Abstract
This article presents a retrospective analysis of a series of nine patients requiring reconstruction of exposed bone, tendons or joint capsules as a result of acute high‐voltage injuries in a single burn centre. As an alternative to free tissue transfer, the dermal substitute Matriderm® was used in a one‐stage procedure in combination with split‐thickness skin grafts (STSG) for reconstruction. Nine patients, in the period between 2005 and 2009 with extensive high‐voltage injuries to one or more extremities which required coverage of exposed functional structures as bone, tendons or joint capsule, were included. A total of 11 skin graftings and 2 local flaps were performed. Data including regrafting rate, complications, hospital stays, length of rehabilitation and time until return to work were collected. Eleven STSG in combination with Matriderm® were performed on nine patients (success rate 89%). One patient died. One patient needed a free‐flap coverage as a secondary procedure. The median follow‐up was 30 months (range 6–48 months). The clinical results of these nine treated patients concerning skin‐quality and coverage of exposed tendons or joint capsule were very good. In high‐voltage injuries free‐flap failure occurs between 10% and 30% if performed within the first 4–6 weeks after trauma. The use of single‐stage Matriderm® and skin grafting for immediate coverage described in this article is a reliable alternative to selected cases within this period.
Keywords: Burns, Dermal substitution, High‐voltage injuries, Matriderm®
INTRODUCTION
The use of free tissue transfer has grown significantly since its introduction in the 1970s (1). Today they are counting to the armamentarium in treating head and neck malignancies, breast neoplasms and upper and lower extremity trauma. The progress of microvascular free tissue transplantation within the last decades has led to an increasing use of free flaps for reconstruction in burn patients 1, 2. Fascial flaps (3), preexpanded flaps 4, 5, composite tissue flaps, multiple flap transplantations in the same patient and combined flaps (‘chimeric’ flaps) based on a single pedicle have been performed in patients with severe burns and high‐voltage injuries (6). However, the risk of flap failure in high‐voltage injuries ranges between 10% and 30% because of microvascular damage by the electrical current 7, 8, 9. Therefore alternative techniques for reconstruction have to be kept in mind.
The purpose of this article is to provide an alternative for the reconstruction of soft tissue defects with exposed structures in these severe traumatised groups of patients.
PATIENTS AND METHODS
Data of patients admitted to the burn unit at the BG Trauma Center Ludwigshafen – Plastic Surgery of the University of Heidelberg – who required reconstruction of soft tissue defects with exposed bone, tendons or joint capsules after high‐voltage injuries were collected in the period between 2005 and 2009. The Trauma Center had a total of 1183 admissions to the intensive care burn unit during the period of 2005 to 2009. The injuries included flame burns (57%), scald burns (18%), electrical burns (9%), contact burns (7%), toxic epidermal necrolysis (6%), explosion injuries (2%) and chemical burns (1%). Of 106 patients with electrical injuries 26 needed soft tissue reconstruction as a result of exposed bone, tendons or joint capsules. Out of 26 patients 9 patients were treated with a combination of split‐thickness skin graft (STSG) and Matriderm® solely. Reasons for this treatment were the presence of only a single vessel of the lower extremity in five cases, high‐dose therapy with catecholamines in three cases and decline to flap surgery in one case respectively. In all nine cases the type of injury was high‐voltage injury with more than 1000 V. Patients' data were analysed retrospectively by chart review and patients interviews. Each patient chart was reviewed for age, gender, type of injury, complications, final outcome and success rate. At admission, patients were resuscitated according to a modified Parkland formula established at our burn unit. The burned areas were treated with closed silver sulfadiazine‐embedded gauze dressings. From admission, patients were given continuous enteral nutritional support. All patients with high‐voltage injuries were screened for inhalation injury by the use of arterial blood gas levels, carbon monoxide haemoglobin levels, chest radiographs and flexible fiberoptic bronchoscopy. None of the patients received steroids. For renal protection, forced diuresis and alkalisation of the urine with a minimum turnover of 7 l/day was performed. If myglobin levels exceeded 10 000 µg/l artificial renal replacement therapy using continuous veno‐venous hemofiltration (CVVHF) was performed. Patients requiring amputation of extremities were not included in the study.
