ABSTRACT
Background: This study was designed to elucidate the in vivo efficacy of epidermal growth factor (EGF) on wound healing in non diabetic and diabetic rats.
Methods: Ninety‐six male Wistar‐Albino rats were randomly divided into six groups. Saline‐moistened gauze, pure gelatin or EGF in gelatin‐microsphere dressings were used in a dermal excision model in both normal and streptomycin‐induced diabetic rats. Wound healing was evaluated on day 7 and 14. Reduction in wound area, hydroxypyroline content and tensile strength of the wound were evaluated in each rat. Tissue samples taken from the wounds were examined histopathologically for reepithelialisation, cellular infiltration, number of fibroblasts, granulation and neovascularisation.
Results: On day 7, the use of EGF‐containing dressing was observed to reduce the wound area better when compared with the other dressings tested. This effect was significant in normal rats rather than diabetic rats. The difference in reduction of wound area did not persist on day 14. No significant effect on hydroxyproline content of the wound was found with EGF‐containing dressing in either normal or diabetic rats. There was a statistically significant increase in tensile strength values of EGF‐applied non diabetic rats over the 14 day period. An increase in tensile strength was prominent in also EGF‐applied diabetic rats on day 14. Histological examination revealed higher histopathologic scores in EGF‐applied diabetic and non diabetic rats.
Conclusion: These findings implicate that use of EGF in gelatin‐microsphere dressings improves wound healing both in normal and diabetic rats.
Keywords: Diabetes mellitus, Epidermal growth factor, Hydroxyproline content, Tensile strength, Wound healing
INTRODUCTION
Tissue repair and wound healing is a complex, dynamic process, involving overlapping steps of haemostasis and inflammation, migration and proliferation of certain cells, angiogenesis, extracellular matrix deposition, maturation and remodelling (1). Normal wound healing requires the interactions of various types of cells, including inflammatory cells, fibroblasts, keratinocytes and endothelial cells, as well as the involvement of growth factors and enzymes. Numerous growth factors are involved in wound healing process and act by stimulating chemotaxis, cellular proliferation, extracellular matrix formation and angiogenesis 1, 2.
Local and systemic factors can disrupt the complex process of wound repair and alter the healing trajectory (3). Diabetes mellitus perhaps is the most common and most studied disease leading to impaired wound healing (4). The compromised wound healing in diabetics is due to immune system and inflammatory defects inherent to diabetes mellitus (5). It was reported that a number of growth factors were markedly reduced in wound fluid from diabetic wounds 6, 7. In diabetic patients, the inflammatory process is abnormal and the proliferation of fibroblasts and endothelial cells is impaired 8, 9, 10. High serum glucose levels lead to a decrease in collagen deposition and impaired wound remodelling. Abnormal wound contraction and a reduction in breaking strength is observed in diabetes 11, 12. It is also well known that, diabetics are more susceptible to wound infection because of impaired neutrophil chemotaxis and phagocytosis 13, 14.
There are many sophisticated dressings in the market to aid in wound management, and several ongoing studies focus on the agents modulating the aspects of wound biology. New and advanced topical dressings are designed to modulate levels of biological molecules, such as growth factors, that may promote wound healing. Growth factor treatments and their potential use in healing chronic wounds have recently been described and both in vivo and in vitro data have demonstrated the efficacy of growth factors in enhancing wound healing 15, 16. Platelet derivated growth factor (PDGF), fibroblast growth factor (FGF), transforming growth factor and epidermal growth factor (EGF) are the most studied growth factors 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31. Early experimental studies have shown the potential of EGF in promoting wound healing but there are only a few clinical trials which have documented the efficacy of EGF in chronic wounds, and more evidence is needed to clarify the clinical recommendations related to the use of EGF in wound management.
We designed this study to elucidate the in vivo efficacy of EGF on epidermal regeneration and wound healing in non diabetic and diabetic rats.
METHODS
Our study protocol was reviewed and approved by Ankara University ethical committee. All animals received humane care in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication No. 85–23, revised 1985).
