Abstract
The nano‐oligosaccharide factor (NOSF) is a new compound aiming to promote wound closure mainly through inhibition of matrix metalloproteinase (MMP) activity. This factor is incorporated within a lipido‐colloid matrix (Techonology Lipido‐Colloid‐NOSF matrix) and locally released in the wound. The objective of this study was to document the performance (non inferiority or superiority) of the NOSF relative to the Promogran® matrix (oxidised regenerated cellulose, ORC) effect in the local management of venous leg ulcers (VLUs). This was a 12‐week, open, two‐arm, multicentre, randomised study. Patients were selected if the area of their VLU [ankle brachial pressure index ≥0·80] ranged from 5 to 25 cm2 with a duration ≥3 months. Ulcers had to be free from necrotic tissue. In addition to receiving compression bandage therapy, patients were randomly allocated to either NOSF matrix or ORC treatment for 12 weeks. The VLUs were assessed on a weekly basis and wound tracings were recorded. Percentage wound relative reduction (%RR) was the primary efficacy criterion. Secondary objectives were wound absolute reduction (AR), healing rate (HR) and % of wounds with ≥40% reduction compared with baseline. A total of 117 patients were included (57 NOSF matrix and 60 ORC). Mean population age was 71·3 ± 13·5 years, body mass index was ≥30 kg/m2 in 39·3% and 15·4% were diabetics. Fifty‐six per cent of the VLUs were present for >6 months, 61% were recurrent and 68% were stagnating despite appropriate care. Mean wound area at baseline was 11·2 ± 7·4 cm2. At the last evaluation, mean difference between the groups for %RR was 33·6 ± 15·0% in favour of NOSF matrix with a unilateral 95% confidence interval (CI) lower limit of 8·6% not including the null value. Therefore, a superiority of NOSF matrix effect compared with ORC was concluded (P = 0·0059 for superiority test). The median of the wound area reduction was 61·1% and 7·7% in the NOSF matrix and control groups, respectively (per‐protocol analysis), or 54·4% versus 12·9% in intent‐to‐treat analysis (p = 0·0286). Median AR was 4·2 cm2 in the NOSF group and 1·0 cm2 with ORC (P = 0·01). Median HR was −0·056 and −0·015 cm2/day in NOSF and ORC groups, respectively (P = 0·029). By logistic regression, the NOSF versus control odds ratio to reach 40% area reduction was 2·4 (95% CI: 1·1–5·3; P = 0·026). In the oldest and largest VLUs, a strong promotion of healing effect was particularly observed in the NOSF matrix group compared with the control group. NOSF matrix is a very promising option for the local management of chronic wounds, especially for VLUs with poor healing prognosis.
Keywords: Randomised clinical trial, Venous leg ulcer, Metalloproteinase matrix (MMP), Nano‐oligosaccharide (NOSF)
Introduction
The most prevalent chronic wounds in Western population are related to vascular diseases such as venous disease and peripheral arterial disease (1). In a systematic review of prevalence studies of lower limb ulceration in the adult population conducted by Graham et al. (2), the reported prevalence rates of open ulcers ranged from 0·12% to 1·1% of the population in studies with clinical validation and that of open or healed ulcers was reported to be 1·8%. In addition, some studies suggest that chronic leg ulcer prevalence might be underestimated 3, 4, 5. Furthermore, recurrence rates are high with estimates in the range of 59–67% 1, 6, 7.
A large majority of these wounds will not heal properly: on average, in favourable cases, 20–24 weeks of treatment is frequently necessary, despite best applied care including compression therapy, to obtain 100% reepithelialisation. No more than 50–60% of venous leg ulcers (VLUs) will follow this expected healing trajectory 8, 9, 10, meaning that numerous patients will have an open wound for many months. Various factors widely contribute to the chronicity of these wounds: the cellular and systemic effects of ageing, repeated ischaemia–reperfusion injuries and bacterial contamination, all resulting in a persistent and inappropriate inflammatory response 11, 12, 13, 14, 15. Typically, non healing wounds display decreased growth factors 16, 17, 18, decreased keratinocytes migration, increased reactive oxygen species (19) and increased tissue proteases 20, 21.
Therefore, in addition to appropriate contention wearing, numerous efforts have been made to develop pharmacological or non pharmacological tools in order to improve the healing prognosis. Few of them have been shown as fully successful 22, 23, 24, 25 in clinical practice. Among the very latest developments is the nano‐oligosaccharide factor (NOSF), a synthesised component identified by the Urgo Laboratories Research Unit (chenôve, France). This factor has been documented in experimental models as presenting matrix metalloproteinase (MMP‐1 in particular) inhibitory properties and is able to increase the local residential time of various growth factors released in situ (26). The NOSF is incorporated within a lipido‐colloid matrix from which it is locally released when the dressing is in contact with wound fluids.
The objective of the present clinical trial was to compare the healing‐promoting effect of the NOSF matrix to a collagen–oxidised regenerated cellulose (ORC) one (Promogran®) in the treatment of VLUs.
Patients and methods
This was a two‐arm, parallel group, randomised trial conducted in 22 French hospital units (dermatology and vascular departments) and 5 UK wound specialised centres. This study was designed to show non inferiority or superiority of the NOSF matrix compared with the control treatment, Promogran.
