Abstract
The association between chronic ulcers and squamous cell carcinomas (SCCs) is well established. Their clinical presentations, however, are varied, ranging from innocuously appearing lesions to overtly exophytic growths. We present a series of cases with heterogeneous clinical presentations and different treatment outcomes. Case series – patient 1 was a 69‐year‐old man with an 18‐month history of static non healing venous leg ulcer, but no sinister features, biopsy was performed to rule out Marjolin's transformation, histology revealed SCC and treatment was simple excision and skin grafting; patient 2 was a 73‐year‐old lady with an 18‐month history of non healing ulcer (innocuous appearance) over distal interphalangeal joint of index finger, histology revealed SCC with deeper extension and treatment was amputation of distal half of finger; patient 3 was a 73‐year‐old lady with a 12‐month history of non healing fungating leg ulcer with irregular borders and everted edges, histology revealed SCC (tumour eroding tibia and distant metastasis) and treatment was above‐knee amputation, radiotherapy and palliation. Whilst SCC is amenable to simple excision in the early stages, delay in diagnosis could result in loss of the affected digit or limb; an SCC which has metastasised is also life threatening. Therefore, a low threshold to biopsy static non healing ulcers or ulcers in unusual sites should be adopted even in those not manifesting any evidence of malignancy.
Keywords: Biopsy, Malignancy, Malignant transformation, Marjolin's ulcer, Non healing ulcer
Introduction
The association between chronic ulcers and cutaneous malignancy is well established; an ulcer may transform into a malignancy or a malignancy may present as an ulcer. A malignant clone may arise within an ulcer over time, possibly through chronic antigenic or non specific stimulation (1), as seen in Marjolin's ulcer — the development of a squamous cell carcinoma (SCC) from long‐standing, non healing ulcers or scars. In addition, SCCs could also develop from a wide variety of chronic conditions such as pressure ulcers (2), pilonidal sinus wounds (3) and osteomyelitis (4). However, the most common types of SCC arise de novo, from premalignant lesions such as Bowen's disease or from pre‐existing skin lesions such as actinic keratosis (5). For SCC arising de novo, various risk factors have been recognised including solar radiation, chemical carcinogens, cytotoxic drugs and human papilloma virus infection 5, 6, 7).
The clinical presentations of SCCs are varied ranging from innocuously appearing lesions (simulating simple ulcers in the early stages) to overtly fungating and exophytic (mushroom‐like) growths. Although an ulcerated SCC is classically described to have prominent everted edges and a necrotic tumour base, these features are not always present, particularly in the early stages of their development. Similarly, SCCs which develop from long‐standing benign ulcers seldom have raised and everted edges. Therefore, irrespective of whether an ulcer undergoes malignant transformation to an SCC or an SCC presenting as an ulcer, their diagnosis can be challenging, even for the experienced clinician. In addition, the ulcer from which the SCC arose may even show transient reduction in size if only part of a large ulcer has undergone malignant transformation.
SCC is amenable to simple surgical excision if detected early; however, due to the tumour's propensity for local destruction, deeper invasion and the potential for metastasis, delay in diagnosis and management would necessitate a more radical approach and may even result in loss of the affected digit or limb. In addition, an SCC which has metastasised could be life threatening. Therefore, early accurate diagnosis and definitive management is imperative.
We present a series of cases of patients with SCC who attended our specialist wound clinic with heterogenous clinical presentations and who had varied clinical outcomes. We then review the literature of SCC, discuss the management options and highlight the need to adapt a low threshold to biopsy chronic non healing ulcers, even those not manifesting any evidence of a malignant change.
Case series
Patient 1
A 69‐year‐old man had been treated in our unit for 18 months with a venous leg ulcer confirmed by colour flow duplex imaging. The ulcer showed progress to healing initially following use of compression treatment but later became a static non healing wound. It was shallow, had well‐defined borders, flat sloping edges, islands of granulation tissue and some evidence of reepithelialisation (Figure 1). Because the healed areas were repeatedly breaking down, a biopsy was performed to rule out Marjolin's transformation, despite the ulcer not showing any features suggestive of a sinister pathology. The histology revealed the ulcer to be a poorly differentiated invasive SCC. There was no regional lymphadenopathy. He had excision of the lesion with 1‐cm margins, and the defect was covered with a split‐thickness skin graft (Figure 2). The margins were clear on histological examination and he remains symptom‐free at present.
