Abstract
A sandwich graft was applied to the debrided cortical bone layer of the tibia in the case of a 72‐year‐old male patient with full‐thickness necrotic burn injury. The combined graft consisted of a dermal template material and autologous split thickness skin graft. After application, the graft was found totally accepted and provided good functionality with acceptable appearance. Histopathologic evaluation revealed a complete take with revascularisation of the implant. Supporting lamellar bony trabecules were also seen in the deep dermal dermis representing a connection to the underlying bone. The use of the dermal matrix in deep burn exposing the bone provides a satisfactory functional result and good cosmetic appearance.
Keywords: Acellular dermal matrix, Bone remodelling, Burn, Dermal substitution
Introduction
The stability of skin is provided by the specific lamellar structure of the epithelium, anchored to the dermis by interdigitating papillae. The papillary dermis is formed by a well‐organised, three‐dimensional system of fine elastic fibres and is linked to the epithelium with the special structure of dermal‐epidermal junctions and anchoring fibrils of the basal lamina. In deep burn injuries, continuity of the skin is lost. The golden standard of wound coverage is still the application of autologous meshed split‐thickness skin grafts (STSG). The thin layer of skin is harvested in the layer of papillary dermis, allowing fast re‐epithelisation of the donor site, but it only provides the grafted skin with a minimal amount of dermal component. The use of this technique leads in most cases to adequate results but in some instances, e.g. to avoid contracture above joints, replacement of the dermal layer is beneficial. It is now an everyday practice of several burn centres to take advantage of biotechnologically derived materials for dermal replacement. A relatively ‘low‐tech’ but user‐friendly member of the family of such agents is AlloDerm®.
AlloDerm® is derived from de‐epithelised acellular human cadaver dermis and can be successfully used in many fields of surgery. It was first described used in burns (1, 2). It is also used in various aspects of plastic surgery, for example, in the management of cranial fossa defect replacement (3), facial surgery 4, 5, 6) and chest wall reconstruction as a base for muscle flap (7). It has been used in neurosurgery to replace dura mater 8, 9, 10, 11), and as a connective tissue interposing graft in the repair of nasal septal perforations (12, 13). Cosmetic surgery can also benefit from its utilisation in case of soft tissue augmentation (14). It is widely used in periodontology for root coverage (15, 16), ridge augmentation and also as a substitute for subepithelial connective tissue graft (17, 18). In animal models, it has been used in tympanoplasty after tympanic membrane perforations (19) and palate anomalies or defects (20). Of all these areas of surgery, the treatment of burns has remained the main field for the everyday use of the acellular dermal matrix 21, 22, 23, 24).
In many cases, the main goal of the surgeon is to replace missing or atrophic connective tissue to maintain the continuity of a certain organ, where the lack of skin/epithel is caused by injury, genetic disorder or surgical intervention. The replacement can be achieved by a well‐organised, artificially produced layer of connective and/or supportive tissue which can be integrated into the physiological surrounding environment. The technique will be regarded as successful if the resulting tissue is similar to normal skin both functionally and in appearance.
Without exception, in all of the above‐mentioned cases, the acellular matrix was used as a scaffold or support for the new viable dermis or subepithelial tissue to form.
Despite the widespread use of AlloDerm® in burns, there has not been any published case of its use explicitly for the coverage of exposed denuded bone.
We report a third‐degree contact burn in an elderly patient that deeply affected the cortical bone of the right tibia. Although there were many plastic surgical operations on that limb, the tibia was still exposed in an approximately 18 cm2 area. After careful debridement of the cortical layer of the bone, AlloDerm® matrix was directly applied with an autologous thin STSG overlay in a one‐stage procedure, and the wound successfully healed.
