effect by the procedure.
We had a poor bleeding of the ulcer bed that stopped spontaneously; a simple elastic bandage placed on the affected limb was usually effective in achieving haemostasis.
Electrocautery to achieve the haemostasis was necessary only in one occasion in which we performed a sharp debridement in the surgical theatre and cut a small vessel.
Discussion and conclusion
Versajet seems to be a valuable equipment in necrotic and sloughy vascular ulcer debridement; we could often achieve an adequately clean bed with a single debridement step and were able to prepare a smooth surface ready to receive a skin graft.
In three cases, the debridement with Versajet was immediately followed by a skin graft, but we usually preferred to wait one day to let the bleeding, if it occurred, completely stop (5, 6).
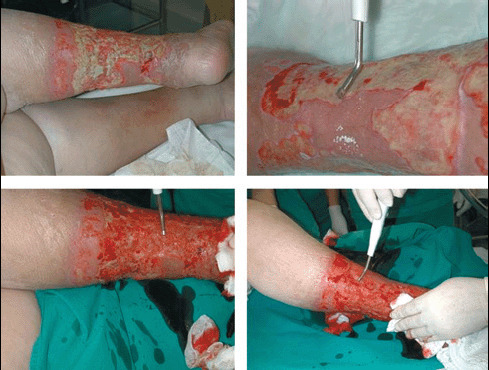
Figure 5.
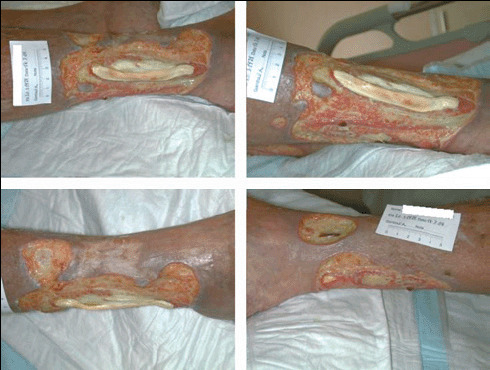
Large vasculitic ulcer in a 75‐year‐old woman; in clockwise order: lateral view, detail, antero‐lateral and medial views.
Figure 6.
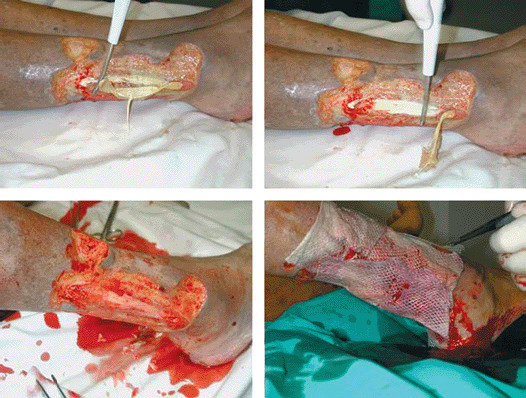
Single‐step procedure: debridement with Versajet with complete cleaning of ulcer bed followed by allograft.
The mean time‐per‐procedue is short (about 5 minutes), but this is just a rough indication depending on the ulcer size and the patient's tolerance and anaesthesia method: it was shorter in small and unpainful ulcers, in patients with high pain threshold and in patients submitted to a general anaesthesia because a high waterjet speed and a orthogonal handpiece position could be held. It was longer in large ulcers or when a low waterjet speed and an oblique handpiece setting was necessary because of the patient's pain: in that case Versajet is not less effective but needs a longer application time.
When Versajet is properly used, the debridement can be selective because it is possible to remove only the tissue centred in the working end sparing the healthy tissue; different handpiece sets (with the tip angled 15° or 45° and working end 8 or 14 mm in length) help to minimise the damage of uninvolved contiguous tissue.
The treated ulcer bed remains clean, although bleeding slightly, because of the suction effect at the collecting point that removes excised tissue, debris and contaminants.
The bleeding almost never requires haemostatic procedure but stops spontaneously and is aided by the elastic bandaging of the affected leg.
The bacterial load is dramatically reduced, thus justifying and promoting the use of Versajet also in infected ulcers.
The pain caused by Versajet was well tolerated (considering its powerful action) by the majority of patients when we used the recommended pump speed setting, equal to or below 5 and if we steer the working end obliquely with respect to the ulcer surface, but a more extensive use of some anaesthetic procedure (local by ointment or infiltration) is preferable in order to further minimise pain.