Matriderm® (Dr Suwelack Skin and Health Care AG, Billerbeck, Germany) is a highly porous dermal substitute consisting of a collagen matrix (collagen type I, III and V) cross‐linked to an elastin hydrolysate. It is available in sheets of 1 and 2 mm thickness, and may be covered in a single‐step procedure with immediate STSG10, 11. We treated nine patients with defects resulting from a high‐voltage injury in the combination of 1:1·5 meshed autologous STSG and 1‐mm‐thick Matriderm® sheets in a single‐stage procedure. Patients treated in this way had defects which otherwise could only be reconstructed with local‐ or free flaps. The size of the defects ranged from 240 to 630 cm2, the size of the exposed structures‐like tendons, capsule or bone ranged from 6 to 22 cm2. The reasons for using above‐described procedure as salvage were already mentioned above. Large full‐thickness wounds without vital dermal and epidermal remnants were excised and grafted within the first week after admission, if the condition of the patient permitted surgery. Following wound excision and haemostasis, the dermal substitute was applied to the wound bed in the study group and soaked with 0·9% saline solution. Autologous 1:1·5 meshed STSG were put on top of the dermal substitute. The split‐thickness grafts were fixed with staples, and fatty gauze was used for dressing of the autograft. At the end of surgery, a vacuum‐assisted therapy (VAC) device with an intermitting negative pressure of 125 mmHg was applied to reduce shearing forces to the skin transplants and oedema, and thereby minimise the distance of diffusion through the dermal substitute. The first change of the dressing, which included the removal of VAC device, was performed 5 days postoperatively. Staples were removed between the 5th and 7th postoperative day. As soon as the STSG was considered to be stable, pressure garments were worn for at least 16 hours/day. Panthenol was applied three times a day as a topical agent to moisturise the transplanted areas.
The take‐rate of the split‐thickness auto‐grafts was defined as the percentage of the graft that was considered to be vital and showed good adherence to the wound bed 5 to 7 days after surgery. Wounds were considered as closed when more than 95% of the grafted surface area was epithelialised and physiotherapy could be started. The necessity of regrafting was registered and regarded as an indicator of the success of the initial transplantation.
RESULTS
The patient group (Table 1) consisted of nine men between the ages of 19 and 54. All the patients were referred for reconstruction of exposed bone, joint capsule or tendons. Five patients underwent only one operative debridement, two patients had two operations and another two patients had three operations before closure with Matriderm® and STSG in a single‐stage procedure. The wounds were temporary closed with cadaveric skin before definite wound closure. So the mean operative procedures before wound closure was 1·6.
Table 1.
Complications
| Complication | Number of cases (n = 9) |
|---|---|
| Death | 1 (Patient 5) |
| Secondary free‐flap coverage (>5 weeks after trauma) | 1 (Patient 3) |
| Wound infection | 1 (Patient 3) |
| Regrafting | 2 (Patient 5 + 8) |
Six of the nine patients needed only one skin grafting including dermal substitution with Matriderm® for a successful reconstruction, in two patients a second procedure with regrafting and Matriderm® was necessary for success, one patient suffering a postoperative wound infection needed a secondary free flap (Table 2). Therefore the success rate was 89%. During the treatment, one patient (patient 5) died after successful reconstruction with STSG and Matriderm® by a severe intracerebral bleeding. Patient 3 needed a free flap as a secondary procedure 5 weeks after trauma, because of superinfection with acinetobacter baumanii, which needed further debridement. After debridement, an area of 32 cm2of exposed bone was present; this was evaluated to be too large for a secondary reconstruction with Matriderm®. Initially, this patient had high‐dose catecholamines, so we decided not to perform free‐flap surgery at the early stage.
Table 2.