Preparation of EGF‐containing microspheres, gelatin sponge and gelatin sponge with EGF‐loaded microspheres
Gelatin microspheres were prepared by a modified coacervation technique reported by Nastruzzi's (32). In order to prepare the EGF‐containing microspheres, EGF (human recombinant EGF, Sigma®, USA) (50 or 750μg in 1 ml phosphate buffer, pH = 7· 4) and heparin (50μl) were added into the aqueous 10% gelatin (Difco®, USA) solution, then the solution was added dropwise into paraffin oil while the mixture was mechanically stirred at 1000 rpm to form a water–oil emulsion. The solution was rapidly cooled by immersing in ice‐water medium. The formed EGF‐containing gelatin microspheres were filtered, washed with acetone and dried at room temperature .
In order to prepare gelatin sponges (GSs), aqueous gelatin solutions stirred at about 2000 rpm for 30 minutes at room temperature and glutaraldehyde solutions were added to form cross‐linkings. Then the solutions were poured into molds, frozen in liquid nitrogen and freeze‐dried for 24 hours. GSs with EGF‐containing microspheres were prepared by adding the microspheres containing EGF just before pouring it into molds. Then the general procedure of sponge formation was followed (33). GSs were sterilised by UV exposure and 1 cm3 of the sponges were applied to the full thickness skin wounds.
Formation of non diabetic and diabetic rat groups
Adult male Wistar–Albino rats weighing between 250 and 300 g were used in this study. All rats were housed in temperature‐ and humidity‐controlled rooms and were allowed free access to standard rat chow and tap water ad lib for 1 week before the start of the experiment.
Diabetes was induced by a single dose of 45 mg/kg streptozocin (Zanosar®, Pharmacia & Up John, USA) through tail vein of the rats. Diabetes was defined as a blood glucose level of greater than 200 mg/dl as measured by glucometer (Glucotrend®, Isse, Turkey). The study was initiated four weeks after the diabetic and non diabetic rat groups were firmly established and blood glucose readings of the rats were taken routinely during the study period.
Ninety six rats were randomly divided into six (three non diabetic and three diabetic) groups. Moistened gauze (MS), GS and GS with EGF‐loaded microspheres (GS‐EGF) were applied to non diabetic and diabetic rat groups. Rats in each group were further divided into two groups in order to evaluate the wound healing on 7th and 14th days.
Surgical procedure
Under ketamine anesthesia (80 mg/kg), asepsis was provided by 10% povidine iodine (Betadine®) and the back of rats were shaved. A circular, full thickness, standard wound was created on the back of each rat by using a 0·5‐cm sterile punch (18 mm2). Three different techniques of wound dressing were applied to non diabetic and diabetic groups of rats. MG was applied to group 1 and 4, GS was applied to group 2 and 5, and GS‐EGF was applied to group 3 and 6, and then covered with polythene sheet (Op‐Site®, Smith & Nephew, UK). The wounds were evaluated for the evidence of infection and the decrease in wound size every 3 days. Wound size was measured by using a grid scale.
Wound healing was assessed on 7th and 14th days. A rectangular shaped 10‐mm wide skin samples, each having the original wound at its center, were excised. Mechanical test was performed on samples immediately, and tensile strength of the wound tissue was calculated. After mechanical measurements, the rectangular samples were divided into two equal parts and one‐half was preserved in 10% formalin solution for histopathological examination. A tissue sample containing wound and normal skin excised from other‐half was preserved in liquid nitrogen tanks for determination of hydroxyproline levels. Finally, rats were euthanised by administering an overdose of sodium pentothal.
Mechanical tests
Lloyd LRX 5 K® mechanical test device was used for the assessment of tensile strength (Lloyd Instruments Limited, Hampshire, UK). Tensile force was applied to the long edge of the rectangular skin samples. The pull interval and pull rate were designated as 30 ± 2 mm and 30 mm/minute, and the test was terminated as the skin sample was completely torn apart (34).
The formula σ = Fmax / A was utilised in calculation of tensile force. In this formula, σ, Fmax and A, respectively denote tensile force (Mega Pascal), maximum force encountered during pull test (Newton) and section surface area of skin sample before the pull test (mm2). Section surface area was calculated by multiplying the short edge of rectangle (10 mm) with skin thickness (2 mm on average).
Determination of hydroxyproline levels
Hydroxyproline content of the wounds were determined by using the Bergman method (35). Absorbance was assessed by ‘Shimatzu® spectrophotometer UV‐120‐02′ device.
