Abstract
The aim of this analysis was to examine the cost‐effectiveness of Contreet Foam (A) in comparison with three other commonly used venous leg ulcer treatment protocols: Aquacel Ag (B), Actisorb Silver (C) and Iodoflex (D). A health‐economic analysis reflecting the UK treatment practice and cost structure was performed. The analysis was set up to assess the cost of relative wound area reduction over a 4‐week treatment period. The model was validated by a UK expert panel consisting of four wound care specialists. To assure that the 4‐week model had a realistic link to cost‐effectiveness of complete wound healing, a Markov analysis was also performed. Sensitivity analyses were carried out to ensure validity. Protocol A and C proved to be the most effective treatments. The mean relative reduction in wound area after 4 weeks of treatment was 50·2% (protocol A), 23·9% (protocol B), 44·6% (protocol C) and 36·0% (protocol D). Cost‐effectiveness ratios showed that protocol A proved to be the most cost‐effective treatment, and protocol B the least. The cost per percentage reduction in wound area was £9·50 for protocol A, compared to £16·50–17·60 for the other treatment options. The cost‐effectiveness of complete healing (Markov analysis) and sensitivity analyses confirmed these results. Using Contreet Foam instead of the other dressing alternatives may imply savings of £2·2–4·4 million per year to the National Health Service.
Keywords: Cost‐effectiveness, Contreet Foam, Silver, Venous leg ulcer, Antimicrobial treatment
Introduction
Leg ulceration associated with venous insufficiency affects approximately 1% of the Western population (1, 2), and with an expected increase in the number of older people over the next decades, a corresponding increase in age‐associated medical problems is to be expected (3). Venous leg ulceration imposes a large economic burden on society. In the UK, treatment costs for chronic venous leg ulcers have been estimated to be as high as £5200 per year per patient (4), of which nursing time has been identified as one of the main cost drivers (5, 6).
A recent review of the research in this field (2) has shown that 13–29% of venous leg ulcers may take more than 2 years to reach complete healing, whereas 50–75% of the ulcers healed within 1 year. An acceptable healing time is hard to define, and chronic leg ulcers have been defined as being of a chronic nature if they remained unhealed for 4 weeks by some (7) and by others as chronic if they remained unhealed for 3 months (8). It is widely accepted that many ulcers with delayed healing are bacterially challenged, and it may well be that excess bacteria cause the delay in healing (9). In a normal situation, the natural microbial flora of a wound is in balance. However, when the bio‐burden exceeds a host‐manageable level, a wound may become clinically infected (10). The microbial progression preceding wound infection is described by Bowler (2001) (10) and is referred to as ‘critically colonised’ (9, 11) and is a condition without signs of clinical infection in which the bio burden is close to or at the maximum manageable level by the patient (11).
Recently, different opportunities have emerged to support the healing of critically colonised ulcers. The combination of the well‐accepted antibacterial effect of sustained‐release silver with effective exudate management has proven to be a clinically effective means of treating delayed healing venous leg ulcers (12, 13). The price of a dressing alone is not an accurate reflection of the cost‐effectiveness of treating a wound, and new innovative wound care dressings should not only prove to be clinically effective to be regarded as feasible treatment alternatives, but also prove to be cost‐effective (14, 15).
The objective of this analysis was to examine the cost‐effectiveness of Contreet Foam, a new sustained silver‐releasing dressing, compared to three other commonly used antibacterial dressings in venous leg ulcer treatment protocols.
Methods
Traditional clinical research answers the question ‘Is the treatment efficacious and safe?’ However, the questions ‘Is it cost‐effective?’ and ‘Is it an efficient use of society's resources?' are normally not answered by clinical trials. Both these questions can be answered by performing an in‐depth health‐economic analysis. To be useful, economic studies must include clinical outcomes with specific endpoints such as reduction in wound area or healing rates for example. However, many studies have not considered outcomes with specific endpoints. As a result, lack of comparable data from wellconducted randomised trials makes it difficult to make evidence‐based purchasing and treatment decisions.
To investigate the cost‐effectiveness of Contreet Foam in patients with critically colonised venous leg ulcers, a health‐economic analysis was performed. The analysis was performed with a societal perspective in a UK context to specifically reflect the UK treatment practice and cost structure. The four treatment protocols are described in Table 1.
Table 1.
Treatment protocols
| Compression therapy | ||||
|---|---|---|---|---|
| Wound contact dressing 0–4 weeks* | Absorbent dressing 0–4 weeks* | 0–4 weeks* | 8–26 weeks† | |
| Protocol A | Contreet Foam | Not needed | Comprilan | Profore |
| Protocol B | Aquacel Ag | Combiderm‐N | Comprilan | Profore‡ |
| Protocol C | Actisorb Plus§ | Tielle Plus Borderless¶ | Comprilan | Profore |
| Protocol D | Iodoflex | N‐A Dressing | Comprilan | Profore |
0–8 weeks in 26‐week sensitivity analysis.