Patients
Adults, hospitalised or outpatients, were selected if they were suffering from a VLU with an ankle‐ brachial pressure index (ABPI) ≥0·8 and were concordant with wearing compression therapy. Patients had to be free from recent venous surgery (<2 months) or from any deep venous thrombosis episode (<3 months). Ulcer area at baseline was required to range between 5 and 25 cm2 with wound duration of 3–24 months. In case of multiple ulcers, a single ulcer was selected as the target wound. Ulcers had to be free from any necrotic/devitalised tissues (no black at colorimetric evaluation). The main exclusion criteria were suspicion of clinical infection or malignant wound degeneration, poor health status, current treatment with immunosuppressive agents, radiotherapy or high dose of oral corticosteroids.
Design and procedures
After checking that patients met the selection criteria and obtaining their written consent to participate in the clinical trial, they were randomly allocated to be treated with either the NOSF matrix or the collagen–ORC matrix (control group) until a complete reepithelialisation was obtained or up to a maximum of 12 weeks. At the inclusion visit, patients’ demographics, ulcer characteristics and medical history were precisely recorded. The selection of compression bandaging was left to the investigators’ decision and its concordance was verified. An acetate tracer of wound area (planimetry) was performed and an ulcer photograph was taken. Following this, wounds were medically evaluated on a weekly basis during the first 4 weeks of the treatment and then every 2 weeks. At each visit, wound status including colorimetric scale evaluation, surrounding skin condition, acetate tracing and wound photograph was assessed. During the study, acceptability of the dressings was monitored by using open questions, and the patients’ concordance to compression therapy was verified at any medic or nurses’ visits.
Concomitant local and general treatments as well as local wound cares were also documented during the whole study. Investigators were allowed to interrupt allocated dressing treatment in the case of an occurrence of unacceptable dressing‐related adverse events or if they considered that wound aggravation required a more appropriate treatment.
Endpoints
Efficacy, which was the primary endpoint of the study, was assessed by the investigating physician at each weekly clinical evaluation (until week 4 and thereafter every 2 weeks until week 12) through the measurement of wound area (judgement criteria).
As secondary endpoints, absolute area reduction, the healing rate (HR), the tolerance (occurrence of local adverse events documented by the investigating physician) and the acceptability of the tested dressings were assessed (by the nursing staff) during the 12‐week follow‐up.
Tested dressings and local cares
The NOSF matrix (Laboratoires URGO, Chenôve, France) was presented as 10 × 10‐cm dressings composed of a lipido‐colloid dressing impregnated with NOSF.
The collagen–ORC matrix Promogran® (Johnson & Johnson Medical, Skipton, UK) is composed of collagen of bovine origin (55%) and of ORC (45%). The selected size of the control matrix was 28 cm2. In contact with wound fluids, the collagen–ORC matrix forms a gel that may be removed by cleansing the ulcer with a saline solution.
NOSF and control matrices were recommended to be changed every 3 days or more frequently if required. Before each dressing change, wounds were inspected and cleansed exclusively with normal saline. If necessary, mechanical debridement was performed to remove slough and necrotic tissues. The matrices were applied to completely cover the wound surface and covered with a non woven absorbent pad (Tetracelle®; Tetra Medical, Annonay, France).
Sample size determination
Sample size was calculated to document non inferiority of the NOSF matrix compared with control treatment on 12‐week ulcer area relative reduction (RR) from baseline value. Based on previous clinical experiences with Promogran (27), the expected reductions in the NOSF matrix and control groups were 60% and 55%, respectively, with a 35% standard error. To show that NOSF matrix was at least not inferior to control relative to a 10% prespecified non inferiority margin, 138 patients were required (alpha and beta risks fixed at 5% and 20%, respectively).
Data processing and statistical analysis
A statistical analysis was conducted by a company independent of the sponsor, according to the statistical analysis plan drawn up and approved by the different parties involved in the trial. Data analyses were conducted with SAS 8·2 software. If a patient withdrew prior to the 12th week of the study, the efficacy analysis took into account the last available evaluation (last observation carried forward).
Comparability of the two groups was verified using the Student’s t‐test or the non parametric Wilcoxon test for continuous variables and chi‐squared test for categorical variables.
The main efficacy outcome was the wound area RR at the last available planimetry compared with baseline (% decrease from baseline). According to recommendations 28, 29, 30, the non inferiority test was evaluated on the per‐protocol (PP) population defined as the intent‐to‐treat population (ITT; all randomised patients with at least one follow‐up planimetry) with ulcer area within the pre‐defined ranges, and so, the PP analysis considered only those ulcers with an initial surface area between 4 and 30 cm2 (as noted in the protocol of the study and in accordance with the trial coordinator, an error of less than 20% was tolerated with respect to this initial surface area criterion of 5–25 cm2).
The test for the non inferiority of NOSF matrix to collagen–ORC matrix in area reduction was carried out through a confidence interval (CI) approach. Non inferiority was established if the lower unilateral limit of the 95% CI for the between‐treatment difference in the mean area change from baseline did not exceed 10%. If non inferiority was documented, then it was assessed whether the new treatment appeared superior to that of the reference treatment and these analyses were performed on ITT population. These superiority analyses were conducted on the primary and secondary outcomes.