Figure 1.
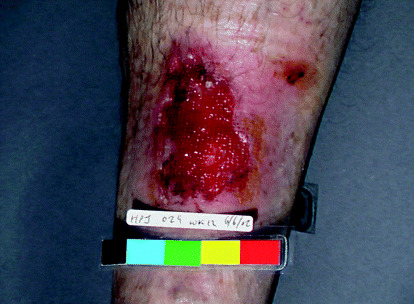
(Patient 1): Ulcer over the anterior aspect of left lower leg.
Figure 2.

(Patient 1): Area in left lower leg after excision of the ulcer (malignancy) and treatment using split skin grafting.
Patient 2
A 73‐year‐old lady was referred to our unit from primary care with an 18‐month history of non healing ulcerative lesion over her left ring finger. Because it was recurrently over‐granulating, it was being cauterised with silver nitrate by the health care professionals (HCPs) in the community. On examination, the lesion (8 mm × 7 mm in maximum diameter) was over the dorsal aspect of the distal phalynx and the distal interphalangeal joint (IPJ) of the left ring finger (Figure 3). It had regular borders, flat edges, and the ulcerated area was covered by over‐granulating tissue. It was fixed to the deep tissue and she had reduced range of movements in her distal IPJ. There was no regional lymphadenopathy. Biopsy for histopathology was performed which revealed this lesion to be a moderately differentiated SCC with deeper extension (to the bone). She underwent amputation of the finger, just distal to the proximal IPJ. The excision margins were clear of tumour and the patient remains symptom‐free at present.
Figure 3.

(Patient 2): Lesion over the distal phalynx and distal interphalangeal joint of the left ring finger.
Patient 3
A 73‐year‐old lady was referred to our unit by her general practitioner with a 12‐month history of a non healing leg ulcer, clinically diagnosed as a venous ulcer. Although it started insidiously as an innocuous lesion on the leg, it continued to grow despite conservative treatment with dressings and bandages by the HCPs in the community. On examination, the ulcer covered the anterior and lateral aspects of her left lower leg, extending from 6 cm above the ankle joint to 13 cm below the knee joint. It measured 17·5 × 16 cm in maximum diameter (Figure 4a). The ulcer had irregular borders, everted edges, necrotic tissue and greyish dried exudate covering the ulcer bed (Figure 4b); the ulcer was malodorous. In addition, there was a satellite lesion in the superior aspect of the ulcer. A four‐quadrant biopsy was done which revealed this to be a moderately differentiated SCC. A bone scan showed that the tumour was eroding the tibia. Enlarged groin lymph nodes were also detected in the same side, biopsy of which revealed metastatic SCC. In addition, computerised tomographic scan revealed enlarged mass of lymph glands along the external iliac chain and metastatic deposits in the lungs.
Figure 4.

(Patient 3): (a) Ulcer over anterior aspect of the left lower leg. Note a satellite lesion in the superior aspect. (b) Close‐up view of the above ulcer. Note the everted edges, necrotic tissue and excess greyish slough.
Because no limb‐saving procedure was possible, she underwent above‐knee amputation. Block dissection of the groin lymph nodes was contemplated, but the procedure was considered too risky because of the close proximity of the lymph nodes to the femoral vessels. She hence received a course of radiotherapy for treatment of the groin lymph nodes. Her hospital stay was further complicated by deep venous thrombosis and a non healing, discharging (sinus) groin wound. She is currently undergoing palliative treatment.