Only used in 26‐week sensitivity analysis.
No 26‐week analysis was made due to lack of healing data.
§Actisorb Plus was renamed Actisorb Silver 220 in 2000 ( 38).
An analysis was also made using Mesorb Mölnlycke as absorbent dressing.
Contreet Foam is manufactured by Coloplast A/S. Aquacel Ag and Combiderm‐N are manufactured by Convatec, Actisorb Plus and Tielle Plus Borderless are manufactured by Johnson & Johnson. Iodoflex, NA dressing, and Profore are manufactured by Smith & Nephew, Comprilan is manufactured by Beiersdorf.
It has been shown that the percentage change in wound area in venous leg ulcers predicts whether a wound is progressing towards complete healing (16, 17). Therefore, the endpoint of this health‐economic analysis is cost per percentage reduction in wound area. Thus, a model was set up to assess the cost of relative wound area reduction over a 4‐week period (Figure 1A). Exact clinical trial data and costs associated with the ulcer treatment were compiled in a health‐economic model to compare the four treatment alternatives in a 4‐week time horizon. Subsequently, to assure that this 4‐week model had a realistic link to cost‐effectiveness of complete wound healing, a Markov analysis was used. No studies following patients to complete healing were available, and therefore a Markov model was used, which is a standard approach in health‐economic research when an intervention or treatment takes place over a long period of time or when there is no long‐term data available (18). The model was used to test the relevance and validity of the 4‐week model. This model is described in detail in the sensitivity analysis section.
Figure 1.
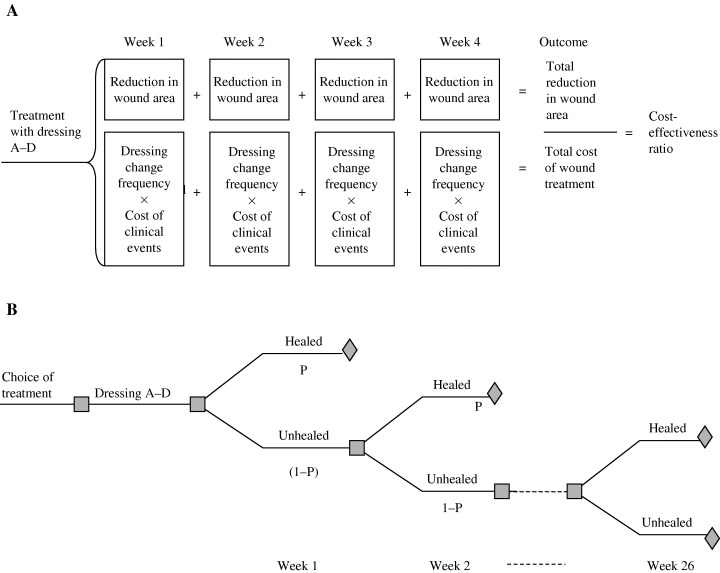
(A) A 4‐week health‐economic model. The model calculates the cost‐effectiveness of protocol A–D by relating reduction in wound area to cost of treatment and dressing change frequency. (B) The Markov model was used to estimate cost‐effectiveness of complete healing. Based on this model, the 4‐week model (A) was shown to be a valid predictor of the cost‐effectiveness of healing critically colonised venous leg ulcers.
Expert validation of models
An expert panel consisting of four wound care experts, their selection being based on their research profiles and clinical experience, was used to validate the analysis. The role of the panel was to provide details on national treatment standards, to reach consensus where information was lacking in the literature, to evaluate data used in the models, to secure the relevance of the models and to provide details and verification of costing procedures, and hereby to optimise the conclusions of the analyses. The members of the expert panel are marked with a star on the authors' list.
Clinical events and costs
Treatment protocols
In the health‐economic analysis, it was assumed that all patients went through an initial wound assessment by a district nurse. As the majority of treatments for leg ulceration in Western countries take place in the community (19), both models assume that a qualified nurse performs all dressing changes in the patient's home. In the UK, the use of physicians' time is effectively zero in the management of venous leg ulcers (20), and therefore only nursing time has been included in the models.