Secondary outcomes were the absolute decrease in wound area and the 12‐week wound closure rate calculated as (value W 12w − value W baseline)/t, where t was the number of days between the two measurements. The results are expressed in cm2/day. Non parametric Wilcoxon rank test was used. Chance to observe a 40% wound area reduction at the last available planimetry was evaluated by binary logistic regression including in the model the applied dressings, the aetiology of ulcer (venous, post‐phlebitic or ulcer with arterial participation, i.e. ABPI < 0·9), the duration of current ulcer (≤6 or >6 months), the presence of granulation tissue (covering 50% or more of ulcer or less than 50%) and the perilesional skin aspect (healthy or not). Median time to reach this endpoint was compared with the log‐rank test. As this analysis documented a significant impact of ulcer duration, a post hoc evaluation was conducted on subgroups stratified according to the ulcer duration.
Local adverse events were descriptively analysed. All superiority tests were bilateral, and a P value of less than 5% was considered as significant.
Ethics
The study protocol and documents were submitted and approved by the Medical Ethics Committee of Lorraine (France) and by the Multicentre Research Ethics Committee (Scotland) for the UK centres. This clinical trial was then conducted in compliance with Good Clinical Practice and with the principles laid down in the Declaration of Helsinki.
All subjects received detailed information on study conduct and gave their written consent to participate in the trial before their inclusion.
Results
Between October 2004 and June 2006, 117 patients were randomised: 57 and 60 patients in the NOSF matrix and control groups, respectively. Twenty‐four patients in the control group and 17 in the NOSF matrix group were withdrawn before week 12 mainly because of the occurrence of a local adverse event (Table 1). All randomised patients were included in the ITT analysis and 99 patients in the PP analysis (50 patients in the NOSF matrix group and 49 in the control group). During the overall 12‐week study period, 93% of patients from both groups were concordant with compression therapy, as noted at all medic and nurse evaluations.
Table 1.
Patient’s disposal
| Reason for withdrawal before week 12 | Control (n = 60), n (%) | NOSF (n = 57), n (%) | Total (n = 117) n (%) |
|---|---|---|---|
| Informed consent withdrawal | 3 (5·0) | 2 (3·5) | 5 (4·3) |
| Ulcer aggravation | 5 (8·3) | 7 (12·3) | 12 (10·3) |
| Local adverse event | 13 (21·7) | 6 (10·5) | 19 (16·2) |
| General intercurrent event | 1 (1·7)* | 0 (0·0) | 1 (0·9) |
| Other reasons | 2 (3·3)† | 2 (3·5)‡ | 4 (3·4) |
| Total | 24 (40·0) | 17 (29·8) | 41 (35·0) |
NOSF, nano‐oligosaccharide factor.
Death because of cerebral haemorrhage.
Lost to follow‐up (1); moved out (1).
Treatment stopped by the patient’s general practitioner (1); stagnation and treatment stopped by another hospital unit (1).
Baseline characteristics
Eighty‐two per cent of the patients were outpatients. No significant difference was observed at baseline between the two treatment groups for demographics or leg ulcers characteristics (Table 2). The population was predominantly female with a mean age of 71·3 ± 13·5 years. Body mass index was ≥30 kg/m2 in 39·3% of cases, and 15·4% of the patients were diabetic. At inclusion, all VLUs were appropriately debrided (no necrotic tissue and 69·1 ± 28·8% of wound area covered with a granulation tissue). More than 56% of the ulcers were present for more than 6 months (median 10 months) and 61% were recurrent. Mean wound area at baseline was 10·9 ± 9·3 cm2, and ulcer area was superior to 10 cm2 in 41% of patients. Sixty‐eight per cent of these wounds were considered by the investigators as stagnating or worsening despite appropriate local and general management.
Table 2.
Patients and ulcers’ characteristics at baseline*
| Control (n = 60) | NOSF (n = 57) | Total (n = 117) | |
|---|---|---|---|
| Sex (F/M) | 36/24 | 33/24 | 69/48 |
| Age (years) | 71·0 ± 13·9 | 71·5 ± 13·1 | 71·3 ± 13·5 |
| Weight (kg) | 84·7 ± 26·2 | 81·2 ± 21·8 | 83·0 ± 24·2 |
| Height (cm) | 168·3 ± 9·5 | 168·0 ± 10·6 | 168·2 ± 10·0 |
| BMI (kg/m2) | 29·8 ± 8·7 | 28·6 ± 6·8 | 29·3 ± 7·8 |
| BMI ≥ 30 kg/m2, n (%) | 26 (43·3) | 20 (35·1) | 46 (39·3) |
| History of vein thrombosis, n (%) | 23 (38·3) | 22 (38·6) | 45 (38·5) |
| Diabetes, n (%) | 12 (20·0) | 6 (10·5) | 18 (15·4) |
| Outpatients, n (%) | 52 (86·7) | 44 (77·2) | 96 (82·1) |
| ABPI | 1·03 ± 0·17 | 1·01 ± 0·10 | 1·02 ± 0·14 |
| Compression bandage wearing, n (%)† | 57 (95·0) | 50 (87·7) | 107 (91·5) |
| Ulcer characteristics | |||
| Recurrent ulcer, n (%) | 40 (66·7) | 31 (54·4) | 71 (60·7) |
| Ulcer duration, months (median) | 12·1 ± 7·7 (12·0) | 10·4 ± 7·1 (8·0) | 11·2 ± 7·4 (10·0) |
| Duration >6 months, n (%) | 35 (58·3) | 31 (54·4) | 66 (56·4) |
| Stagnating/worsening ulcer, n (%) | 43 (71·7) | 37 (64·9) | 80 (68·4) |
| Ulcer area, cm2 (median) | 10·4 ± 8·4 (7·9) | 11·4 ± 10·1 (9·0) | 10·9 ± 9·3 (8·1) |
| Surface area ≥10 cm2, n (%) | 22 (36·7) | 26 (45·6) | 48 (41·0) |
| Condition of the perilesional skin, n (%) | |||
| Healthy | 10 (16·7) | 4 (7·0) | 14 (12·0) |
| Erythematous | 30 (50·0) | 30 (52·6) | 60 (51·3) |
| Oedematous | 10 (16·7) | 14 (24·6) | 24 (20·5) |
| Eczematous | 8 (13·3) | 9 (15·8) | 17 (14·5) |
| Others | 16 (26·7) | 17 (29·8) | 33 (28·2) |
| Ulcer aetiology, n (%) | |||
| Venous | 32 (53·3) | 32 (56·1) | 64 (54·7) |
| Post‐phlebitic | 12 (20·0) | 8 (14·0) | 20 (17·1) |
| Arterial participation | 16 (26·7) | 17 (29·8) | 33 (28·2) |
ABPI, ankle brachial pressure index; BMI, body mass index; NOSF, nano‐oligosaccharide factor.