Overview of the affected total body surface of the nine patients. Outcome after initial grafting need for secondary procedures and woundhealing in the postoperative course after initial reconstruction with Matriderm® and STSG. Woundhealing was judged as completed when the STSG or the flap in patient 3 was clinically stable and allowed at the beginning of physioterapy
| Patient | Age | Mechanism | Outcome | Secondary Procedures | Woundhealing completed |
|---|---|---|---|---|---|
| 1 | 34 | 38% high‐voltage distribution box | Successful grafting | Not needed | 9 days p.o. |
| 2 | 19 | 46% contact to train power line | Successful grafting | Not needed | 10 days p.o. |
| 3 | 27 | 91% contact to train power line | Secondary free flap | Free flap | 26 days p.o. |
| 4 | 54 | 84% high‐voltage distribution box | Successful grafting | Not needed | 9 days p.o. |
| 5 | 44 | 82% contact to power line | Successful regrafting | 1 × Regrafting | 18 days p.o. |
| 6 | 35 | 16% high‐voltage distribution box | Successful grafting | Not needed | 9 days p.o. |
| 7 | 29 | 27% high‐voltage current | Successful grafting | Not needed | 10 days p.o. |
| 8 | 42 | 48% high‐voltage current | Successful regrafting | 1 × Regrafting | 19 days p.o. |
| 9 | 48 | 64% contact to train power line | Successful grafting | Not needed | 10 days p.o. |
The median follow‐up was 30 months (range 6–48 months). Local flap types included a gastrocnemius muscle flap and a peroneus longus flap which were combined with the treatment of Matriderm® and STSG in large defects in two cases. The mean hospital stay was 61 days, with a mean rehabilitation time of 12·7 months. Sixty percent of the patients returned to work.
Case 1
A 34‐year‐old man sustained a 38% total body surface area (TBSA) electrical burn to the trunk and both lower extremities by contact to a high‐voltage distribution box. After necrectomy most areas were transplantable with regular STSG. At the lateral part of the right lower extremity, an area of about 10 cm2 of the extensor‐tendons was exposed (Figure 1A and B). The patient underwent a single‐stage Matriderm® and STSG 1:1·5 meshed reconstruction (Figure 1C and D) with a VAC on top of the graft. Figure 1C shows the Matriderm® on top of the wound, as visible in Figure 1D after hydration Matriderm® gets transparent, identically to the colour of the wound bed. Wound closure was achieved without regrafting (Figure 1E). The patient was discharged after a 48‐day hospitalisation. After 1 year of rehabilitation the patient returned to full‐time employment in his field of work.
Figure 1.
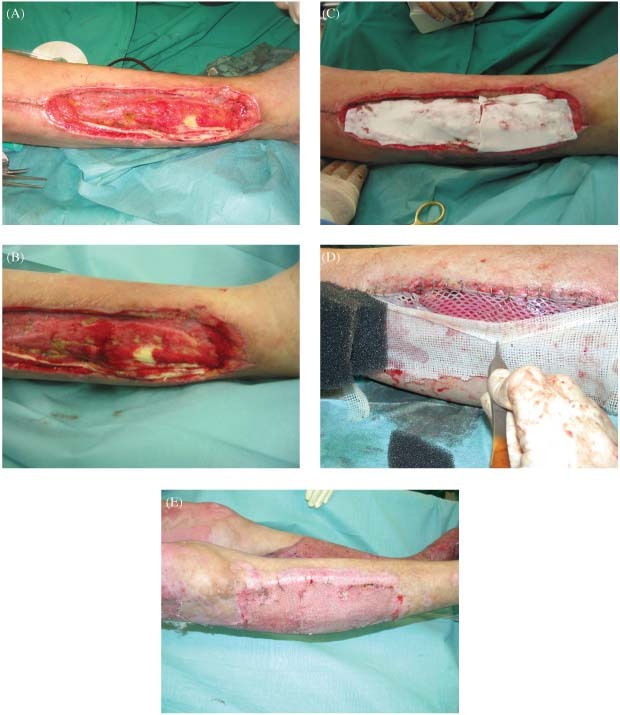
(A–E) At the lateral part of the right lower extremity an area of about 10 cm2of the extensor‐tendons are exposed (A and B). Single‐stage Matriderm® and STSG 1:1·5 meshed reconstruction (C and D) with a vacuum‐assisted therapy on top of the graft; C shows the Matriderm® on top of the wound, as visible in D after hydration Matriderm® gets transperent, identically to the colour of the wound bed. Wound closure was achieved without regrafting (E).