The mean wear time of the individual treatments determined the dressing change frequency, and thereby the visiting frequency of the district nurses. Treatment protocols applied in the model were based on the actual procedures described in published reports for the dressings in protocol A–D, manufacturers' instructions for use, and the expert panel's advice. In all protocols, short stretch bandages (Comprilan, Beiersdorf, AG, Hamburg, Germany) were applied over the primary dressing. The risk of a clinical wound infection occurring (Table 2) was integrated into both models and was in accordance with standard practice treated with amoxycillin 500 mg three times a day for 10 days (20, 21). A general risk of infection was applied, as no dressing‐specific data of infection risk were available (22).
Table 2.
Clinical data used in the 4‐week model
| Treatment | Parameter | Basic analysis | Data used for sensitivity analyses | |
|---|---|---|---|---|
| Protocol A (12, 13) | Reduction in wound size after 4 weeks* | 50·2% | 40·12–60·24% | |
| Weekly healing rate* | – | 4·45% | ||
| Dressing change frequency* | 2·19 per week | 1·75–2·63 | ||
| Protocol B (30) | Reduction in wound size after 4 weeks | 23·9% | 19·12–28·68% | |
| Weekly healing rate | – | Not available | ||
| Dressing change frequency | 1·9 per week | 1·52–2·28 per week | ||
| Protocol C (32) | Reduction in wound size after 4 weeks | 44·63% | 35·70–53·56% | |
| Weekly healing rate | – | 6·13% (31) | ||
| Dressing change frequency | 3·6 per week | 2·9–4·3 per week | ||
| Protocol D (39) | Reduction in wound size after 4 weeks | 36% | 28·8–43·2% | |
| Weekly healing rate | – | 1·28% | ||
| Dressing change frequency | 2·7 per week | 2·16–3·24 per week | ||
| Four‐layer bandage system (29) | Weekly healing rate | – | 7·4% | |
| Dressing change frequency | – | 1·1 per week | ||
| Infection rate (22) | Weekly risk of infection | 2·3% | – |
The table also shows the data used to test the strength of the 4‐week model in the applied sensitivity analyses.
*Mean of data from several studies.
Wound progression data
Healing progression based on the different treatment options was derived from published studies found after performing a thorough literature search (Table 3). The search was based on a database search (Medline, Cinahl, Embase, year 1966–2003) supplemented with a relevant hand search. Studies with a minimum of 15 patients with venous leg ulcers could be included in the analysis. Furthermore, studies where dressing change frequency was based on prefixed intervals were not included, as they do not reflect clinical practice. Wounds should preferably be described as critically colonised or delayed in healing. Where possible, data from more than one study were combined to determine the treatment‐specific healing patterns. For protocol A (Table 2), data from two clinical trials could be combined. Weekly relative reduction in wound area was calculated based on the information of absolute reduction.
Table 3.
Overview of the different studies used in the analysis
| Protocol | Reference | Study design | Type of wound | Duration of study and use of active treatment | Evaluated patients (total number included) | Ulcer size reported at inclusion (cm2) |
|---|---|---|---|---|---|---|
| A | Jørgensen et al. ( 13 ) | Comparative, randomisedcontrolled multicentre | Moderate‐to‐high exuding leg ulcerswith characteristics of delayed healing | 4 weeks | 109 evaluated (129 included)52 treated with Contreet Foam | Median 5·9 (range 1·9–37·4) |
| Karlsmark et al. ( 12 ) | Non comparative prospectivestudy, multicentre | Moderate‐to‐high exuding leg ulcerswith characteristics of delayed healing | 4 weeks | 23 evaluated (25 included) | Mean 15·6 (range 3·0–58·1) | |
| B | Vanscheidt et al. ( 30 ) | Open, multicentre, | Chronic leg ulcers, mixed ethiology, | 4 weeks | 15 evaluated (18 included) | Not reported |
| non randomised, | critically colonised | |||||
| non comparative | ||||||
| C | Wunderlich & Orfanos (31) | Randomised controlled | High exudate level | 6 weeks | 40 evaluated (40 included), | Mean 30 |
| multicentre comparative study | (‘bakteriell super‐in‐fiziert’) | 19 treated with Actisorb Silver | ||||
| Tebbe & Orfanos (32) | Prospective, non randomised,non comparative | Venous leg ulcers (critically colonised) and pressure ulcers (only results from leg ulcer patients are used) | 4 weeks | 156 evaluated with leg ulcersand 68 with pressure ulcers | Mean leg ulcers 24·2 | |
| D | Hansson et al. (39) | Randomised, controlled, | Exudating or sloughy ulcers. | 12 weeks or until | 125 evaluated (153 included), | Mean 8·8 (SD 11·9) |
| multicentre | Clinical infection was an exclusion criterion | the wound is dry | 49 treated with Iodoflex for 4 weeks |
Cost of wound treatment
The cost of wound treatment was divided into the cost of an initial assessment, the cost of dressing change and the cost of treatment of a clinical wound infection (Table 4). No cost of hospitalisation in relation to wound infection was applied. The short stretch compression bandage was reused 10 times for each patient as recommended by the manufacturers. Direct costs of dressings and other products used during a dressing change were mainly derived from the National Health Service Drug Tariff, March 2004 (23) and from other publicly available sources (Table 5). The nursing time needed to perform the initial wound assessment and a typical dressing change was estimated by the expert panel to be 30 min and 40 min, respectively. Debridement was assumed to be autolytical and not requiring further resources. Nursing time costs and nurse transportation costs were based on the annually published ‘Unit costs of health and social care’ (Personal Social Services Research Unit, 2003) (24).