Histological examination
For histological examination, tissue samples were fixed in 10% phosphate‐buffered formalin, dehydrated and embedded in paraffin. Sections were cut at 4–5μm and stained with hematoxylin–eosin and Masson's trichrome reagents. Photomicrographs were obtained under light microscope. At least 10 sections from each wound were examined. Histological evaluations were performed by modified method of Tsuboi and Rifkin (36). The parameters measured were degree of reepithelialisation, granulation tissue thickness, number of infiltrated cells, and neovascularisation for the entire wound area. Each of the parameters was graded numerically to permit average scores to be complied. Degree of reepithelialisation was rated on a scale of 0–4 (0 = no closure; 1 =< 30%; 2 => 31, < 60%; 3 => 61%, < 99%; and 4 =complete reepithelialisation by keratinocytes). Granulation tissue formation was rated on a scale of 0–3 (0 = not found; 1 = thin granulation layer; 2 = moderate granulation; and 3 = thick granulation layer). As an index of degree of cellular infiltration, the number of fibroblasts and macrophages were estimated. Polymorphonuclear cells and lymphocytes were not counted. Cellular infiltration was rated on a scale of 0–3 (1 = few cells, 2 = moderate number of cells, 3 = many cells). Neovasculature were scored by counting the number of capillary lumens in the complete wound section at ×100 magnification. Only mature vessels that contained erythrocytes were counted and given a value of 0–3. (0 = 0− 4 capillaries per wound, 1 = 5− 14 capillaries per wound, 2 = 15− 24 capillaries per wound, 3 => 24 capillaries per wound. Five micron‐thick sections were stained with hematoxylin–eosin and Mallory Azan. Afterwards, they were examined and photographed under Olympus‐BH2® light microscope (Olympus, Tokyo, Japan).
Statistical analysis
Differences among groups for wound area, hydroxyproline level and histopathological scores were evaluated by Kruskal–Wallis variance analysis. When the P‐value from the Kruskal–Wallis test statistics is statistically significant, multiple comparison test was used to know which groups differ from which others (37). Student's t‐test was used for evaluation of mechanical test results. Comparison between day 7 and day 14 was assessed by Mann–Whitney U‐test. The Bonferroni correction was applied for all possible multiple comparisons. Because of the number of tests undertaken, the level of significance is set at 0.008 for Mann–Whitney U‐test. SPSS 11.5 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. A P‐value < 0· 05 was considered significant.
RESULTS
Macroscopic observations
One rat in N MG day 14, two rats in N GS‐EGF day 14 and two rats in D GS‐EGF day 7 and another two rats in D GS‐EGF day 14 groups died during the course of the study. Death rats were not replaced and the study was completed with the remaining ones.
On 7th day, skin defects were observed in all lesions and all the wounds were covered with hemorrhagic crutes. Wound infection was observed in one rat from D GS‐EGF group. On 14th day, size of the lesion was significantly reduced but there were no complete healing in non diabetic and diabetic rats treated with MG and GS. In N GS‐EGF group, size of the lesion was reduced in all rats except the two with wound infection. Furthermore, no skin defect was visible in three rats from this group. In D GS‐EGF group, size of the lesion was reduced in all rats except the one with wound infection. Skin defects were also not visible in the three rats from this group.
Reduction in wound area
On 7th day, for non diabetic rats, the reduction in the mean surface area of wounds in GS‐EGF group was significantly higher than that in MG (P = 0· 008) and GS (P = 0· 001) groups. For diabetic rats, decrease in wound size on 7th day was more prominent in GS‐EGF group, but this difference did not reach statistical significance. On 14th day, no statistically significant difference was found between any of the groups by means of wound size (P > 0· 05) (Figure 1).
Figure 1.
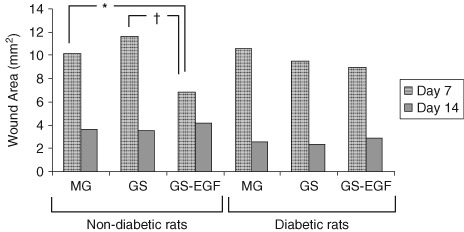
Wound areas of non diabetic and diabetic rats on day 7 and 14. (* P = 0· 008; † P = 0· 001).