Results are presented as mean ± SD or as indicated.
After study inclusion, all patients except one in each group received compression.
Wound area RR
In the PP population (99 patients in total), the median of the wound area RR was 61·1% and 7·7% in the NOSF matrix and control groups, respectively. The mean difference between groups was 33·6 ± 15·0% with a unilateral 95% CI lower limit of 8·6% not including the null value. Therefore, a superiority conclusion of NOSF effect relative to the control effect could be drawn (P = 0·0059 for the Wilcoxon superiority test). The analysis on the ITT population (117 patients) conducted came to the same conclusion (median of area reduction: 54·4% versus 12·9% in the NOSF matrix and control groups, respectively P = 0·0286). Throughout the 12‐week study period, the NOSF matrix effect was greater compared with the regression observed in control group (Figure 1).
Figure 1.
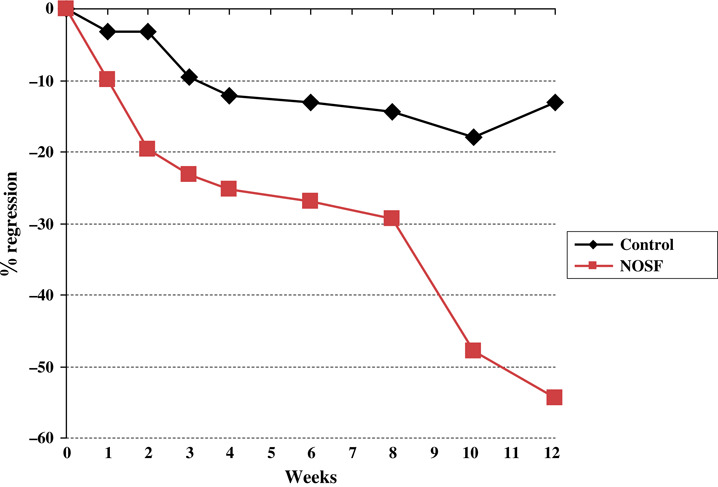
Median of wound area regression (%) in the intent‐to‐treat population over 12 weeks.
Wound area absolute reduction
The mean wound area absolute reduction (AR) (Figure 2) was 2·3 ± 10·2 cm2 (median: 4·2 cm2) at the last planimetry in the NOSF matrix group compared with 0·2 ± 10·4 cm2 (median: 1·0 cm2) in the control group, a significant difference (P = 0·01; Wilcoxon test).
Figure 2.
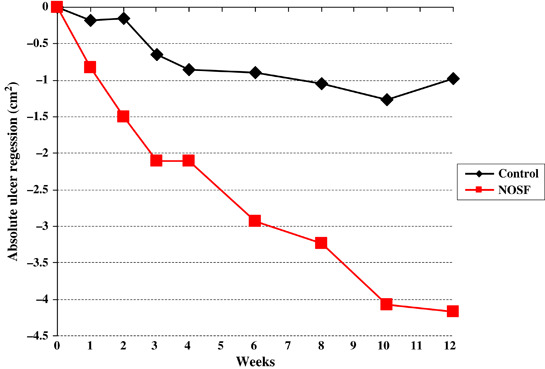
Median of absolute wound area regression (cm2) in the intent‐to‐treat population over 12 weeks. NOSF, nano‐oligosaccharide factor.
Healing rate
The mean wound HR was significantly higher in the NOSF matrix group than in the control group: −0·016 ± 0·285 cm2/day (median: −0·056) and +0·075 ± 0·475 cm2/day (median: −0·015), respectively (P = 0·029; Wilcoxon test).
Chances to reach a 40% wound area reduction
Eighteen ulcers (10 in the NOSF and 8 in the control group) were 100% reepithelialised at the last evaluation. Overall, 56% of leg ulcers treated with the NOSF matrix reached the 40% wound area reduction endpoint compared with 35% in the control group (P = 0·022; Figure 3). By logistic regression, including the parameters ulcer aetiology, ulcer duration, extent of baseline granulation tissue and perilesional skin aspect in the model, the NOSF versus control odds ratio (OR) was 2·4 (95% CI: 1·1–5·3; P = 0·026) in favour of the NOSF matrix group. The median time to reach a 40% wound reduction was 42 days in the NOSF matrix group and 84 days in the control group (P = 0·06; log‐rank test). The ulcer duration was also a significant predictive factor for this endpoint with an OR of 2·2 (95% CI: 1·0–4·9; P = 0·043) for ulcer of less than a 6‐month duration compared with that of more than 6 months.