Table 4.
Parameters related to cost of clinical events
| Initial wound assessment |
| Nursing/physician time |
| Dressing change |
| Dressing change frequency |
| Nursing/physician time |
| Transportation of nurse |
| Wound cleansing and autolytical debridement |
| Dressing materials |
| Ancillary supplies |
| Care of wound infection |
| Treatment with systemic antibiotics |
Table 5.
Unit costs (£) used in the analyses
| Basic analysis (£) | Data use for sensitivity analyses | |
|---|---|---|
| Product costs | ||
| Contreet Foam (10 × 10 cm)* | 6·95 | Constant |
| Aquacel Ag (10 × 10 cm)* | 3·93 | Constant |
| CombiDERM‐N (14 × 14 cm)* | 1·94 | Constant |
| Iodoflex (10 g)† | 7·80 | Constant |
| N‐A Dressing (9·5 × 9·5 cm)* | 0·32 | Constant |
| Actisorb Silver 220 (10·5 × 10·5 cm)* | 2·35 | Constant |
| Tielle Plus Borderless (11 × 11 cm)* | 2·88 | Constant |
| Comprilan, reused 10 times (10 cm × 5 cm)* | 0·33 | Constant |
| Profore (four‐layer bandage system)* | 8·76 | Constant |
| Saline for irrigation* | 0·27 | Constant |
| Gloves‡ | 0·03 | Constant |
| Mesorb (15 × 20 cm)* | 1·22 | Constant |
| Labour cost | ||
| District nurse cost per visit (40 min)§ | 41·95 | £33·56–50·34¶ |
| Transportation cost | ||
| Cost per visit§ | 1·17 | Constant |
Prices are from National Health Service Drug Tariff, March 2004.
Prices are from British National Formula (BNF) August 2002.
National Health Service, Logistic Authority, April 2004.
Personal Social Services Research Unit, 2003.
Equal to a variation in nursing time from 32 min to 48 min.
These data were used to calculate a weekly cost of wound management for each protocol and further to calculate the cost per percentage reduction in wound area.
Due to the relatively high average age of people with venous leg ulcers, no costs of lost workdays were applied, and no discounting of costs were performed due to the relatively short time horizon of the model, as is normal practice in health‐economic models.
Sensitivity analysis
The robustness and validity of the 4‐week cost‐effectiveness analysis was tested through systematic variation of key parameters and extrapolation of healing rates to complete healing. Using this approach, any uncertainty related to the clinical data (actual or estimated) and cost data was challenged. Parameters associated with uncertainty (clinical data and nursing time) were varied ±20% as listed in 2, 5. The use of gauze dressing (Mesorb, Mölnlycke Healthcare, AB Gothenburg, Sweden) as an absorbent dressing, instead of Tielle Plus Borderless in protocol C, was tested as well.
To effectively test the link between cost‐effectiveness of reduction in wound area and complete healing, a model predicting complete healing was used. As the median healing time for venous leg ulcers generally varies between 12 and 26 weeks (4, 25, 26), the time horizon for this model was chosen to be 26 weeks (6 months) The well‐established principle of a Markov chain model (18) was used to develop a decision model which reflects the possible clinical outcomes and costs in a hypothetical cohort of patients (27) with delayed healing venous leg ulcers. The patients were modelled to be in one of two ulcer states; healed or not healed. Transition from not healed to healed could be made on a weekly basis determined by the probability of complete healing for each treatment (Figure 1B).
Clinical events and costs associated with these ulcer states were aggregated with a computerised Markov model (28). The endpoint for this analysis was cost per healed wound.
Weekly complete healing percentages were calculated from information on the number of healed ulcers using standard economic methods. This method takes into account that the number of unhealed wounds is reduced each week in the 26‐week model as wounds progress towards healing and has also been used by Carr et al. (29). In the studies where complete healing percentages were not available (12, 30), individual patient data on wound area reduction was needed to extrapolate to complete healing percentages. Such data were not available in the study by Vanscheidt et al. (30). Consequently, protocol B could not be incorporated in the 26‐week model.