Literature review
Incidence and aetiology
SCC is the second most common cutaneous malignancy after basal cell carcinoma (BCC). It has a 2 : 1 male‐to‐female preponderance and tends to occur in the elderly age group (8). SCC may arise de novo, from pre‐existing skin lesions like actinic keratosis (in situ squamous cell carcinomata), or from a premalignant area of Bowen's disease (intraepidermal carcinoma) (5). Various predisposing factors have been recognised for the development of spontaneous SCC including solar radiation, cytotoxic drugs, human papilloma virus infection, chemical hydrocarbons, tar and mineral oils (7, 9).
SCC also arises as a result of malignant transformation in long‐standing chronic non healing ulcers. Although Marjolin (1827) described the phenomenon of malignant degeneration from burn scars, the term ‘Marjolin's ulcer' currently encompasses SCCs arising from any form of long‐standing chronic ulcers (10) or scars (11), specifically burn scars (12). Other chronic conditions such as pressure ulcers (2), chronic sinuses (13), pilonidal sinuses (3, 14), chronic lymphoedema (15) and osteomyelitis (4) also predispose to the development of SCC.
In addition, SCCs arising from hidradenitis suppurativa (16, 17), from chronic trophic ulcers of leprosy patients (18), from ulcers developing in areas of necrobiosis lipoidica (19), from chronic ulceration secondary to snake bite (20), discoid lupus erythematosus (21) and a wide variety of dermatoses (22, 23) have also been reported.
It has been estimated that the incidence of malignancy in leg ulcers is 2·2 per 100 leg ulcers, (24) although the accurate incidence is difficult to determine and may be overestimated (25). In venous ulcers, it appears that SCC is more frequent than BCC (26), but more cases of BCC arising from chronic venous leg ulcers have recently been reported (27, 28). The malignant transformation of chronic ulcers is related to the duration of the ulcer; the longer the ulcer duration, higher the chances for malignant change (29).
Macroscopic and microscopic features
SCC is a cancer of the epidermis whose cells typically resemble differentiated keratinocytes (30). SCCs arising spontaneously (de novo) begin as small nodules on the skin. As they enlarge, the centre becomes necrotic, sloughs, and the nodule turns into an ulcer. The ulcer has prominent everted edges due to excessive tissue growth, and the base consists of necrotic tumour covered with serum and blood (11). In general, two types of cutaneous SCCs are recognised: (i) a slow‐growing variety that is verrucous in nature and exophytic in appearance; although this type may be deeply locally invasive, it could also metastasise; (ii) the second general type is more nodular and indurated, with rapid growth and early ulceration combined with local invasiveness. The tendency to metastatic disease is greater with this type. However, it needs to be emphasised that all SCCs do not manifest these characteristics and, similarly, SCCs developing from long‐standing benign ulcers seldom manifest the classical features of a malignant ulcer.
Microscopically, SCC is characterised by long irregular tongues of dysplastic‐looking tumour cells spreading in all directions and clusters of cells with concentric rings of flattened squamous cells at their centre, giving rise to the histological metaphor, ‘epithelial pearls’ (11). The tumour cells arise from the epidermis or ulcer base and could extend into the deep dermis and adjacent tissues in a haphazard fashion (5, 6). This is associated with well‐differentiated keratinisation. With poorly differentiated lesions, keratinisation may be minimal or absent, and there is decreased inflammatory response in the dermis (31). A poorly differentiated lesion may have a pseudo‐glandular appearance with marked pleomorphism, hyperchromatic and sometimes bizarre nuclei, and conspicuous mitotic figures (8).
Pathophysiology of SCC arising in chronic ulcers
Although the exact mechanism by which malignancies arise within chronic wounds remains a matter of conjecture, several theories have been postulated. A two‐step process (initiation phase and promotion phase) by which normal cells are converted to malignancy in burn scars was proposed by Arons et al. (32) in 1965. During the initiation phase, normal cells become dormant, neoplastic cells. The promotion phase allows dormant cells to change into a tumour by the stimulation of a cocarcinogen such as infection. Fleming et al. (1990) proposed that toxins released from damaged tissue leads to mutation of cells and eventually a tumour (33).