Figure 3.
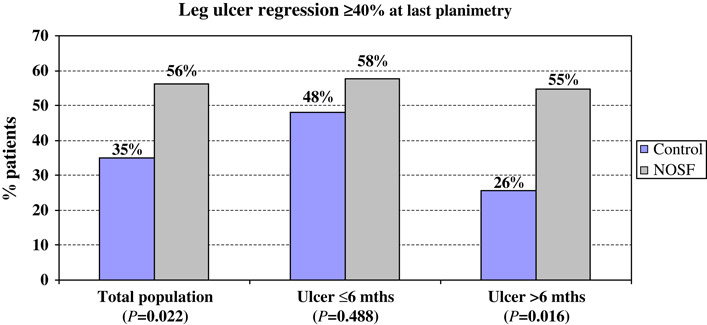
Leg ulcer regression ≥ 40% at the last planimetry.
Subgroup analysis
As ulcer duration appeared as a significant healing prognosis factor in this population, a post hoc analysis was conducted by stratifying population according to this parameter (ulcer duration ≤6 or >6 months). Globally, whereas wound reduction and HR were significantly higher in the NOSF matrix group, all patients considered, no significant difference between groups was detected in the subgroup of ulcers of less than 6‐month duration (Table 3). On the opposite, for ulcers of more than 6‐month duration, results were very significantly in favour of the NOSF matrix‐treated wounds. In this group, 55% and 26% of ulcers decreased by 40% or more in the NOSF matrix and control groups, respectively (P = 0·016; chi‐squared test).
Table 3.
Wound healing parameters according to ulcer duration*
| Ulcer duration ≤6 months | Ulcer duration >6 months | |||||
|---|---|---|---|---|---|---|
| NOSF (n = 26) | Control (n = 25) | P value† | NOSF (n = 31) | Control (n = 35) | P value† | |
| Ulcer area at baseline (cm2) | 8·0 ± 5·8 (5·9) | 9·5 ± 8·1 (5·8) | 0·435 | 14·4 ± 12·0 (11·0) | 11·0 ± 8·7 (8·6) | 0·193 |
| Relative reduction (%) | −36·5 ± 80·2 (−63·5) | −21·6 ± 86 (−28·3) | 0·608 | −26·5 ± 72·9 (−44·3) | 1·0 ± 70·3 (−7·7) | 0·044 |
| Absolute reduction (cm2) | −3·4 ± 5·8 (−4·6) | −1·7 ± 11·4 (−1·9) | 0·559 | −1·3 ± 12·9 (−4·1) | 0·9 ± 9·6 (0·6) | 0·019 |
| Healing rate (cm2/day) | −0·057 ± 0·156 (−0·055) | 0·017 ± 0·487 (−0·034) | 0·224 | 0·358 ± 0·017 (−0·059) | 0·116 ± 0·469 (−0·007) | 0·027 |
| Number and % of ulcers with ≥40% area reduction | 15 (57·7%) | 12 (48·0%) | 0·488 | 17 (54·8%) | 9 (25·7%) | 0·016 |
NOSF, nano‐oligosaccharide factor.
Results are expressed as mean ± SD (median).
Wilcoxon test.
Adverse events and dressings acceptability
A total of 16 local adverse events have been reported in 14 patients (24·5%) in the NOSF matrix group (possibly related to the NOSF matrix) and 27 local adverse events in 23 patients (38·3%) in the control group. The nature of these adverse events differed with the applied dressing (Table 4). Perilesional skin irritations were similar in the two groups; overgranulation was more frequently noted in the NOSF matrix group and pain and infection occurred more frequently in the control group. In 6 NOSF and 14 control group patients, these local events justified discontinuation of the tested matrices treatment.
Table 4.
Local adverse events
| NOSF (n = 57) | Control (n = 60) | |||
|---|---|---|---|---|
| Number of events | Prevalence (%) | Number of events | Prevalence (%) | |
| Number and nature of adverse events | n = 16 | n = 27 | ||
| Perilesional skin irritation | 7 | 12·3 | 8 | 13·3 |
| Pain (between dressing changes) | 4 | 8·8 | 12 | 20·0 |
| Overgranulation | 4 | 7·0 | 1 | 1·7 |
| Infection | 1 | 7·0 | 6 | 10·0 |
| Definitive discontinuation of the treatment | n = 6 | n = 14 | ||
NOSF, nano‐oligosaccharide factor.
The occurrence of severe pain in the control group was the main reason for discontinuation of the treatment. Dressing changes were, overall, easier in the NOSF matrix group compared with the control group (Table 5). The time between two dressing changes was similar in the two treatment groups (every 3·04 versus 2·97 days in the NOSF and control groups, respectively).
Table 5.
Dressing acceptability
| NOSF | Control | |||
|---|---|---|---|---|
| n * | n (%) | n * | n (%) | |
| Difficult dressing removal | 1050 | 4 (0·4) | 984 | 110 (11·2) |
| Dressing adhesiveness to wound bed | 328 | 12 (3·4) | 358 | 56 (17·1) |
| Bleeding at dressing removal | 1045 | 4 (0·4) | 980 | 34 (3·5) |
| Pain at dressing removal† | 1045 | 33 (3·2) | 1092 | 152 (15·4) |
NOSF, nano‐oligosaccharide factor.
Number of documented local cares.
Pain scored as important/very important by nurse.