The rationale for using an antibacterial dressing and/or a dressing designed to manage moderate‐to‐high amounts of exudate depends on several factors, the most important being the bacterial load in the wound, the exudate production in the wound and the wound bed appearance as some of the most important factors (9). When the condition of the wound changes, the treatment has to reflect this change. Therefore, when the exudate level decreases as a consequence of the lowered bacterial load in the wound, the treatment principle should be adjusted. The events occurring from 0 to 26 weeks comprise a switch to a four‐layer bandage system after 8 weeks of treatment. When this switch occurs, the four‐layer bandage system replaces the antimicrobial dressings the patients are treated with in the beginning of the models, as this system also includes a wound‐contact layer (Table 1). In the UK, the four‐layer compression bandage system (Profore, Smith and Nephew) is the most widely used compression principle for moderate‐to‐low exuding venous leg ulcers (assumption by expert panel) and was therefore used in this model.
Results
The wound‐healing progress for the different treatment options is shown in Figure 2. Protocol A and C proved to be the most effective treatments. The mean relative reduction in wound area after 4 weeks of treatment was 50·2% (protocol A), 23·9% (protocol B), 44·6% (protocol C) and 36·0% (protocol D) (Table 2). The cost per dressing change and cost per week of treatment (Table 6) demonstrate that when the dressing change frequency was integrated in the cost calculation, the weekly cost of using Contreet Foam was £111·1, compared to £96·9–176·4, for the other dressing alternatives.
Figure 2.
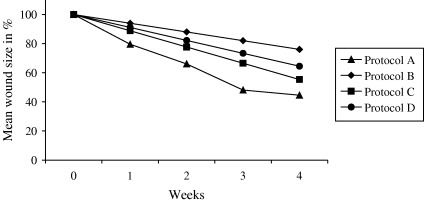
Reduction in wound area using the different protocols. Data are obtained from the sources listed in Table 2.
Table 6.
Cost‐effectiveness data
| Protocol | Cost per dressing change (£) | Number of dressing changes per week | Cost per week of treatment (£) | Reduction in wound size after 4 weeks (%) | Cost per percentage reduction in wound area (£) |
|---|---|---|---|---|---|
| A | 50·75 | 2·19 | 111·13 | 50·2 | 9·51 |
| B | 49·67 | 1·90 | 96·86 | 23·9 | 17·58 |
| C | 49·01 | 3·60 | 176·42 | 44·6 | 16·54 |
| D | 51·91 | 2·70 | 140·15 | 36·0 | 16·48 |
More important, when relating healing‐progression data to the cost of treatment by calculating the cost‐effectiveness ratios, protocol A proved to be the most cost‐effective treatment (Figure 3). The cost per percentage reduction in wound area was £9·5 for protocol A, compared to £16·5–17·6, for the other treatment options (Table 6).
Figure 3.
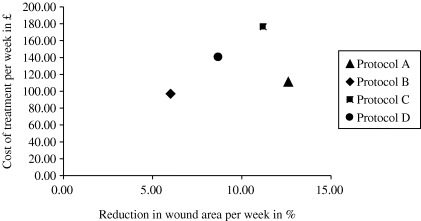
Relation between cost‐effectiveness and reduction in wound area.
Sensitivity analyses
The sensitivity analyses on wound‐progression data did not change the overall results of protocol A being the most cost‐effective choice of treatment, and protocol B being the least cost‐effective treatment choice.
It is evident that the main cost driver was nursing time, i.e. the hierarchy changed markedly when dressing change frequency was incorporated in the analysis.
When wear time and thereby dressing change frequency varied, it did not change the overall results that protocol A was the most cost‐effective treatment choice.
A relevant variation in treatment practice was tested for protocol C. The absorbent dressing used in protocol C was changed from a foam dressing (Tielle Borderless Plus) to a less costly gauze pad (Mesorb), which lowered the weekly costs of wound care management using protocol C to £170·4. However, this did not change the conclusion of protocol A being the most cost‐effective alternative.
The prediction of cost‐effectiveness of complete wound healing confirmed the clinical findings in the 4‐week model. The proportion of healed wounds after 26 weeks of treatment was 81·9% (protocol A), 84·6% (protocol C) and 75·7% (protocol D).
When costs were combined with the healing data, the cost‐effectiveness results of the special sensitivity analysis also substantiated the hierarchy from the 4‐week model. However, the data used for prediction of the healing in model C was taken from another source (31) than the data used for protocol C in the 4‐week model, because the study by Tebbe & Orfanos (32) did not report complete healing percentages. However, the Wunderlich & Orfanos (31) data are based on fixed dressing changes (one time per day) and do as such not fulfil the criteria set up for the basic analysis and have therefore not been incorporated in the basic 4‐week analysis.