In case of large, necrotic and painful ulcers, the patient has to be moved to the surgical theatre and submitted to a general anaestesia because the pain could become untolerable.
When we have to get rid of hard necrotic tissue and/or fibrin slough firmly stuck to the ulcer bed, we can use Versajet as a surgical tool and sharply debride the ulcer in a single step after having submitted the patient to a general anaesthesia; but we could also waste healthy tissue.
In this case, when possible, we prefer to perform daily, less aggressive treatments with Versajet and use moist dressings to prepare the ulcer bed for the next treatment.
The combined use of moist dressings and Versajet is synergistic: Versajet mechanically removes the unwanted tissue making the following lytic action of the moist dressings easier; the dressings soften the necrotic tissue making the following Versajet debridement action more complete.
This attitude allows us to exert a more gentle debridement and spare the healthy tissue as much as possible.
Comparing Versajet with other debridement types we noticed that simple mechanical rinsing and firmly wiping and scrubbing the wound bed with gauze and saline solution is less effective and more painful.
Versajet allows a more effective and much shorter debridement with regard to moist dressings; on the other hand, it is more painful and less selective; decreasing the debridement time with respect to the moist dressings, it allowed us to shorten considerably the in‐hospital stay.
Versajet has several advantages over standard surgical scalpel debridement; it can be done in the ward, like a usual dressing; it is less aggressive and more selective, allowing a superior sparing on healthy tissue; it prevents the diffusion of microbial contamination deeper into the wound; it induces only minor bleeding and is less painful; Versajet can create a smoother, less irregular ulcer surface ready to receive a skin graft or other suitable dressings.
We think Versajet presents many advantages over other equipment based on pulsed high‐pressure waterjet (29) because these are more painful as the waterjet is driven to the ulcer surface at very high pressure (between 200 and 400 mbar), cause abundant vapourisation and aerosol effect that inhibits a good view of the treated area and causes risk for the surgeon and his staff to inhale devitalised tissue and contaminants spread in the ambient; it is noisy and needs a long time (up to 20 minutes) to get the right pressure regime; furthermore, there is a possible concern regarding high‐pressure irrigation that may drive bacteria and contaminants deep into the wound and adjacent tissues.
The high cost of the disposable handpiece represents a disadvantage of Versajet but, from a pharma‐economical point of view, it shortens the hospitalisation and the healing time, promoting a quicker ulcer debridement, thus allowing a total saving in the wound management.
For all these reasons, despite of the high cost of the handpiece, Versajet can be recommended in chronic ulcer debridement.
References
- 1. Clark RAF. Wound repair: overwiew and general considerations. In: Clark RAF, editor. The molecular and cellular biology of wound repair, 2nd edition. New York: Plenum Press, 1996:. 3–50. [Google Scholar]
- 2. Eaglstein WH, Falanga V. Chronic wounds. Surg Clin North Am 1997;77: 689–97. [DOI] [PubMed] [Google Scholar]
- 3. Stadelmann WK, Digenis AG. Impediments to wound healing. Am J Surg 1998;176: S39–47. [DOI] [PubMed] [Google Scholar]
- 4. Abatangelo G, Davidson JM. Cutaneous Development, Aging and Repair. Springer‐Verlag, Berlin, Heidelberg, New York 1989. [Google Scholar]
- 5. Robson MC. Wound infection. Surg Clin North Am 1997;77: 637–50. [DOI] [PubMed] [Google Scholar]
- 6. Mulder GD, Brazinsky BA. Factors influencing wound healing. In: Leaper, DJ , Harding, KG , editors Wounds: biology and management. Oxford: Oxford University Press, 1998:. 52–70. [Google Scholar]