Discussion
This 12‐week, open, randomised trial was designed to document the non inferiority or superiority of a new wound dressing (the NOSF matrix) relative to a reference matrix, the Promogran device, in the treatment of leg ulcers of venous origin. The NOSF is an oligosaccharide that decreases the activity of MMPs, especially MMP‐1 in experimental cell culture models (26). The NOSF is incorporated within a lipido‐colloid dressing to form a matrix from which it is released in the wound microenvironment as the wound exudates come in contact with the dressing.
Targeting MMPs to promote chronic wound healing is an option that is the subject of numerous fundamental research projects 31, 32, 33, 34, 35, 36. Indeed, compared with acute wounds, chronic wound fluids are characterised by prolonged high MMP activities resulting in low levels of newly synthesised extracellular matrix components, an excess of growth factor degradation and increased proinflammatory cytokine release, all participating in blocking the natural healing process by maintaining the wound in the inflammatory phase. However, few MMP modulators or inhibitors have been clinically evaluated. The more recently introduced tool is a collagen–ORC matrix (Promogran). This matrix forms a gel in contact with wound fluids and is able to absorb and inhibit activity of various MMPs 21, 37. In diabetic foot ulceration, a controlled study has suggested a potential healing‐promoting effect of Promogran compared with saline gauze (38). In another randomised trial in the treatment of venous leg ulceration, Promogran was compared with a petrolatum‐impregnated dressing and a significant increase in HR was documented (27). For these reasons, this dressing was selected as the control treatment in this study.
Leg ulcers included in this clinical trial can be considered as difficult‐to‐heal wounds. Indeed, the mean age of the population was more than 70 years, ulcers were present for 11 months on average, 61% were recurrent and the baseline mean ulcer area was superior by 10 cm2. All these criteria are known to predict poor healing 39, 40, 41, which may explain why around 70% of the selected wounds were considered as stagnating or worsening at baseline by experienced physicians despite adequate local and general management.
Per cent decrease of wound area from baseline was the main efficacy criterion. Whereas this study was open, this criterion was centrally and blindly evaluated by an independent investigator and the statistical analysis was made by an independent company. This endpoint was selected as an indicator of healing promotion in stagnating wounds. In fact, a 20–40% wound area reduction at 3–4 weeks has been shown to be highly predictive of complete closure at 20–24 weeks for leg ulcers 42, 43, 44, 45. All other secondary endpoints (absolute wound reduction, HR and per cent of wounds reaching ≥40% wound area decrease) are related to the main efficacy parameter and reflect the potential of the tested matrix to initiate the healing process.
For all these previous primary or secondary endpoints, superiority of the NOSF matrix effect was shown compared with Promogran in the treatment of VLUs. These results may suggest than the incorporation of the NOSF compound into a lipido‐colloid matrix has increased the clinical benefits of this matrix, previously shown and documented in the management of acute or chronic wounds 46, 47, 48. The effect of the Promogran matrix on wound area reduction was, overall, lower than that observed in the study by Vin et al. (27). These authors showed a 12‐week percent wound area decrease of 54·4% on average (median: 82·4%) with Promogran, somewhat higher than that observed in our study (mean reduction 8·3% with a median of 13·0%). This discrepancy may be related to differences in recruited populations. Indeed, in the study by Vin et al., healing prognosis of included ulcers was more favourable in the Promogran group with a mean duration of the ulcer of 8·4 ± 11·0 months (median 3 months) compared with 12·1 ± 7·7 months (median 12 months) in our study. In the same way, ulcer area was lower in the Vin et al. study: 7·0 ± 6·8 cm2 (median 4·5 cm2) compared with 10·4 ± 8·4 cm2 (median 7·9 cm2) in the Promogran group of this study.
While not initially planned, subgroup analyses may support this hypothesis. For recent ulcers (duration of 6 months or less), baseline ulcer area was closest to the figures noted in the study by Vin et al. In this subgroup, there was a trend in favour of NOSF treatment but no significant differences between dressings were observed for all parameters. Additionally, Promogran results were obviously better than those in the subgroup of older ulcers, which were also the largest wounds. In this latter population, Promogran effect was clinically weak, whereas a clear and clinically relevant stimulating property of NOSF matrix on healing process was noted with 55% of ulcers reaching a ≥40% wound regression.
The documented acceptability of the NOSF matrix was better than that in the control group: the local management appeared to be easier with the NOSF matrix. The presentation of the NOSF matrix as a wound dressing could explain this fact. By contrast, the collagen–ORC matrix forms a gel and requires more local actions during dressing change, which may potentially be more traumatic and painful.
In terms of local tolerance, the most striking difference between the groups was the percentage of reported pain and infection in the control arm: this was more than double. The frequent pain events reported in the Promogran group have already been observed (27). As regards infection, there is no obvious reason to explain this difference. Perhaps, the overall lower efficacy of Promogran on the healing process may increase the risk of the occurrence of local infection.
In conclusion, this study has documented the effect of the new NOSF matrix to promote wound closure in VLUs. This efficacy was superior to the one noted with a collagen–ORC matrix. Furthermore, even those ulcers with the lowest healing prognosis (old and large wounds) have benefited from this dressing. NOSF matrix thus appears as a very promising option for the treatment of VLUs, especially those presenting with a poor healing prognosis.