Based on this analysis, where complete healing was the endpoint, the cost of healing one wound is predicted to £1521 for protocol A as opposed to £1892–2276, if using the other dressing alternatives (Table 7).
Table 7.
Results of sensitivity analyses after ±20% variation in key parameters and prediction to complete healing
| Protocol | Parameter varied | Cost per percentage reduction in wound area (£) | Cost per healed wound (£) |
|---|---|---|---|
| A | After variation of reduction in wound area | 7·9–11·8 | – |
| After prediction to complete healing | – | 1521 | |
| After variation of dressing change frequency | 7·7–11·3 | – | |
| After variation of nursing time | 7·9–11·1 | – | |
| B | After variation of reduction in wound area | 14·6–22·0 | – |
| After prediction to complete healing | – | Not available | |
| After variation of dressing change frequency | 14·3–20·8 | – | |
| After variation of nursing time | 14·5–20·7 | – | |
| C | After variation of reduction in wound area | 13·8–20·7 | – |
| After prediction to complete healing | – | 1892 | |
| After variation of dressing change frequency | 13·4–19·7 | – | |
| After variation of nursing time | 13·8–19·3 | – | |
| D | After variation of reduction in wound area | 20·6–13·7 | – |
| After prediction to complete healing | – | 2276 | |
| After variation of dressing change frequency | 13·4–19·6 | – | |
| After variation of nursing time | 13·7–19·2 | – |
Discussion and limitations
This health‐economic analysis examined the costs associated with the management of critically colonised leg ulcers and has established a hierarchy of cost‐effectiveness amongst the dressings studied. Contreet Foam proved to be the most cost‐effective treatment among these widely used wound management alternatives.
Furthermore, the analysis showed that dressing selection should not be based on the cost of individual dressings alone. A wider perspective is needed to assess the true cost and benefit relationship. The present analysis includes among others nursing time, dressing change frequency, dressings and ancillary supplies, and most importantly, relates these cost factors to the clinical outcomes of the treatment protocol used.
The results of this analysis are generally in line with other studies. Cost per percentage reduction in wound area in venous leg ulcers has been reported to be in the range of £8–11 (20), which is similar to the range found in our analysis (£9.5–17.6).
Bosanquet (33) has suggested that the cost of leg ulceration to the UK health service is between £1000 and £5200 per patient per year, and Harding et al. (2001) (20) have found that the cost of healing an ulcer with moist wound healing dressings approximates £1200. In comparison, the majority of leg ulcers in this analysis healed within 6 months at a cost of £1,521–2334.
The 4‐week model calculating cost per percentage reduction in wound area is a very accurate and definite measurement of the cost‐effectiveness, because it is based on factual data. No extrapolations were necessary, because the time horizon of this model did not go beyond the research period of the clinical studies included in the analysis. The results of this 4‐week model were also confirmed in the 26‐week model where complete healing was evaluated. The strength of the 26‐week model is that it describes the consequences progressing from wounds being critically colonised over chronic wounds being in bacterial balance, to completely healed wounds to reflect a real life situation, i.e., all sensitivity analyses performed contribute to the reliability of the results.
Conclusion and recommendations
To more realistically assess the true societal impact of adopting a new treatment such as Contreet Foam, the results from this analysis should be further contemplated asking: ‘What is the practical impact if choosing to treat delayed healing, critically colonised venous leg ulcers with a new treatment’? The literature indicates (17, 34) that a wound area reduction of less than 20–40% over the initial 2–4 weeks is a reliable indicator that the wound is not responding well to treatment. In protocol B (Aquacel Ag), where the reduction after 4 weeks treatment was 23·9%, reaching complete healing within an acceptable time horizon, is questionable. Protocol B could unfortunately not be tested in the model set up to predict complete healing, as the required data was not available. Therefore, it remains unclear what the clinical and financial impact of using this dressing has on achieving complete healing. Furthermore, the clinical relevance of the relatively long wear time reported in protocol B could be questioned, since a relatively high frequency of leakages (30%) was reported in that study (30). This may indicate that the dressing, if used optimally, should be changed more frequently. As nursing time and thereby the dressing wear time has been shown in this analysis and elsewhere (5, 6) to have a considerable impact on the cost‐effectiveness of wound management, a shorter wear time could lead to even higher weekly costs if choosing protocol B.
The incidence of venous leg ulcers has been reported to be around 0·76% per year among people aged 65 and older (35). This equates to a total of 63 000 ulcers diagnosed in the UK (0·76% of 8·31 million people; number of people in UK over 65 years) (36).