Case 2
A 19 year‐old man climbed on a train to take a picture of the city skyline and thereby got into contact with a train powerline. He sustained a 46% TBSA electrical burn to the left hand, forearm, trunk and both lower extremities. Owing to the drop from the rail car he also suffered a complex elbow luxation fracture, which was stabilised by an external fixation device. Most burned areas could be transplanted with STSG. On the dorsum of the hand and the thumb, the paratenon was partially debrided. Instead of choosing a free‐flap coverage we decided to treat this area with Matriderm® and STSG in a single‐step procedure because the patient had high‐dose catecholamines at the time of operation (Figure 2A–D). The time of reconstruction in the above‐mentioned way was on day 8 after trauma, with a high risk of flap loss especially with the mentioned high‐dose catecholamines 7, 8, 9. The patient was discharged after a 54‐day hospitalisation. After 9 months of rehabilitation the patient started his studies at the Mannheim Business School.
Figure 2.
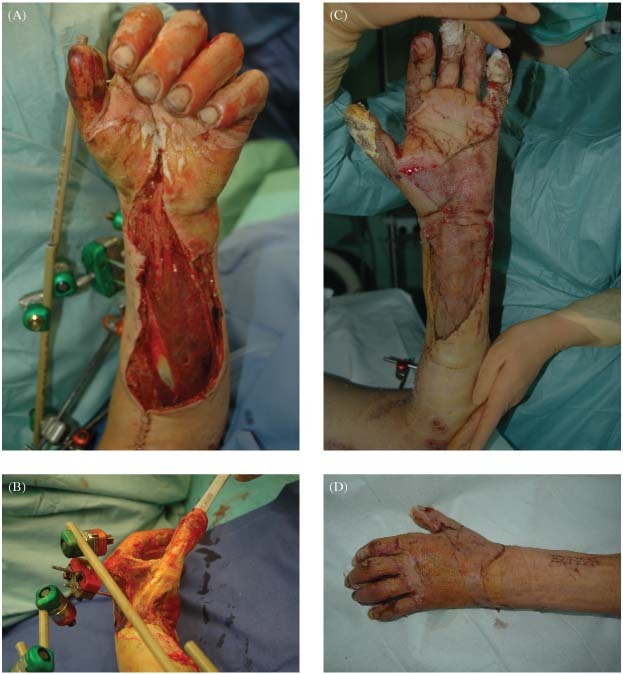
(A–D) On the dorsum of the hand and the thumb the paratenon was partially debrided (A/B). Instead of choosing a free‐flap coverage, we decided to treat this area with Matriderm® and STSG in a single‐step procedure because the patient had high‐dose catecholamines at the time of operation. Wound closure was achieved without regrafting (C/D).
DISCUSSION
The majority of patients undergoing treatment for electrical and thermal burn injuries are usually treated successfully with debridement and coverage with STSG. However, a minority sustain injuries with large soft tissue defects and exposed structures such as bone, tendons or joint capsules require more complex treatments than simple skin graft coverage. In the past, these wounds were treated with preservation of eschar to generate granulation tissue, decortication or drilling of bone cortex to prepare a viable bed for skin grafts and limited local flap reconstruction (12). This frequently leads to long‐term morbidity, including persistent skin ulceration with potential for malignant transformation, tendon loss, loss of tendon excursion and fused joints (12). In the most severe cases, amputation of the limb was the only solution. Concerning limb salvage, the progress in microvascular free tissue transplantation has led to an increasing use of free flaps for tissue reconstruction in burn patients 1, 2. The literature and our own experience show that patients with high‐voltage injuries have a flap failure risk of 10–30% during the first 4–6 weeks after trauma according to the prolonged intimal trauma of the affected vessels induced by the electrical current 7, 8, 9. Because of a low resistance of about 200–500 Ohm blood vessels are a very good conductor for the electrical current and therefore, depending on the blood flow rate of the affected vessels, the intima is altered or not (13). Exposure of vital structures such as tendons, bone or capsule and their inability to sustain simple skin grafts are the most important indications for free tissue transfer in these types of injuries 7, 8, 9. Unfortunately, there are situations when patients decline flap surgery, they need high‐dose catecholamines, or there are no reliable vascular preconditions for flap surgery. Besides these unfavourable preconditions, the above‐described vulnerable period of 4–6 weeks after an high‐voltage injury is additionally associated with an elevated risk of flap loss 7, 8, 9.