Chronic irritation with repeated damage and attempted repair of the damaged cutaneous tissue was purported to be a contributing factor in the initiation of carcinogenesis by Hill et al. (1990) (34). Similarly, Ozek et al. (2001) proposed the mechanism of malignant change to a sequence of repeated ulceration and healing (35). It has also been suggested that trauma to the skin results in the implantation of epidermal cells into the dermis. This causes a foreign body reaction within the dermis and alters the normal regenerative process of the tissue. Further injury and subsequent healing in this tissue will not be endured in the same manner as normal tissue, resulting in malignancy (32, 33).
Management
In the early stages, SCC is amenable to simple excision with primary closure or a split‐thickness skin graft, as observed in patient 1 in the case series. On the contrary, as observed in patients 2 and 3, any delay would necessitate a more radical approach and could result in loss of the affected digit or limb. In addition, as observed in patient 3, an SCC which has metastasised is life threatening. Therefore, to achieve an optimal outcome, it is important to obtain an early accurate diagnosis of the condition.
Biopsy of the suspicious lesions for histopathology remains the ‘gold standard’ for diagnosis. Excision biopsy is the preferred method but is not always practical either due to size or location of the lesion, or due to the lack of technical expertise to attain wound closure. In such circumstances, a full‐thickness incisional or punch (4–6 mm is sufficient) biopsy is a reasonable alternative (36). Small, isolated skin ulcerations and lesions suspicious for carcinoma could be treated conservatively for 2–3 weeks with a bland antibiotic ointment and a continuous light dressing. Any lesion that has not healed after 2 or 3 weeks of conservative treatment must be considered a skin cancer until proven otherwise (37).
Various modalities of treatment have been advocated including surgical excision, fractionated radiotherapy, Mohs micrographic surgery (MMS), cryosurgery, electrodessication and curettage, topical chemotherapy, carbon dioxide laser, intralesional interferon and photodynamic therapy. The last three, however, remain largely experimental. Topical 5‐fluorouracil (topical chemotherapy) is effective for small in situ lesions (38) and is contraindicated in invasive SCC due to risk of continued, subclinical spread (39). In addition, Sheen et al. (2003) reported successfully treating an SCC of the toe with intra‐arterial infusion of methotrexate (40).
The principles of management of an SCC depend on the age and general health of the patient, site and size of the lesion, and the presence or absence of metastasis. In general, treatment should primarily involve complete eradication of the tumour. Surgical excision continues to remain the preferred and most appropriate treatment for SCC of the skin, with the surgical margin being dependent on the tumour diameter and the site of the lesion. The issue of margins is mostly discussed in the literature in terms of ‘melanotic (melanoma) skin cancers (MSK)’ and ‘non melanotic skin cancers (NMSK)’, which encompasses SCC and BCC. This article focuses only on the management of NMSK.
As a rule of thumb, the greater the exophytic character of the lesion, the less invasion there is and the less margin required (37). When the primary lesion is a nodule, as in a well‐demarcated BCC, extending into the dermis, a 2–3‐mm margin is adequate (37). Thomas et al. (2003) in a prospective study of 150 NMSK observed that 4‐mm surgical margins gave a microscopic lateral margin beyond one microscopic high‐power field (0·5 mm) in 96% of cases of BCC and 97% of cases of SCC (41). Consequently, they suggest a 4‐mm margin as the optimal treatment in the management of uncomplicated BCC and SCC. In more invasive or ulcerative types, a greater margin is required. A margin of 5 mm for BCC and a margin of 10 mm for SCC are suggested, although to state that this categorically is an oversimplification and could result in excess or inadequate excision of the adjacent tissues. When determining the margins for excision, various factors need to be considered including the type of tumour, location, its size, whether it is primary or recurrent, and the age and general health of the patient. The operating surgeon should make a clinical decision on an individual patient basis, taking all variables into account. After excision of the lesion with adequate margins, the wound is either closed primarily or if the edges cannot be approximated without tension, then a skin graft (split thickness or full thickness) may be required. In certain circumstances, closure of the defect using local flaps (e.g. V‐Y advancement flap and rhomboid flap) may be warranted.