Patient treatment
A 72‐year‐old male patient suffered mostly deep partial thickness burns on both of his lower extremities and full thickness burn on his right foot and lower leg, covering 32% of total body surface area in a flame and contact burn. He received paramedic first aid and was brought into our burn unit. His previous medical history revealed essential hypertension disease, two acute myocardial heart attacks and non insulin‐dependent diabetes mellitus. The patient received fluid resuscitation and was placed in an air‐fluidised bed, according to the intensive care protocol of our department. Upon admission necrotomy was performed on both lower extremities, insulin therapy introduced and administration of antibiotics started. His condition was successfully stabilised, and during the course of treatment, necrectomy of devitalised skin, muscle and tendons was performed, first on day 4 and later three more times with temporary porcine xenograft coverage of debrided areas. The first debridement cleared most of the wound, while the further surgical necrectomies were performed in an effort to salvage the patient's right foot. Despite these efforts, the distal part of the right foot required amputation at the level of the tarso‐metatarsal joints (Lissfranc's line) on day 30 of hospitalisation. Pressure sores developed on both heels that required repeated skin grafting and eventually healed. After 10 applications of autologous meshed STSG (first transplantation on day 8), most of the burned area was epithelised. Extensive excision of necrotic tissue resulted in exposure of the tibia on the anterior aspect of the lower leg above the metaphysis and proximal epiphysis (Figure 1A). The distal defect (not shown) was covered with a transposition flap. At the proximal defect, we removed the periosteum and a necrobiotic layer of the tibial cortex at an approximately 18 cm2 large area (Figure 1B). The defect was covered with meshed (unexpanded) AlloDerm® dermal template (Figure 1C) and an additional layer of 1:1·5 meshed and expanded 0·2 mm thin autologous STSG in the same time (Figure 1D). The sandwich graft was secured with sutures, and a multilayer wet bandage applied (Figure 1E). The graft healed completely in 14 days (Figure 1F). Full epithelisation was followed by a course of emollients and compression stockings administered over a period of 1 year after the trauma.
Figure 1.
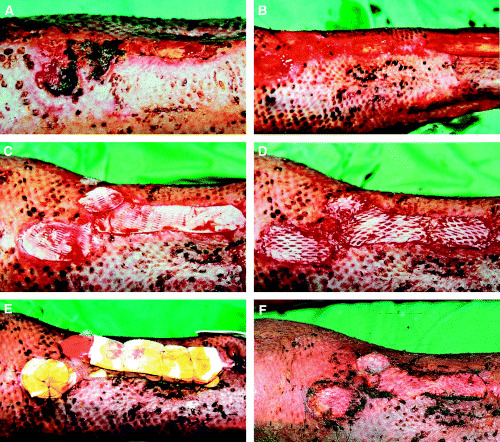
(A) Extensive tissue loss resulted in exposure of the tibia on the anterior aspect of the lower leg above the proximal epiphysis. (B) The periosteum and a necrobiotic layer of the tibial cortex was removed at an approximately 18 cm2 large area. (C). The defect was covered with meshed (unexpanded) AlloDerm® dermal template and (D) an additional layer of 1:1·5 meshed and expanded 0·2 mm thin autologous split‐thickness skin grafts. (E) The sandwich graft was secured with sutures, and a multilayer wet bandage applied. (F) The graft healed completely in 14 days.
Materials and methods in histology
The punch biopsy specimen obtained 2 years after healing from the AlloDerm® located at the anterior surface of the lower extremity was fixed in 4% paraformaldehyde (pH 7·4). The tissue was embedded in paraffin. After sectioning (4 µm), haematoxylin‐eosin staining was made with standard techniques. Other stainings were also used to judge the grade of fibrosis (van Gieson) and presence of haemosiderin (Prussian blue). The tissue was also studied with indirect immunohistochemistry according to the manufacturer's (DAKO, Glostrup, Denmark) guideline for standard ABC technique and antigen retrieving as previously described (25). The vasculature was identified with monoclonal antibodies to CD31 (endothelial marker) and smooth muscle actin (SMA). Those elements showing active cell cycle were immunostained with monoclonal antibody to Ki‐67 (Mib‐1, DAKO, Glostrup, Denmark). The antibody binding was visualised with biotin‐linked secondary antibody using peroxidase‐based kit (LSAB, DAKO, Glostrup, Denmark) and VIP substrate (Vector Lab., Burlingame, CA, USA) according to the manufacturer's instructions (streptavidine‐biotin immunoperoxidase technique). To assess the specificity of immunostainings, we included negative and positive controls (not described or shown). The microscopic analysis and the photography were made through Olympus BX51 microscope equipped with digital camera.