In this article, nine patients with high‐voltage injuries were treated with a combination of Matriderm® as a dermal substitute and STSG as a single‐stage procedure to cover exposed structures as an alternative to microvascular free tissue transfer. The use of Matriderm® and STSG presented in these cases showed an alternative wound closure. Our experience shows that the exposure of smaller areas of bone, tendons and capsula is a valid indication for the use of Matriderm® and STSG.
In this context, Matriderm® might be more applicable than alternative dermal templates because of the single‐stage use, but clinical comparative studies between Matriderm® and these templates have to be done, to state this finally. One frequently used template is Integra®. The difference between Matriderm® and Integra® for these indications is found in the way of their cross‐linking chemical process. Matriderm® consists of collagen I, III and V which is cross‐linked with elastine by a cryotechnical process, which results in a distinct porous ultrastructure. This ultrastructure allows an early migration of fibroblasts and angioblast into the porous membrane 10, 14. Therefore vascularisation is provided at day 5. This is the basis for the one step procedure with Matriderm® 10, 14. Integra® is mostly made of collagen and chondroitine‐6‐sulfate, which are cross‐linked by glutar‐aldehyde. This cross‐linking process is the reason for the long period of 3–4 weeks for vascularisation of Integra until it can be skin grafted in the second step. This time is needed to break up the glutar‐aldehyde cross‐links for cellular and vascular ingrowth 10, 14.
Collagen seems to be the optimal biocompatible material for dermal substitution in full‐thickness wounds (14). It is important that a dermal matrix does not degrade too quickly in the wound environment (14). Reconstituted non cross‐linked collagen matrixes degrade within the 1st week after transplantation and do not contribute to the formation of mature collagen bundles (14). Reconstituted collagen is made of soluble collagen fragments, whereas collagen used for Matriderm® is built up from insoluble collagen fibres, which are cross‐linked to elastine. Dermal substitution of full‐thickness skin defects with such a collagen matrix, which is coated with elastin thereby leads to skin regeneration (14). The collagen fibres form a scaffold that directs fibroblasts and possibly other cells towards dermal regeneration. The presence of elastin particles in the collagen matrix diminishes the formation of granulation tissue in an early phase of wound healing. As a result, a neodermis of high quality with random organised collagen bundles regenerates. By diminishing the expression of myofibroblasts, elastin reduces wound contraction (14).
Therefore, the single‐stage procedure showed in this article is an alternative for free‐flap coverage in selected cases. Resection of exposed tendons to make simple STSG possible should be avoided in these cases, because the long‐term functional outcome by fibrosis of the affected residual musculature is not well predictable. Therefore functional structures should be saved to a maximum. Electrical and deep thermal injuries often lead to extensive necrosis of underlying structures and resultant infections. Case 1 illustrates lower extremity wounds in a patient with tendon and bone exposure and heavy bacterial contamination. After 2 necrectomies and VAC the final reconstruction with Matriderm® and STSG was performed, and complete wound closure was achieved without any further skin grafting. Case 2 illustrates a patient who sustained a high‐voltage injury to the hand and forearm with exposition of multiple tendons after necrectomy. With the use of Matriderm® and STSG sheets as a single‐stage procedure a successful reconstruction was achieved, providing tendon coverage and excursion with minimal bulkiness of the hand and forearm. Following the acute management of these critical ill patients, the next challenging step is the reconstruction of the tissue defects. Comparable to deep dermal burns in these cases there is a loss of important dermal structures, which is associated with reduced skin‐elasticity after simple skin grafting 10, 11. As shown in the cases reported in this article the reconstruction with Matriderm® and STSG is a sufficient and reliable alternative in selected cases.