Tensile strength
The maximum pull force of normal skin sample was found to be 102· 82 Newton (kgm/sn2). Maximum pull force of skin samples of non diabetic and diabetic rats are shown in Figure 2.
Figure 2.
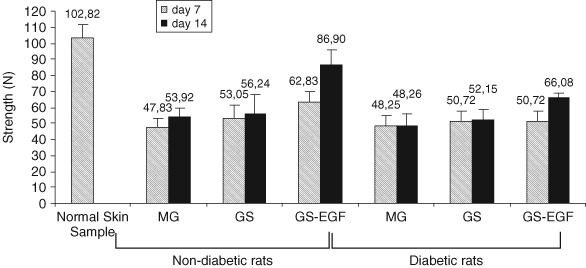
Maximum pull force of normal skin sample and skin samples from non diabetic and diabetic rats.
Tensile strength was calculated relying on pull force and area of skin slice. In non diabetic rats, 7th and 14th day assessments revealed that tensile strength of wounds treated with GS‐EGF was higher than those treated either with MG or GS (P < 0· 05), but there were no difference among groups treated with MG or GS (P > 0· 05) (Figure 3). In diabetic rats, no difference was observed between the tensile strengths of skin samples between MG‐, GS‐ and GS‐EGF‐applied groups on 7th day (P > 0· 05) . However, tensile strength of samples from GS‐EGF‐applied rats were found to be higher than those treated with MG or GS on 14th day (P < 0· 05) (Figure 3).
Figure 3.
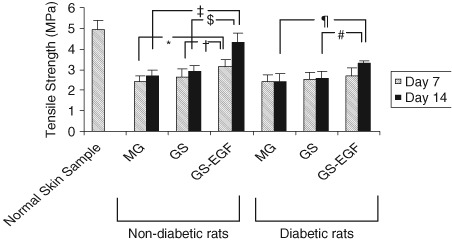
Tensile strenght values of the normal skin sample and samples from non diabetic and diabetic rats on day 7 and 14. (* P = 0· 002; † P = 0· 021; ‡ P = 0· 00002; § P = 0· 00003; ¶ P = 0· 00081; # P = 0· 00078).
Hydroxyproline content of wound
For both non diabetic and diabetic rats, hydroxyproline contents of wounds treated with the three different modalities were similar on 7th day (P > 0· 05). For the 14th day assessment, we noted that type of the wound dressing in non diabetic rats had no significant effect on hydroxyproline contents of wounds (P > 0· 05). However in diabetic rats, hydroxyproline contents of wounds in GS‐applied group were statistically significantly higher than both MG (P = 0· 004) and GS‐EGF‐applied groups (P = 0· 011) (Figure 4).
Figure 4.
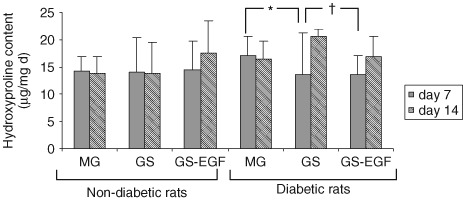
Hydroxyproline content of wounds on day 7 and 14. (* P = 0· 004; † P = 0· 011).
Histological findings
There were significant differences in comparisons of normal and diabetic rats, particularly in terms of reepithelialisation, granulation tissue formation and neovascularisation (5, 6). On day 7, scores for reepithelialisation , granulation tissue formation and neovascularisation were significantly higher in diabetic rats treated with GS‐EGF than diabetic rats treated with GS (P = 0· 002, P < 0· 001 and P < 0· 001, respectively). In non diabetic rats scores for reepithelialisation were not different in GS‐EGF group than the other groups, but granulation tissue formation (P = 0· 01) and neovascularisation (P < 0· 001) were enhanced. On day 14, scores for reepithelialisation (P = 0· 008) and granulation tissue formation (P = 0· 011) were higher in diabetic rats treated with GS‐EGF than the rats treated with other dressings. In non diabetic groups also higher scores for reepithelialisation (P = 0· 036) and granulation tissue formation (P = 0· 011) were observed.
Figure 5.