- 7. Agren MS, Eaglstein WH, Ferguson MW, Harding KG, Moore K, Saarialho‐Kere UK, Schultz GS. Causes and effects of the chronic inflammation in venous leg ulcers. Acta Derm Venereol Suppl 2000;210: 3–17. [PubMed] [Google Scholar]
- 8. Enoch S, Harding KG. Wound bed preparation: the science behind the removal of barriers to healing. Wounds 2003;15: 213–29. [Google Scholar]
- 9. Shultz GS, Sibbald GR, Falanga V, Ayello EA, Dowsett C, Harding KG, Romanelli M, Stacey MC, Teot L, Vanscheid W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11: S1–28. [DOI] [PubMed] [Google Scholar]
- 10. Herrick SE, Sloan P, McGurk M et al. Sequential changes in histologic pattern and extracellular matrix deposition during the healing of chronic venous ulcers. Am J Pathol 1992;141(4):1085–95. [PMC free article] [PubMed] [Google Scholar]
- 11. Phillips TJ, Palko MJ, Bhawan J. Histologic evaluation of chronic human wounds treated with hydrocolloid and nonhydrocolloid dressings. J Am Acad Dermatol 1994;30(1):61–4. [DOI] [PubMed] [Google Scholar]
- 12. Yager DR, Nwomeh BC. The proteolytic environment of chronic wounds. Wound Repair Regen 1999;7(6):433–41. [DOI] [PubMed] [Google Scholar]
- 13. Hart J. Inflammation 2: its role in the healing of chronic wounds. J Wound Care 2002;11: 245–9. [DOI] [PubMed] [Google Scholar]
- 14. Baharestani M. The clinical relevance of debridement. In: Baharestani M, Gottrupp F, Holstein P, Vansheidt W, editors. The clinical relevance of debridement. Berlin, Heildelberg, New York: Springer‐Verlag, 1999: 23–80. [Google Scholar]
- 15. Falanga V. Occlusive dressings: why, when, which. Arch Dermatol 1998;124: 872–7. [PubMed] [Google Scholar]
- 16. Falanga V. Wound bed preparation and the role of enzymes: a case for multiple actions of therapeutic agents. Wounds 2002;14: 47–57. [Google Scholar]
- 17. Ovington LG. Dressings and adjunctive therapies: a BCPR guidelines revisited. Ostomy Wound Manage 1999;45: 94s–106s. [PubMed] [Google Scholar]
- 18. Harding KG, Jones V, Price P. Topical treatment: which dressing to choose. Diabetes Metab Res Rev 2000;16(Suppl. 1):S47–50. [DOI] [PubMed] [Google Scholar]
- 19. Bolton LL, Johnson CL, VanRijswijk L . Occlusive dressings: therapeutic agents and effects on drug delivery. Clin Dermatol 1992;9: 573–83. [DOI] [PubMed] [Google Scholar]
- 20. Wiseman DM, Rovee DT, Alvarez OM. Wound dressings‐design and use. In: Cohen IK, Digelman R, Linblad WJ, editors. Surgical aspects of wound healing. Philadelphia, PA: W.H. Saunder, 139–67. [Google Scholar]
- 21. Falanga V. Wound bed preparation and the role of enzymes: a case for multiple action of therapeutic agents. Wounds 2002;14: 47–57. [Google Scholar]
- 22. Himel H. Wound healing: focus on the chronic wound. Wounds 1995;. 7(Suppl. A):70A–77A. [Google Scholar]
- 23. Sieggreen MY, Makelbust J. Debridement choices and challenges. Adv Wound Care 1997;10: 32–7. [PubMed] [Google Scholar]
- 24. Jeffrey J. Metalloproteinases and tissue turnover. Wounds 1995;7(Suppl. A):13A–22A. [Google Scholar]
- 25. Koeveker GB. Surgical debridement of wounds. In: Falanga V, editor. Cutaneous wound healing. London: Martin Dunitz, 2001: 233–45. [Google Scholar]
- 26. Steed DL, Donohoe D, Webster MW, Linsdley L, the Diabetic Ulcer Study Group. Effects of extensive dèbridement and treatment on the healing of diabetic foot ulcer. J Am Cell Surg 1996;183: 61–4. [PubMed] [Google Scholar]
- 27. Levine SN, Lindberg RB, Mason AD, Pruitt BA. The quantitative swab culture and smear: a quick simple method for determining the number of viable aerobic bacteria on open wounds. J Trauma 1976;16: 89–94. [PubMed] [Google Scholar]
- 28. Bill TJ, Ratcliff CR, Donovan AM, Knox LK, Morgan RF, Rodeheaver GT. Quantitative swab culture versus tissue biopsy: a comparison in chronic wounds. Ostomy Wound Manage 2001;47: 34–7. [PubMed] [Google Scholar]
- 29. Palmier S, Trial C. Use of high pressure waterjets in wound debridement. In: Téot L, Banwell P, Ziegler U, editors. Surgery in wounds. Berlin‐New York: Springer; 2004. : 72–6. [Google Scholar]