Results
A photograph taken 2 years after surgery reveals the graft site almost unnoticeable (Figures 2A,B). All treated surfaces were covered with functionally and cosmetically acceptable skin (Figure 2C) which is soft and pliable at the site of the AlloDerm® implant (Figure 2D). The implanted dermal material served as a template to the formation of a neo‐dermis that connects the previously denuded bone and the transplanted thin autologous skin with its patchy minimal dermal content. Clinically, it gives a firm support to the overlaying skin and protects the bone effectively. To further investigate the characteristics of this neo‐dermis, a punch biopsy specimen was taken at this stage from the graft site and evaluated histopathologically. Hematoxilin‐eosin stain of the graft site reveals atrophic but intact and inflammation‐free skin (Figure 3A) showing normal epidermal structure and keratinisation (black arrows). Apparent cell proliferation is detected as indicated by Ki67 monoclonal antibody (mAb) expression (Figure 3B, black arrows). Moderate amounts of loose collagen fibrils are present at the dermo‐epidermal junction (not demonstrated in the figures), as well as an oligocellular elastic connective tissue (displayed as eosinophilic bundles) in the deep dermis as detected by van Gieson's staining (Figure 3C). In the deeper dermal layers, mature lamellar bony trabeculae are present (arrows) surrounded by a well‐developed neo‐dermis. This neo‐dermis represents the implant material transformed into the recipient's own dermis with ample vascularisation. The vasculature of the dermal implant can be visualised with mAb to SMA in the vessel walls (Figure 3D, asterisk) or by CD 31 immunostaining of the endothelial cells (not shown). The above appears to demonstrate a completed remodelling phase of the wound healing, indicating that the AlloDerm® could be converted into a compact, functionally and morphologically live tissue as an interconnection between the epidermal–dermal layer of the skin and the underlying bony tissue. The patient was also satisfied with the functional result and good cosmetic appearance of the resulting scar.
Figure 2.
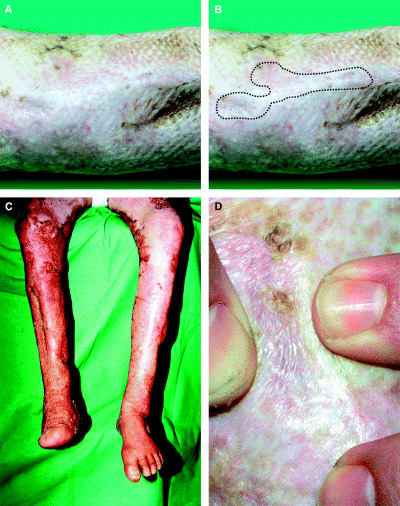
(A) Control photograph taken 2 years later reveals the graft site almost unnoticeable. (B) Marking shows the exact site where the sandwich‐graft was applied. (C) All treated surfaces are covered with functionally and cosmetically acceptable skin which presents as (D) soft and pliable at the site of the acellular dermal matrix implant as well.
Figure 3.
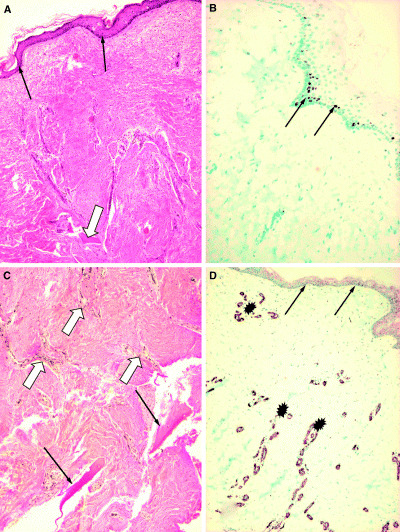
Punch biopsy specimen taken from the site of the acellular dermal matrix implant 2.5 years after grafting. Histopathological evaluation: (A) Hematoxilin‐eosin stain of the graft site shows atrophic but intact and inflammation‐free skin with normal epidermal structure and keratinisation. The arrows indicate the intact epidermal layer. The open arrow points to a bony trabecule (original magnification ×10). (B) The basal layer of the epidermis shows normal proliferation capacity of cells, as detected by Ki67 monoclonal antibody (mAb) expression (arrows). The rate of proliferation is comparable with normal epidermis (not shown) (original magnification ×10). (C) Van Gieson staining reveals an oligocellular elastic connective tissue (eosinophilic bundles) in the deep dermis. In the deeper dermal layers, at the osteo‐neo‐dermal junction, mature lamellar bony trabeculae are present (arrows) surrounded by a well‐developed neo‐dermis. Open arrows indicate blood vessels (original magnification ×20). (D) The vasculature of the neo‐dermis at the implant site is shown with mAb to smooth muscle actin in the vessel walls (asterisk) indicating sufficient angiogenesis in the graft during remodelling (original magnification ×10).
Discussion
It can be a difficult task to cover deep burn wounds that affect, in addition to the skin, the underlying bone structure as well. Therapeutic options range from healing by second intention for rather small lesions with a great risk of osteomyelitis and/or disfiguring scars, to various complicated surgical interventions. The use of local flaps is usually limited, due to simultaneous injury of the surrounding areas. The survival of a free artery flap is dependent on several factors such as comorbidity, and age of the patient; these may significantly reduce its chances to take.