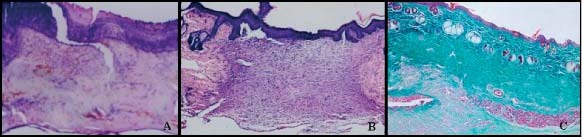
(A) N‐MG on day 7. Section from healing region covered by thick crust and a vascular fibrin remnants. A few number of cells can be seen (haematoxylin–eosin, ×10). (B) N‐SF on day 14. Completed epithelialisation and granulation tissue with vascularisation and inflammatory cell is observed. Also a dermal papilla can be noticed (haematoxylin–eosin, ×4). (C) N‐GS‐EGF on day 14. Epithelialisation is completed. Multiple dermal papilla are present. Connective tissue appearance of dermis hair follicules and sebaceous glands of the surrounding peripheral normal skin can also be noticed (Masson's trichrome, ×4).
Figure 6.
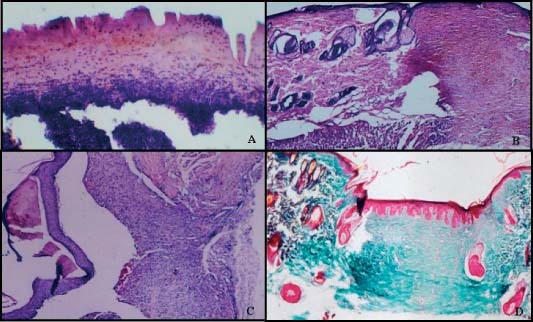
(A) D‐MG on day 7. Wound area is filled with necrotic cells and covered by crust. (haematoxylin–Eosin, ×20). (B) D‐MG on day 14. Epithelialisation is completed, granulation tissue rich in vascularisation and inflammatory cell infiltration can be seen (haematoxylin–eosin, ×4). (C) D‐GS‐EGF on day 7. Completed epithelialisation was seperated from granulation artificially during sectioning. Crust covers epidermis but is surrounded and limited by the corneum layer. A piece of gelatin and infiltrative cells can be noticed in highly vascularised granulation tissue (haematoxylin–eosin, ×4). (D) D‐GS‐EGF on day 14. Completed epithelialisation and formation of multiple dermal papilla can be seen, collagen rich connective tissue in dermis is prominent. (Masson's trichrome, ×4).
DISCUSSIONx
Wound healing is a complex cellular and biochemical process. Cytokines and growth factors are soluble factors that regulate wound healing. Application of exogenous growth factors has been shown to positively influence wound healing in animal models and also in clinical trials 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31. Acute wound therapy with exogenous growth factors accelerates the appearance of fibroblasts and collagen into the wound, shortening the natural inflammatory phase for gain in injured tissue tensile strength (38).
Over the past two decades several recombinant growth factors have been tested for their ability to accelerate wound healing. EGF, the first‐discovered growth factor, is known to stimulate fibroblast replication, collagen formation and reepithelialisation. Laato et al. (22) confirmed that EGF is a potent dose‐dependent mitogen for the granulation fibroblast. Previous studies have demonstrated that a number of growth factors were markedly reduced in wound fluid from chronic wounds compared with acute wounds (28). Bennett and Schultz (15) postulated increased destruction or inhibition of growth factors by elevated levels of proinflammatory cytokines and metallomatrix protein following repeated trauma and infection. Early experimental studies have shown the potential of EGF in promoting wound healing 23, 24. However, there are also some studies in the literature which failed to show any benefit of EGF in wound healing 39, 40, 41. In these studies EGF was applied with a simple vehicle that does not provide sustained release. Buckley et al. (22) and Cohen and Carpenter (42), based on in vitro results, concluded that these failures may be because of the experimental conditions that did not provide sufficient continuous exposure of residual epithelial cells to EGF. It is known that mitogenic effect of EGF requires continuous exposure of target cells to EGF for a minimum 6–12 hours. The half life of EGF in the body is too short when applied via injection or in free form. Many enzymes are increased within the early wound because of cytokines and growth factors. It is known that topical applications of growth factors are generally ineffective, because they are rapidly degraded by local enzymatic activity (38). Sufficient continuous exposure of epithelial cells to EGF is required for effect. Therefore, incorporation or encapsulation of EGF into a polymer matrix and its sustained release from this matrix may enhance its in vivo efficiency. It has been shown that sustained release of growth factors leads to superior outcome 25, 26, 27, 43, 44, as a matter of fact that, the vehicle that will be used to deliver the growth factor requires attention when constructing a study design. Strength of the current report is the use of a novel drug delivery preparation—gelatin microspheres—with which to introduce the growth factor into the wound. GS was used as the wound dressing. Gelatin is a non toxic, non immunogenic and biodegradable material. Prepared sponges have a very soft, porous and highly elastic structure (33). A porous and biodegradable matrix would serve as the host for the proliferating cells and would degrade spontaneously without creating any adverse effects while the tissue regenerates. EGF was added into these sponges in gelatin microspheres that would act as sustained release vehicle.