MMS has recently been established as a ‘gold standard’ in the management of all NMSK in terms of cure rates, margin control and tissue conservation. They are useful in treating cancers in difficult anatomical locations, particularly the face, and in recurrent lesions. It is clearly superior for treatment of locally recurrent SCC, with a 5‐year cure rate of up to 90%, compared with 76·7% obtained with standard surgical excision (42). It is also an excellent choice for early SCCs of the digits without bony involvement, especially in the periungual region, where it avoids amputation of the digit without compromising cure rates (43). Kirsner et al. (1996) have also advocated this technique as a potential limb saving procedure in patients with SCC complicating osteomyelitis (44). MMS, however, because of its high cost, length of procedure and the need for special equipment and highly trained personnel, is best reserved for specific indications.
The role of radiation therapy per se in cutaneous SCC in fairly limited. It is, however, useful in elderly patients with poor general health who may not be fit for surgical intervention and in massive unresectable tumours in critical anatomical sites such as the face (45). Yaparpalvi et al. (2003) reported successfully treating a case of subungual SCC with osseous involvement using radiotherapy, thus preserving the digit (46). Radiotherapy is particularly useful in conjunction with other modalities such as surgery in recurrent or highly aggressive lesions and in metastatic cutaneous SCC to achieve loco‐regional control (47). Although radiotherapy could be used to debulk tumour volume before surgical excision, as in rectal cancers, the role of radiotherapy for such indication is uncommon in cutaneous SCC.
Prophylactic lymph node dissection is not thought to be of benefit except for large anaplastic tumours in patients under 50 years of age (48). If the regional lymph nodes are found to be enlarged, then a diagnostic procedure like a fine‐needle aspiration cytology or an incisional biopsy is indicated to confirm a metastatic deposit. Regional lymph node spread is treated by surgical block dissection, or radiotherapy, or a combination of both.
Prognosis
The average rate of metastasis is quoted to be 3·6% for SCCs commonly involving the regional lymph nodes, lungs and liver (36). However, for lesions in high‐risk areas such as the face or scalp, the rate of metastasis could be as high as 30% (49). Similarly, the rate of metastasis in SCCs arising from chronic ulcers, scars and sinuses is high (50) and estimated to be around 20% (51), with the lymph nodes being the most commonly involved. The mean latent period between treatment of the primary and diagnosis of the metastasis has been estimated to be around 11 months (52). Perineural, lymphatic and mucoperiosteal invasions are usually indicative of advanced disease with metastasis and thus a poor prognosis. Recurrent SCCs tend to be more aggressive (37).
The prognosis of SCC without metastasis is good with conventional treatment with a 5‐year survival rate of about 90%. Friedman et al. in a retrospective review of 44 patients with SCC observed that at 5 years, 86% of patients were free of recurrence following primary surgical therapy and 14% of patients had recurrence or metastasis (53). If there is lymph node metastasis, but the tumour is operable, then the 5‐year survival falls to 39% in SCC of the extremities (54); if the tumour is inoperable, the mean survival time of the patients is only 12·2 months (52). The local recurrence for SCC is twice that of BCC (5), and it has been estimated that almost 50% of patients with one NMSK develop another one within the next 5 years (38).
The tumour grade and degree of differentiation is also a valuable prognostic marker in SCC. Baldursson et al. (1999) in a study of 25 patients with SCC complicating chronic venous leg ulceration observed that the disease was lethal in ten patients who had poorly differentiated tumours and died within a year of diagnosis (55). The tumour behaviour is also correlated with the level of dermal invasion and the vertical tumour thickness. Friedman et al. (1985) found that the tumours which recurred were 4 mm or more thick and involved the reticular dermis. The thickness and level of invasion of cutaneous SCC appear to represent important prognostic factors and may be relevant indicators for wide field resection and/or elective lymph node dissection (53).