In case of bones with massive spongiosa layer, e.g. calvaria, or epiphysis of skeletal tubular bones, a widely accepted albeit not problem‐free method is covering the debrided spongiosa surface with STSG. The resulting surface, however, is very fragile. The thin epithelium that covers the decorticated bone can be damaged very easily. By cutting thicker grafts, its dermal component can be increased, at the cost of poor take and significant donor site morbidity.
In recent years, a growing number of modalities have emerged to substitute the lost dermis in full‐thickness skin defects. Bio‐engineered human fibroblast‐derived skin substitutes as well as specifically processed human dermis‐based skin templates are currently available. One of many possibilities is AlloDerm®, a dermal substitute processed from human allogenic acellular cadaver dermis. It allows coverage with ultra‐thin STSG or cultured keratinocytes. Many previous reports mention the use of the acellular matrix in dermal replacement; however, there has to date been no report of its use for covering exposed denuded bone.
Our burn team used this material for dermal replacement for the first time in our country in 1996. Since that time, we carried out this treatment for several more burn patients, either in primary closure or in reconstruction, including difficult‐to‐heal areas, e.g. in the vicinity of joints (26). In all our cases, in accordance with previous reports, we used a one‐step procedure for implantation of the dermal replacement material and coverage with autologous meshed skin. The material itself is bipolar; its surface where the epithelium was removed from, is easily distinguishable from its bottom. Due to its porosity, the nutrition of the graft is undisturbed in the early period, and later on, perhaps due to the special processing, some remaining extracellular matrix structures promote the rapid re‐growth of vessels into the material. Clinically, we did not ever see any hindering of graft take that theoretically would have been presented by the administration of an extra layer under the STSG. So the decision was made to apply a sandwich‐graft for wound coverage in this patient with extensive deep burn of the lower extremities. This multilayer graft consisted of a primary layer of meshed unexpanded allogenic dermal template material and an additional layer of thin 1:1·5 meshed autologous STSG (Figure 1).
The outcome of the application of the described sandwich graft was not only a full epithelisation but also a relatively well‐cushioned surface more resistant to everyday wear and tear than STSG alone. The use of a microvascular free flap for reconstruction in our case would carry a great risk of graft failure because of the advanced atherosclerosis and diabetes of the patient.
In summary, we found no previous report in medical history in which AlloDerm® was applied directly to the denuded bone to provide a substitute for its periosteum, while at the same time serving as a supportive tissue to the STSG overlay. We recommend the described technique for reconstruction because of its minimal invasiveness and excellent long‐term results.
References
- 1. Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full‐thickness burns. Burns 1995;21:243–8. [DOI] [PubMed] [Google Scholar]
- 2. Wainwright D, Madden M, Luterman A, Hunt J, Monafo W, Heimbach D, Kagan R, Sittig K, Dimick A, Herndon D. Clinical evaluation of an acellular allograft dermal matrix in full‐thickness burns. J Burn Care Rehabil 1996;17:124–36. [DOI] [PubMed] [Google Scholar]
- 3. Lorenz RR, Dean RL, Hurley DB, Chuang J, Citardi MJ. Endoscopic reconstruction of anterior and middle cranial fossa defects using acellular dermal allograft. Laryngoscope 2003;113(1):496–501. [DOI] [PubMed] [Google Scholar]
- 4. Cox AJ III, Wang TD. Skeletal implants in aesthetic facial surgery. Facial Plast Surg 1999;15(1): 3–12. [DOI] [PubMed] [Google Scholar]
- 5. Abenavoli FM, Corelli R. Use of AlloDerm and polytetrafluoroethylene together to correct a depression of the frontal bone. Plast Reconstr Surg 2004;113(2):759–60. [DOI] [PubMed] [Google Scholar]
- 6. Batista EL Jr, Batista FC. Managing soft tissue fenestrations in bone grafting surgery with an acellular dermal matrix: a case report. Int J Oral Maxillofac Implants 2001;16(6):875–9. [PubMed] [Google Scholar]