The present study was aimed to evaluate the effectiveness of EGF on wound healing. We used GS with EGF‐loaded microsphere for wound closure in a dermal excision model in both normal and streptozocin‐induced diabetic rats. We evaluated the reduction in wound area, hydroxypyroline content and tensile strength of the wound. The tissue samples taken from the wounds were examined histopathologically for inflammatory cell infiltration, fibroblast activity, granulation, vascularisation and reepithelialisation. These assessments were performed on 7th day and again on the 14th day after wounding.
On 7th day the use of GS with EGF‐loaded microsphere was observed to reduce the wound area better when compared with the other dressings tested. This effect was significant in normal rats rather than diabetic rats. The difference in reduction of wound area did not persist on day 14. Although, no significant effect on hydroxyproline content of the wound was found with GS with EGF‐loaded microsphere in either normal or diabetic rats; there was a statistically significant increase in tensile strength values of EGF‐applied non diabetic rats over the 14 day period. An increase in tensile strength was prominent in also EGF‐applied diabetic rats on day 14. This result is consistent with the previous studies 29, 45. The balance between synthesis and breakdown and so deposition of collagen is important in wound healing and development of wound strength. Previous studies have reported that, EGF application increased hydroxyproline and collagen levels in wounds 17, 46. In our study, we observed no benefit of EGF on hydroxyproline content of the wounds. It is well known that healthy dermis mostly consists of type I collagen however, in granulation tissue there are 30% type III collagen. Studies designed to assess the specific collagen level of wounds may be needed to clarify this issue.
EGF is known as a potent mitogen for epidermal cells in normal skin, but there is less knowledge about its effect on poor granulation tissue and remodelling in diabetics (29). In the present study, histopathological examination and scoring revealed that there was a significant difference by means of wound healing scores in EGF‐applied normal and diabetic rats on day 7 and also 14. GS with EGF‐loaded microsphere seems to decrease the maturation time of granulation tissue and wound contraction which means that it enhances reepithelialisation, but no significant effect was detected in inflammatory infiltration and number of fibroblasts in time‐dependent activity.
In conclusion, results of the current study provide evidence to suggest that EGF in gelatin‐microsphere dressings may be used in wound management to enhance the healing process. Although these results are promising, there is a need for much more research in this area for integration of this experience in clinical practice. There are many sophisticated dressings in the market to aid in wound management. Growth factors may be used in combination with alternative wound dressings or activators of other signal transduction pathways such as hyperbaric oxygen to potentiate their effects. Clearly, this would be another interesting area of future research.