A draw back is the costs of 4·5US$/cm2. Therefore in the above‐described cases the costs for the substitution ranged between 25 and 100US$ (6–22 cm2) for the exclusive coverage of the exposed structures. If larger areas are treated like in case 1, where an area of 140 cm2 (630US$) was covered with Matriderm® the costs rise rapidly. On the other hand the procedure is performed within minutes, and free‐flap surgery is often time consuming and expensive as well. The treatment with Matriderm® similar to the treatment with other dermal substitutes is probably reserved to the industrial countries, because of the cost in tens, but those who have the opportunity to use such products should keep this alternative as a treatment option in mind. Of course, it is not a standard alternative method to local or free‐flap surgery in larger non transplantable areas; at our Centerwe perform 250–300 free flaps per year if indicated, but it is a good option in the cases shown above. As in deep dermal burns 10, 11, the reconstruction and substitution of dermal structures is crucial for long‐term functional outcome, skin‐quality and skin‐elasticity. We therefore recommend the use of dermal substitutes in selected cases like high‐voltage injuries to optimise skin‐quality, to avoid the well‐known risk of flap failure during the first 4–6 weeks. VAC should be used in these cases to minimise oedema, which compromises graft take and to achieve a better contact to the wound surface in these frequently irregular wound beds. The VAC device is not a inalienable precondition as Matriderm® and STSG shows excellent take rates without VAC (10), but it is beneficial for the management of these complex wounds. The clinical results of these nine treated patients concerning skin‐quality and coverage of exposed tendons or capsule were very good. It is a simple and time‐efficient procedure for defect coverage. Meticulous and radical necretomy with clean wound conditions is a prerequisite for successful reconstruction in these complex cases.
REFERENCES
- 1. Ohmori K. Application of microvascular free flaps to burn deformities. World J Surg 2;193:1978. [DOI] [PubMed] [Google Scholar]
- 2. Sharzer LA, O’Brien BM, Horton C, Adamson JE, Mladick RA, Carraway JH, Hayhurst JW, McLeod A. Clinical applications of free flap transfer in the burn patient. J Trauma 15;766:1975. [DOI] [PubMed] [Google Scholar]
- 3. Chowdary RP, Chernofsky MA, Okunski WJ. Free temporoparietal flap in burn reconstruction. Ann Plast Surg 25;169:1990. [DOI] [PubMed] [Google Scholar]
- 4. Hallock GG. Preexpansion of free flap donor sites used in reconstruction after burn injury. J Burn Care Rehabil 16;646:1995. [DOI] [PubMed] [Google Scholar]
- 5. Takushima A, Harii, K , Asato H. Expanded latissimus dorsi free flap for the treatment of extensive post‐burn neck contracture. J Reconstr Microsurg 18;373:2002. [DOI] [PubMed] [Google Scholar]
- 6. Germann G, Bickert B, Steinau H. Versatility and reliability of combined flaps of the subscapular system. Plast Reconstr Surg 103;1386:1999. [DOI] [PubMed] [Google Scholar]
- 7. Sauerbier M, Ofer N, Germann G, Baumeister: Microvascular reconstruction in burn and electrical burn injuries of the severely traumatised upper extremity. Plast Reconstr Surg 2007;119:605–615. [DOI] [PubMed] [Google Scholar]
- 8. Handschin AE, Vetter S, Jung FJ, Guggenheim M, Künzi W, Giovanoli P. A case‐matched controlled study on high‐voltage electrical injuries vs thermal burns. J Burn Care Res 2009;30:400–407. [DOI] [PubMed] [Google Scholar]
- 9. Stefanacci HA, Vandevender DK, Gamelli RL. The use of free tissue transfers in acute thermal and electrical extremity injuries. J Trauma 2003;55:707–712. [DOI] [PubMed] [Google Scholar]
- 10. Ryssel H, Gazyakan E, Germann G, Öhlbauer M. The use of MatriDerm® in early excision and simultaneous autologous skingrafting in Burns. Burns 2008;34:93–97. [DOI] [PubMed] [Google Scholar]
- 11. Haslik W, Kamolz LP, Nathschläger G, Andel H, Meissl G, Fray M. First experiences with the collagen‐elastin matrix Matriderm® as a dermal substitute in severe burn injuries of the hand. Burns 2007;33:364–368. [DOI] [PubMed] [Google Scholar]
- 12. Asch MJ, Curreri P, Pruit BA Jr. Thermal injury involving bone: report of 32 cases. J Trauma 1972;12:135–139. [PubMed] [Google Scholar]
- 13. Jaffe RH, Willis D, Bachem A. The effect of electrical current on the arteries: a histological study. Arch Pathol 1928;6:244. [Google Scholar]
- 14. Lamme EN, Van Leeuwen RT, Jonker A, Van Marle J, Middelkoop E. Living skin substitutes: survival and function of fibroblasts seeded in a dermal substitute in experimental wounds. J Invest Dermatol 1998;111:989. [DOI] [PubMed] [Google Scholar]