- 7. Cothren CC, Gallego K, Anderson ED, Schmidt D. Chest wall reconstruction with acellular dermal matrix (AlloDerm) and a latissimus muscle flap. Plast Reconstr Surg 2004;114(4):1015–7. [DOI] [PubMed] [Google Scholar]
- 8. Barret JP, Dziewulski P, McCauley RL, Herndon DN, Desai MH. Dural reconstruction of a class IV calvarial burn with decellularized human dermis. Burns 1999;25:459–62. [DOI] [PubMed] [Google Scholar]
- 9. Costantino PD, Wolpoe ME, Govindaraj S, Chaplin JM, Sen C, Cohen M, Gnoy A. Human dural replacement with acellular dermis: clinical results and a review of the literature. Head Neck 2000;22(8):765–71. [DOI] [PubMed] [Google Scholar]
- 10. Warren WL, Medary MB, Dureza CD, Bellotte JB, Flannagan PP, Oh MY, Fukushima T. Dural repair using acellular human dermis: experience with 200 cases: technique assessment. Neurosurgery 2000;46(6):1391–6. [DOI] [PubMed] [Google Scholar]
- 11. Chaplin JM, Costantino PD, Wolpoe ME, Bederson JB, Griffey ES, Zhang WX. Use of an acellular dermal allograft for dural replacement: an experimental study. Neurosurgery 1999;45(2):320–7. [DOI] [PubMed] [Google Scholar]
- 12. Kridel RWH, Foda H, Lunde KC. Septal perforation repair with acellular human dermal allograft. Arch Otolaryngol Head Neck Surg 1998;124:73–8. [DOI] [PubMed] [Google Scholar]
- 13. Ayshford CA, Shykhon M, Uppal HS, Wake M. Endoscopic repair of nasal septal perforation with acellular human dermal allograft and an inferior turbinate flap. Clin Otolaryngol 2003;28(1):29–33. [DOI] [PubMed] [Google Scholar]
- 14. Jones FR, Schwartz BM, Silverstein P. Use of a nonimmunogenic acellular dermal allograft for soft tissue augmentation: a preliminary report. Aesthetic Surg Q 1996;16:196–201. [Google Scholar]
- 15. Richardson CR, Maynard JG. Acellular dermal graft: a human histologic case report. Int J Periodontics Restorative Dent 2002;22(1):21–9. [PubMed] [Google Scholar]
- 16. Minsk L. The use of acellular dermal connective‐tissue graft for root coverage in periodontal plastic surgery. Compend Contin Educ Dent 2004;25(3) (170):172–6. [PubMed] [Google Scholar]
- 17. Barros RR, Novaes AB, Grisi MF, Souza SL, Taba MJ, Palioto DB. A 6‐month comparative clinical study of a conventional and a new surgical approach for root coverage with acellular dermal matrix. J Periodontol 2004;75(10):1350–6. [DOI] [PubMed] [Google Scholar]
- 18. Harris RJ. A short‐term and long‐term comparison of root coverage with an acellular dermal matrix and a subepithelial graft. J Periodontol 2004;75(5): 734–43. [DOI] [PubMed] [Google Scholar]
- 19. Downey TJ, Champeaux AL, Silva AB. AlloDerm tympanoplasty of tympanic membrane perforations. Am J Otolaryngol 2003;24(1):6–13. [DOI] [PubMed] [Google Scholar]
- 20. Ophof R, Maltha JC, Von den Hoff JW, Kuijpers‐Jagtman AM. Histologic evaluation of skin‐derived and collagen‐based substrates implanted in palatal wounds. Wound Repair Regen 2004;12(5):528–38. [DOI] [PubMed] [Google Scholar]
- 21. Bello YM, Falabella AF, Eaglstein WH. Tissue‐engineered skin. Current status in wound healing. Am J Clin Dermatol 2001;2(5):305–13. [DOI] [PubMed] [Google Scholar]
- 22. Lattari V, Jones LM, Varcelotti JR, Latenser BA, Sherman HF, Barrette RR. The use of a permanent dermal allograft in full‐thickness burns of the hand and foot: a report of three cases. J Burn Care Rehabil 1997;18:147–55. [DOI] [PubMed] [Google Scholar]
- 23. Sheridan RL, Choucair RJ. Acellular allogenic dermis does not hinder initial engraftment in burn wound resurfacing and reconstruction. J Burn Care Rehabil 1997;18:496–9. [DOI] [PubMed] [Google Scholar]
- 24. Griffin JE, Johnson DL. Management of the maxillofacial burn patient: current therapy. J Oral Maxillofac Surg 2005;63(2):247–52. [DOI] [PubMed] [Google Scholar]
- 25. Dezso B, Haas GP, Hamzavi F, Kim S, Minticello E, Benson PD, Pontes JE, Maughan RL, Hillman GG. The mechanisms of local tumour irradiation combined with I.‐2 therapy in murine renal carcinoma: histological evaluation of pulmonary metastases. Clin Cancer Res 1996;2:1534–52. [PubMed] [Google Scholar]
- 26. Juhász I, Erdei I, Szabó É, Hunyadi J. Dermal substitution with lyophilized acellular human dermis in the treatment of scar contractures following thermal injury. J Eur Acad Dermatol Venereol 2001;15(S2):265. [Google Scholar]