Discussion
The diagnosis of ulcerative SCC is relatively easier compared to that of SCC arising from chronic ulcers. There are certain accepted characteristics (Table 1) which suggest an ulcer to be an SCC, of which an everted edge is the most obvious. The ulcer could have one or all the features mentioned in the table. It should, however, be emphasised that an ulcerative SCC could present without any of the recognised features, as observed in patient 2.
Table 1.
Features suggestive of an ulcer to be malignant and features which may suggest a malignant transformation within an ulcer
| Strong association |
| Everted wound edges |
| Exophytic growth |
| Irregular base or margin |
| Excess granulation tissue extending beyond the margins |
| Translucent or shiny granulation tissue affecting the ulcer margins |
| Relatively weak association |
| Increase in |
| Pain |
| Bleeding |
| Exudate |
| Malodour |
| Increase in size despite appropriate treatment |
Bleeding or discharge (excessive) is a feature of malignant ulcers. The whole ulcer may be covered with old coagulated blood or serum. They may also exude small quantities of thick, colourless, foul‐smelling fluid that dries to form a very adherent thick greyish covering (56), as observed in patient 3 (Figure 4b). This feature, though not diagnostic, should nevertheless alert the clinician to the possibility of a malignancy. If the ulcer becomes infected, the discharge can be copious, purulent and foul smelling. The ulcer may become painful if it invades deeper tissue/structures. There are no systemic effects while the tumour is confined to the skin. However, if the ulcer becomes heavily infected, there may be general malaise and pyrexia due to systemic inflammatory response syndrome or sepsis. The regional lymph nodes could also become enlarged either as a result of metastasis or due to superimposed infection in the tumour as happens in one‐third of patients (11).
The diagnosis of SCC arising from chronic ulcers, on the other hand, poses difficult diagnostic dilemmas. The clinical features of an ulcer degenerating into an SCC, though recognised, are not well described. Existing literature consists of case reports, and no series is sufficiently large to evaluate the essential characteristics of a malignant transformation in a non healing ulcer. It might also be precluded by the relative rarity of this association. When malignant transformation occurs in long‐standing ulcers, some of the features listed in Table 1 might be present, but not always, as observed in patient 1.
SCCs arising from chronic ulcers are less invasive, slow growing (11), and the edges may not be everted (9). Malignant transformation or dysplastic changes in chronic ulcers are known to occur mainly from its edges. This is because wounds heal from the edges and hence there is a rapid turnover of cells in an attempt towards reepithelialisation. In long‐standing ulcers, only one edge of the ulcer may undergo dysplastic changes, while the rest of the ulcer may continue to improve and may even reduce in size with appropriate treatment – classically observed in a venous leg ulcer improving with rest and compression; this phenomenon was observed in patient 1. This may result in the spurious appearance of a healing ulcer and the diagnosis of a carcinoma may be missed, particularly in the absence of the classical features of malignancy.
Any ulcer or part of an ulcer which breaks down recurrently after healing should also be viewed with suspicion. Similarly, the appearance of unusual nodules in long‐standing ulcers or any changes in the skin edge suggest a sinister pathology (9, 11). Biopsy should be contemplated even if the ulcer fails to demonstrate any of the recognised features of a malignant transformation.
The diagnosis of an SCC in the lower limb in elderly patients, in addition, poses a problem. If a lesion develops in a more conspicuous area such as the face, then it is more easily noticed by the patient or the attending HCP. On the other hand, a small lesion or an ulcer in the leg is likely to be missed. Furthermore, because leg ulcers due to various aetiologies are prevalent in the community, the diagnosis of an SCC masquerading as an ulcer or an SCC arising within an ulcer is likely to be overlooked, as they could be mistakenly diagnosed as a simple leg ulcer. This might be prevented if the HCPs are aware of the association and have a high‐index of suspicion in any ulcer failing to heal despite receiving appropriate treatment.