REFERENCES
- 1. Singer AJ, Clark RAF. Mechanisms of disease: cutaneous wound healing. N Engl J Med 1999;341:738–46. [DOI] [PubMed] [Google Scholar]
- 2. Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am 1997;77:509–28. [DOI] [PubMed] [Google Scholar]
- 3. Steed DL. Wound‐healing trajectories. Surg Clin North Am 2003;83:547–55. [DOI] [PubMed] [Google Scholar]
- 4. Hinchliffe RJ, Valk GD, Apelqvist J, et al. A systematic review of the effectiveness of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev 2008;24:S119–44. [DOI] [PubMed] [Google Scholar]
- 5. Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med 2005;23:594–608. [DOI] [PubMed] [Google Scholar]
- 6. Werner S, Breden M, Greenhalg DG, et al. Induction of keratinocyte growth factor is reduced and delayed during wound healing in genetically diabetic mouse. J Invest Dermatol 1994;103: 469–72. [DOI] [PubMed] [Google Scholar]
- 7. Brawn DL, Kanc CD, Chernausek SD, et al. Differential expression localization of IGF‐1 and IGF‐2 in cutaneus wounds of diabetic versus nondiabetic mice. Am J Pathol 1997;151: 715–24. [PMC free article] [PubMed] [Google Scholar]
- 8. Brown DL, Kao WW‐Y, Greenhalgh DG. Apoptosis downregulates inflammation under the advancing epithelial wound edge: delayed patterns in diabetes and improvement with topical growth factors. Surgery 1997;121:372–80. [DOI] [PubMed] [Google Scholar]
- 9. Loots MAM, Lamme EN, Mekkes JR, et al. Cultured fibroblasts from chronic diabetic wounds on the lower extremity (non‐insulin‐dependent diabetes mellitus) show disturbed proliferation. Arch Dermatol Res 1999;291:93–9. [DOI] [PubMed] [Google Scholar]
- 10. Lerman OZ, Galiano RD, Armour M, et al. Cellular dysfunction in the diabetic fibroblast. Am J Pathol 2003;162:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schaffer MR, Tantry U, Efron PA, et al. Diabetes‐impaired healing and reduced wound nitric oxide synthesis: a possible pathophysiologic correlation. Surgery 1997;121:513–9. [DOI] [PubMed] [Google Scholar]
- 12. Galeano M, Torre V, Deodato B, et al. Raxofelast, a hydrophilic vitamin E‐like antioxidant, stimulates wound healing in genetically diabetic mice. Surgery 2001;129:467–77. [DOI] [PubMed] [Google Scholar]
- 13. Delamaire M, Maugendre D, Moreno M, et al. Impaired leucocyte functions in diabetic patients. Diabet Med 1997;14:29–34. [DOI] [PubMed] [Google Scholar]
- 14. Wysocki J, Wierusz‐Wysocka B, Wykretowicz A, et al. The influence of thymus extracts on the chemotaxis of polymorphonuclear neutrophils (PMN) from patients with insulin‐dependent diabetes mellitus (IDD). Thymus 1992;20: 63–7. [PubMed] [Google Scholar]
- 15. Bennet NF, Schultz GS. Growth factors and wound healing. Part II: role in normal and chronic wound healing. Am J Surg 1993;166:74–81. [DOI] [PubMed] [Google Scholar]
- 16. Cross KJ, Mustoe TA. Growth factors in wound healing. Surg Clin North Am 2003;83:531–46. [DOI] [PubMed] [Google Scholar]
- 17. Grotendorst GR, Martin GR, Pencev D, et al. Stimulation of granulation tissue formation by platelet‐derived growth factor in normal and diabetic rats. J Clin Invest 1985;76:2323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Broadley KN, Aquino AM, Hicks B, et al. Growth factors bFGF and TGF beta accelerate the rate of wound repair in normal and in diabetic rats. Int J Tissue React 1988;10:345–53. [PubMed] [Google Scholar]
- 19. Robson MC, Payne WG, Garner WL, et al. Integrating the results of phase IV (post‐marketing) clinical trial with four previous trials reinforces the position that Regranex (becaplermin) gel 0· 01% is an effective adjunct to the treatment of diabetic foot ulcers. J Appl Res 2005;5:35–45. [Google Scholar]
- 20. Steed DL. Diabetic ulcer study group. Clinical evaluation of recombinant human platelet‐derived growth factor for the treatment of lower extremity diabetic ulcers. J Vasc Surg 1995;21:71–8. [DOI] [PubMed] [Google Scholar]
- 21. Richard JL, Parer‐Richard C, Daures JP, et al. Effect of topical basic fibroblast growth factor on the healing of chronic diabetic neuropathic ulcer of the foot. A pilot, randomized, double‐blind, placebo‐controlled study. Diabetes Care 1995;18:64–9. [DOI] [PubMed] [Google Scholar]