References
- 1. Nelzen O, Bergqvist D, Lindhagen A. Venous and non‐venous leg ulcers: clinical history and appearance in a population study. Br J Surg 1994;81:182–7. [DOI] [PubMed] [Google Scholar]
- 2. Graham ID, Harrison MB, Nelson EA, Lorimer K, Fisher A. Prevalence of lower‐limb ulceration: a systematic review of prevalence studies. Adv Skin Wound Care 2003;16:305–16. [DOI] [PubMed] [Google Scholar]
- 3. Walker NK, Vandal AC, Holden JK, Rodgers A, Birchall N, Norton R, Triggs CM, MacMahon S. Does capture‐recapture analysis provide more reliable estimates of the incidence and prevalence of leg ulcers in the community? Aust N Z J Public Health 2002;26:451–5. [DOI] [PubMed] [Google Scholar]
- 4. Marklund B, Sulau T, Lindholm C. Prevalence of non‐healed and healed chronic leg ulcers in an elderly rural population. Scand J Prim Health Care 2000;18:58–60. [DOI] [PubMed] [Google Scholar]
- 5. Nelzen O, Bergqvist D, Lindhagen A, Hallböök T. Chronic leg ulcers: an underestimated problem in primary health care among elderly patients. J Epidemiol Community Health 1991;45:184–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Callam MJ, Harper DR, Dale JJ. Chronic ulcer of the leg: clinical history. Br Med J (Clin Res Ed) 1987;294:1389–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baker SR, Stacey MC. Epidemiology of chronic leg ulcers in Australia. Aust N Z J Surg 1994;64:258–61. [DOI] [PubMed] [Google Scholar]
- 8. Steed DL, Hill DP, Woodske ME, Payne WG, Robson MC. Wound‐healing trajectories as outcome measures of venous stasis ulcer treatment. Int Wound J 2006;3:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steed DL. Wound‐healing trajectories. Surg Clin North Am 2003;83:547–55, vi–vii. [DOI] [PubMed] [Google Scholar]
- 10. Robson MC, Hill DP, Woodske ME, Steed DL. Wound healing trajectories as predictors of effectiveness of therapeutic agents. Arch Surg 2000;135:773–7. [DOI] [PubMed] [Google Scholar]
- 11. Whitney JD. Overview: acute and chronic wounds. Nurs Clin North Am 2005;40:191–205, v. [DOI] [PubMed] [Google Scholar]
- 12. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366:1736–43. [DOI] [PubMed] [Google Scholar]
- 13. Sibbald RG, Orsted H, Schultz GS, Coutts P, Keast D; International Wound Bed Preparation Advisory Board; Canadian Chronic Wound Advisory Board . Preparing the wound bed 2003: focus on infection and inflammation. Ostomy Wound Manage 2003;49:23–51. [PubMed] [Google Scholar]
- 14. Mulder GD, Vande Berg JS. Cellular senescence and matrix metalloproteinase activity in chronic wounds. Relevance to debridement and new technologies. J Am Podiatr Med Assoc 2002;92:34–7. [DOI] [PubMed] [Google Scholar]
- 15. Bowler PG. Wound pathophysiology, infection and therapeutic options. Ann Med 2002;34:419–27. [DOI] [PubMed] [Google Scholar]
- 16. Murphy MO, Ghosh J, Fulford P, Khwaja N, Halka AT, Carter A, Turner NJ, Walker MG. Expression of growth factors and growth factor receptor in non‐healing and healing ischaemic ulceration. Eur J Vasc Endovasc Surg 2006;31:516–22. [DOI] [PubMed] [Google Scholar]
- 17. Chan RK, Liu PH, Pietramaggiori G, Ibrahim SI, Hechtman HB, Orgill DP. Effect of recombinant platelet‐derived growth factor (Regranex) on wound closure in genetically diabetic mice. J Burn Care Res 2006;27:202–5. [DOI] [PubMed] [Google Scholar]
- 18. Ovington LG. Overview of matrix metalloprotease modulation and growth factor protection in wound healing. Part 1. Ostomy Wound Manage 2002;48(6 Suppl):3–7. [PubMed] [Google Scholar]
- 19. James TJ, Hughes MA, Cherry GW, Taylor RP. Evidence of oxidative stress in chronic venous ulcers. Wound Repair Regen 2003;11:172–6. [DOI] [PubMed] [Google Scholar]
- 20. Mwaura B, Mahendran B, Hynes N, Defreitas D, Avalos G, Adegbola T, Adham M, Connolly CE, Sultan S. The impact of differential expression of extracellular matrix metalloproteinase inducer, matrix metalloproteinase‐2, tissue inhibitor of matrix metalloproteinase‐2 and PDGF‐AA on the chronicity of venous leg ulcers. Eur J Vasc Endovasc Surg 2006;31:306–10. [DOI] [PubMed] [Google Scholar]
- 21. Lobmann R, Zemlin C, Motzkau M, Reschke K, Lehnert H. Expression of matrix metalloproteinases and growth factors in diabetic foot wounds treated with a protease absorbent dressing. J Diabetes Complications 2006;20:329–35. [DOI] [PubMed] [Google Scholar]
- 22. Clarke‐Moloney M, Lyons GM, Burke PE, O’Keeffe D, Grace PA. A review of technological approaches to venous ulceration. Crit Rev Biomed Eng 2005;33:511–56. [DOI] [PubMed] [Google Scholar]
- 23. Harding KG, Moore K, Phillips TJ. Wound chronicity and fibroblast senescence – implications for treatment. Int Wound J 2005;2:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O’Donnell TF Jr., Lau J. A systematic review of randomized controlled trials of wound dressings for chronic venous ulcer. J Vasc Surg 2006;44:1118–25. [DOI] [PubMed] [Google Scholar]
- 25. Palfreyman SJ, Nelson EA, Lochiel R, Michaels JA. Dressings for healing venous leg ulcers. Cochrane Database Syst Rev 2006;3:CD001103. [DOI] [PubMed] [Google Scholar]
- 26. Coulomb B, Couty L, Fournier B, Laurensou C, Aillaud C, Gogly B, Lafont A, Evaluation de la matrice lipido‐colloide NOSF (nano‐oligosaccharide factor), dans un modèle de reconstruction dermique in vitro. Oral communication presented in « 12ème Conference Nationale des Plaies et Cicatrisations », Paris, January 2008.