On this basis and the fact that at least 28% (17 640) (4) will not be healed within 1 year, and further assuming that approximately one‐third (estimated by the expert panel) of chronic venous leg ulcers are delayed in healing due to bacteria, antibacterial dressings would be suitable for use in at least 5800 ulcers annually. When using protocols A, C or D, the cost for the health care system to heal 76–85% percent of these wounds during a 26‐week period would be between £8·8–13·2 million, when assuming that 5800 patients would benefit from this treatment. Furthermore, using protocol A instead of the other dressing alternatives may, based on this analysis, imply savings of £2·2–4·4 million per year to the National Health Service.
Although this analysis sets out to analyse the cost‐effectiveness of treating delayed healing, critically colonised venous leg ulcers, which is a subset of venous leg ulcers in general, it is foreseen that the average cost of leg ulcer treatment will be decreased, if these troublesome wounds are treated more effectively. Furthermore, a more rapid and effective healing process will also add to the quality of life for the patients (37). Protocol A has been shown to effectively reduce odour and pain originating from the wound (12, 13), which supports the use of protocol A viewed from the patients' perspective.
In conclusion, Contreet Foam (protocol A) provides a clinically effective and more cost‐conscious treatment alternative in the treatment of bacterially challenged wounds.
Acknowledgements
This study is supported by a grant from Coloplast A/S. The views expressed in this publication are those of the authors and do not necessarily reflect those of Coloplast A/S.
ES, TK and DJL are members of an expert panel.
References
- 1. Callam MJ, Ruckley CV, Harper DR, Dale JJ. Chronic ulceration of the leg: extent of the problem and provision of care. Br Med J 1985;290(6485):1855–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leach MJ. Making sense of the venous leg ulcer debate: a literature review. J Wound Care 2004;13(2):52–6. [DOI] [PubMed] [Google Scholar]
- 3. Burgess B. An investigation of hydrocolloids. Prof Nurse 1993;8(7 Suppl 3):3–6. [PubMed] [Google Scholar]
- 4. Doherty D, Ross F, Yeo L, Uttley J. Leg ulcer management in an integrated service. The South Thames Evidence Based Practice (STEP), Project Report (6), June 2000.
- 5. Dealey C. The cost of wound care. Nursing 1990;4(15):14–6. [PubMed] [Google Scholar]
- 6. National Institute for Clinical Excellence (NICE) London. Guidance on the use of debriding agents and specialist wound care clinics for difficult to heal surgical wounds. NICE, April 2001, Technology Appraisal Guidance No. 24.
- 7. Scottish Intercollegiate Guidelines Network (SIGN). The Care of Patients with Chronic Leg Ulcer. A National Clinical Guideline. Edinburgh: Sign Publication, 1998: 1–21. [Google Scholar]
- 8. Nelzen O, Bergqvist D, Lindhagen A. Leg ulcer ethiology – a cross sectional population study. J Vasc Surg 1991;14(4):557–64. [PubMed] [Google Scholar]
- 9. Hess CT, Kirsner RS. Orchestrating wound healing: assessing and preparing the wound bed. Adv Skin Wound Care 2003;16(5):246–57. [DOI] [PubMed] [Google Scholar]
- 10. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001;14(2):244–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sibbald RG, Orsted H, Schultz GS, Coutts P, Keast D. Preparing the wound bed 2003: focus on infection and inflammation. Ostomy Wound Manage 2003;49(11): 24–51. [PubMed] [Google Scholar]
- 12. Karlsmark T, Agerslev RH, Bendz SH, Larsen JR, Roed‐Petersen J, Andersen KE. Clinical performance of a new silver dressing, Contreet Foam, for chronic exuding venous leg ulcers. J Wound Care 2003;12(9):351–4. [DOI] [PubMed] [Google Scholar]
- 13. Jørgensen B, Price P, Andersen KE, Gottrup F et al. The silver‐releasing foam dressing, Contreet Foam, pramotes faster healing of critically colonised venous leg ulcers: a randomised controlled trial. Int Wound J 2005;2(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hermans M, Bolton L. The influence of dressings on the costs of wound treatment. Dermatol Nurs 1996;8(2):93–100. [PubMed] [Google Scholar]