- 22. Buckley A, Davidson JM, Kamerath TBW, et al. Sustained release of epidermal growth factor accelerates wound repair. Proc Natl Acad Sci USA 1985;82:7340–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laato M, Niinikoski J, Bardin B, Lebel L. Stimulation of wound healing by epidermal growth factor: a dose dependent effect. Ann Surg 1986;203:379–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buckley A, Davidson JM, Kamerath CD, et al. Epidermal growth factor increases granulation tissue formation dose dependently. J Surg Res 1987;43:322–8. [DOI] [PubMed] [Google Scholar]
- 25. Nanney LB. Epidermal growth factor‐induced effects on wound healing. Clin Res 1987;35:706A. [Google Scholar]
- 26. Laato M, Hahari VM, Niinikoski J, Vuorio E. Epidermal growth factor increases collagen production in granulation tissue by stimulation of fibroblast proliferation and not by activation of procollagen genes. Biochem J 1987;247:385–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown LG, Nanney LB, Grillen J, et al. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med 1989;321:76–9. [DOI] [PubMed] [Google Scholar]
- 28. Cooper DM, Yu EZ, Hennessey P, et al. Determination of endogenous cytokines in chronic wounds. Ann Surg 1994;219:688–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown GL, Curtsinger LJ, Brightwell JR et al. Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J Exp Med 1986;163:1319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Afshari M, Larijani B, Fadayee M, et al. Efficacy of topical epidermal growth factor in healing diabetic foot ulcers. Therapy 2005;2:759–65. [Google Scholar]
- 31. Choi JS, Leong KW, Yoo HS. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials 2008;29:587–96. [DOI] [PubMed] [Google Scholar]
- 32. Nastruzzi C, Pastesini C, Cortesi R, et al. Production and in vitro evaluation of gelatin microspheres containing an antitumor tetra‐amidine. J Microencapsulation 1994;11:249–60. [DOI] [PubMed] [Google Scholar]
- 33. Ulubayram K, Eroglu I, Hasırcı N. Gelatin microspheres and sponges for delivery of macromolecules. J Biomet App 2002;16:227–41. [DOI] [PubMed] [Google Scholar]
- 34. Serbetci K, Korkusuz F, Hasırcı N. Thermal and mechanical properties of hydroxyapatite impregnated acrylic bone cements. Polym Test 2003;20:1–11. [Google Scholar]
- 35. Bergman I, Loxley R. Two improved and simplified methods for the spectrophotometric determination of hidroxyproline. Analy Chem 1963;35:1961–5. [Google Scholar]
- 36. Tsuboi R, Rifkin DB. Recombinant basic fibroblast growth factor stimulates wound healing in healing impaired ab/db mice. J Exp Med 1990;172:245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Conover WJ. Some methods based on ranks, Section 5.2 Several independent samples. Practical Nonparametric Statistics 2nd edn. New York: John Wiley & Sons, 1980; 229–39. [Google Scholar]
- 38. Robson MC, Hill DP, Woodske ME, et al. Wound healing trajectories as predictors of effectiveness of therapeutic agents. Arch Surg 2000;135:773–7. [DOI] [PubMed] [Google Scholar]
- 39. Greaves MW. Lack of effect of topically applied epidermal growth factor on epidermal growth in man in vivo. Clin Exp Dermatol 1980;5:101–3. [DOI] [PubMed] [Google Scholar]
- 40. Thornton JW, Hess CA, Cassingham V, et al. Epidermal growth factor in the healing of second degree burns: a controlled animal study. Burns Incl Therm Inj 1982;8:156–60. [DOI] [PubMed] [Google Scholar]
- 41. Arthurson G. Epidermal growth factor in healing of corneal wounds, epidermal wounds and partial thickness scalds. Scand J Plast Reconst Surg 1984;18:33–7. [DOI] [PubMed] [Google Scholar]
- 42. Cohen S, Carpenter G. Human epidermal growth factor: isolation and chemical and biological properties. Proc Natl Acad Sci USA 1975;72:1317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sheardown H, Clark H, Wedge C, et al. A semi‐solid drug delivery for epidermal growth factor in corneal epithelial wound healing. Curr Eye Res 1997;16:183–90. [DOI] [PubMed] [Google Scholar]
- 44. Ulubayram K, Cakar AN, Korkusuz P, et al. EGF containing gelatin‐based wound dressing. Biomaterials 2001;22:1345–56. [DOI] [PubMed] [Google Scholar]
- 45. Paquette D, Badiavas E, Falanga V. Short‐contact topical tretinoin therapy to stimulate granulation tissue in chronic wounds. J Am Acad Dermatol 2001;45:382–6. [DOI] [PubMed] [Google Scholar]
- 46. Klein SA, Anderson GL, Kennedy AB, et al. The effects of a broad‐spectrum matrix metalloproteinase inhibitor on characteristics of wound healing. J Invest Surg 2002;15:199–207. [DOI] [PubMed] [Google Scholar]