- 27. Vin F, Teot L, Meaume S. The healing properties of Promogran in venous leg ulcers. J Wound Care 2002;11:335–41. [DOI] [PubMed] [Google Scholar]
- 28. Wiens BL. Choosing an equivalence limit for noninferiority or equivalence studies. Control Clin Trials 2002;23:2–14. [DOI] [PubMed] [Google Scholar]
- 29. Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ; CONSORT Group . Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA 2006;295:1152–60. [DOI] [PubMed] [Google Scholar]
- 30. Le Henanff A, Giraudeau B, Baron G, Ravaud P. Quality of reporting of noninferiority and equivalence randomized trials. JAMA 2006;295:1147–51. [DOI] [PubMed] [Google Scholar]
- 31. Xue M, Le NT, Jackson CJ. Targeting matrix metalloproteases to improve cutaneous wound healing. Expert Opin Ther Targets 2006;10:143–55. [DOI] [PubMed] [Google Scholar]
- 32. Medina A, Scott PG, Ghahary A, Tredget EE. Pathophysiology of chronic nonhealing wounds. J Burn Care Rehabil 2005;26:306–19. [DOI] [PubMed] [Google Scholar]
- 33. Armstrong DG, Jude EB. The role of matrix metalloproteinases in wound healing. J Am Podiatr Med Assoc 2002;92:12–18. [DOI] [PubMed] [Google Scholar]
- 34. Herouy Y, Trefzer D, Zimpfer U, Schöpf E, Wanscheidt W, Norgauer J. Matrix metalloproteinases and venous leg ulceration. Eur J Dermatol 2000;10:173–80. [PubMed] [Google Scholar]
- 35. Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol 1996;107:743–8. [DOI] [PubMed] [Google Scholar]
- 36. Weckroth M, Vaheri A, Lauharanta J, Sorsa T, Konttinen YT. Matrix metalloproteinases, gelatinase and collagenase, in chronic leg ulcers. J Invest Dermatol 1996;106:1119–24. [DOI] [PubMed] [Google Scholar]
- 37. Cullen B, Smith R, McCulloch E, Silcock D, Morrison L. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen 2002;10:16–25. [DOI] [PubMed] [Google Scholar]
- 38. Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg 2002;137:822–7. [DOI] [PubMed] [Google Scholar]
- 39. Chaby G, Viseux V, Ramelet AA, Ganry O, Billet A. Refractory venous leg ulcers: a study of risk factors. Dermatol Surg 2006;32:512–9. [DOI] [PubMed] [Google Scholar]
- 40. Gohel MS, Taylor M, Earnshaw JJ, Heather BP, Poskitt KR, Whyman MR. Risk factors for delayed healing and recurrence of chronic venous leg ulcers – an analysis of 1324 legs. Eur J Vasc Endovasc Surg 2005;29:74–7. [DOI] [PubMed] [Google Scholar]
- 41. Skene AI, Smith JM, Doré CJ, Charlett A, Lewis JD. Venous leg ulcers: a prognostic index to predict time to healing. BMJ 1992;305:1119–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gelfand JM, Hoffstad O, Margolis DJ. Surrogate endpoints for the treatment of venous leg ulcers. J Invest Dermatol 2002;119:1420–5. [DOI] [PubMed] [Google Scholar]
- 43. Kantor J, Margolis DJ. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br J Dermatol 2000;142:960–4. [DOI] [PubMed] [Google Scholar]
- 44. Margolis DJ, Berlin JA, Strom BL. Which venous leg ulcers will heal with limb compression bandages? Am J Med 2000;109:15–9. [DOI] [PubMed] [Google Scholar]
- 45. Phillips TJ, Machado F, Trout R, Porter J, Olin J, Falanga V. Prognostic indicators in venous ulcers. J Am Acad Dermatol 2000;43:627–30. [DOI] [PubMed] [Google Scholar]
- 46. Letouze A, Voinchet V, Hoecht B, Muenter KC, Vives F, Bohbot S. Using a new lipidocolloid dressing in paediatric wounds: results of French and German clinical studies. J Wound Care 2004;13:221–5. [DOI] [PubMed] [Google Scholar]
- 47. Meaume S, Ourabah Z, Cartier H, Granel‐Brocard F, Bressieux JM, Bohbot S. Evaluation of a lipidocolloid wound dressing in the local management of leg ulcers. J Wound Care 2005;7:329–34. [PubMed] [Google Scholar]
- 48. Smith J, Barrett S, Hayes W, Kirby P, Walsh S, Gittins E, Whitehurst F, Cooper R. Evaluation of Urgotul® plus K‐Four compression for venous leg ulcers. Br J Nurs 2004;13(TVN Suppl):S20–8. [DOI] [PubMed] [Google Scholar]