- 15. Jones AM. Using an economic model to assess cost‐effectiveness. J Wound Care 2004;13(4 Suppl):1–7. [Google Scholar]
- 16. Donohue K, Falanga V. Healing rate as a prognostic indicator of complete healing: a Reappraisal. Wounds 2003;15(3):71–6. [Google Scholar]
- 17. Flanagan M. Wound measurement: can it help us to monitor progression to healing? J Wound Care 2003;12(5):189–94. [DOI] [PubMed] [Google Scholar]
- 18. Sonnenberg FA, Beck R. Markov models in medical decision making: a practical guide. Med Decis Making 1993;13(4):322–38. [DOI] [PubMed] [Google Scholar]
- 19. Cullum N. The nursing management of leg ulcers in the community: a critical review of research. Department of Nursing, University of Liverpool. Leg Ulcers 1994;10(2). [Google Scholar]
- 20. Harding KG, Price P, Robinson B, Thomas S, Hofman D. Cost and dressing evaluation of hydrofiber and alginate dressings in the management of community‐based patients with chronic leg ulceration. Wounds 2001;13(6):229–36. [Google Scholar]
- 21. Meaume S, Gemmen E. Cost‐effectiveness of wound management in France: pressure ulcers and venous leg ulcers. J Wound Care 2002;11(6):219–24. [DOI] [PubMed] [Google Scholar]
- 22. Tammelin A, Lindhold C, Hambraeus A. Chronic ulcers and antibiotic treatment. J Wound Care 1998;7(9):435–7. [DOI] [PubMed] [Google Scholar]
- 23. National Health Service England and Wales, Department of Health. Drug Tariff. London: The Stationary Office, 2004. [Google Scholar]
- 24. Personal Social Services Research Unit. Unit Costs of Health and Social Care 2003. Compiled by Ann Netten and Lesley Curtis. E‐mail: pssru_library@kent.ac.uk.
- 25. Meyer FJ, McGuiness CL, Lagattolla NR, Eastham D, Burnand KG. Randomized clinical trial of three‐layer paste and four‐layer bandages for venous leg ulcers. Br J Surg 2003;90(8):934–40. [DOI] [PubMed] [Google Scholar]
- 26. O'Brien JF, Grace PA, Perry IJ, Hannigan A, Clarke Moloney M, Burke PE. Randomized clinical trial and economic analysis of four‐layer compression bandaging for venous ulcers. Br J Surg 2003; 90(7):794–8. [DOI] [PubMed] [Google Scholar]
- 27. Briggs A, Sculpter M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics 1998;13(4):397–409. [DOI] [PubMed] [Google Scholar]
- 28. DATA 4.0 Treeage Software Inc., Williamstown, MA, 2002.
- 29. Carr L, Phillips Z, Posnett J. Comparative cost‐effectiveness of four‐layer bandaging in the treatment of venous leg ulceration. J Wound Care 1999;8(5):243–8. [DOI] [PubMed] [Google Scholar]
- 30. Vanscheidt W, Lazareth I, Routkovsky‐Norval C. Safety evaluation of a new ionic silver dressing in the management of chronic ulcers. Wounds 2003;15(11):371–8. [Google Scholar]
- 31. Wunderlich U, Orfanos CE. Behandlung der Ulcera cruris venosa mit trockenen Wundauflagen. Der Hautartz 1991;42: 446–50. [PubMed] [Google Scholar]
- 32. Tebbe B, Orfanos CE. Behandlung von Ulcera cruris und Dekubitus mit einem Xerodressing: Phasenübergreifende Wundauflage mit antimikrobieller Wirksamkeit. H+G 1996;71(9):697–702. [Google Scholar]
- 33. Bosanquet N. Venous leg ulcers: 10 years of assessing cost‐effective solutions. The Profore Supplement (sponsored by Smith & Nephew). Br J Community Nurs 2001;6: 27–30. [Google Scholar]
- 34. Flanagan M. Improving accuracy of wound measurement in clinical practice. Ostomy Wound Manage 2003;49(10):28–40. [PubMed] [Google Scholar]
- 35. Margolis D, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer. Incidence and prevalence in the elderly. J. Am Acad Dermatol 2002; 46(3):381–6. [DOI] [PubMed] [Google Scholar]
- 36. Office for National Statistics 2003. Population at mid‐2000: England: Estimated resident population by single year of age and gender. Retrieved from http://www.statistics.gov.uk/statbase/mainmenu.asp
- 37. Price P. Health‐related quality of life and the patient's perspective. J Wound Care 1998;7(7):365–6. [DOI] [PubMed] [Google Scholar]
- 38. White RJ. A charcoal dressing with silver in wound infection: clinical evidence. Br J Nurs 2001;6(12 Silver Suppl 2):4–11. [Google Scholar]
- 39. Hansson C and the Cadexomer Iodine Study Group. The effects of cadexomer iodine paste in the treatment of venous leg ulcers compared with hydrocolloid dressing and paraffin gauze dressing. Int J Dermatol 1998;37: 390–6. [DOI] [PubMed] [Google Scholar]