Currently, in the United Kingdom, leg ulcer care is predominantly provided by nurses in the community (57, 58). With the recent emergence of community leg ulcer clinics, it is estimated that over 80% of all patients with leg ulcers are cared for in the community by district and practice nurses or by the patients' relative (59). Studies have shown reduction in costs and improvements in healing rates in a mature community leg ulcer clinic staffed by specialist leg ulcer nurses (60, 61).
The drive towards community‐centred care for patients with leg ulcers seems to be expanding and must be encouraged. However, all HCPs in the community caring for patents with ulcers should be made aware of complications arising from such ulcers. It should be borne in mind that although the majority of leg ulcers in the community are either venous, arterial or mixed (arterio‐venous) in origin, a small percentage of such ulcers could be a carcinoma.
It also needs to be emphasised that apart from leg ulcers, malignant change could occur in any chronic ulcer such as a pressure ulcer or a pilonidal sinus wound. HCPs should hence have a high index of suspicion in any non healing ulcer receiving appropriate treatment and adopt a low threshold to biopsy such ulcers. The benefit of doing repeated biopsies in suspicious ulcers, even if negative, far outweighs the extremely low complication rate of the procedure. Pain and bleeding are minimal and could be easily controlled with simple analgesia and calcium alginate dressing (e.g. Kaltostat®), respectively. If the facilities or expertise to do biopsy is not available, then the patients should be referred to the appropriate clinic or hospital without undue delay. Similarly, ulcers in unusual sites, refractory to conventional treatment, should be considered malignant until proven otherwise.
Conclusion
Whilst SCC is amenable to simple excision in the early stages, delay in diagnosis could result in loss of the affected digit or limb; SCC which has metastasised is also life threatening. Therefore, early diagnosis is imperative in their management. A low threshold to biopsy static non healing ulcers and ulcers in unusual sites should be adopted even in those not manifesting any evidence of malignancy.
This article was presented in the annual conference of Wounds – UK in Harrogate, UK, November, 2003.
References
- 1. Trent JT, Kirsner RS. Wounds and malignancy. Adv Skin Wound Care 2003;16: 31–4.DOI: 10.1097/00129334-200301000-00014 [DOI] [PubMed] [Google Scholar]
- 2. Ratliff CR, Beitz J. Two case studies of Marjolin's ulcers in patients referred for management of chronic pressure ulcers. J WOCN 2002;29: 266–8.DOI: 10.1067/mjw.2002.127210 [DOI] [PubMed] [Google Scholar]
- 3. Lineaweaver WC, Brunson MB, Smith JF, Franzini DA, Lumley TO. Squamous carcinoma arising in a pilonidal sinus. J Surg Oncol 1984;27(4):239–42. [DOI] [PubMed] [Google Scholar]
- 4. Saglik Y, Arikan M, Altay M, Yildiz Y. Squamous cell carcinoma arising in chronic osteomyelits. Int Orthop 2001;25(6):389–91.DOI: 10.1007/s002640100280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Britto JA, Fenton L, Fenton OM. The skin, skin tumours and plastic surgery (Chap 9). In: Bernard KG, Young AE, editors. The new aird's companion in surgical studies, 2nd edition. London: Churchill Livingstone Publications, 1998: 191–2. [Google Scholar]
- 6. Coleman DJ, Humzah MD. Plastic and reconstructive surgery, skin lesions. In: Russell RCG, Williams NS, Bulstrode CJK, editors. Bailey and love's short practice of surgery, 23rd edition. London: Arnold Publications, 2000: 181–2. [Google Scholar]
- 7. Mork J, Lie AK, Glattre E, Hallmans G, Jellum E, Koskela P, Moller B, Pukkala E, Schiller JT, Youngman L, Lehtinen M, Dillner J. Human papillomavirus infection as a risk factor for squamous‐cell carcinoma of the head and neck. N Engl J Med 2001;344(15):1125–31.DOI: 10.1056/NEJM200104123441503 [DOI] [PubMed] [Google Scholar]
- 8. Du Vivier A. Non‐melanoma skin cancer (Chap 10). In: Du Vivier A, editor. Atlas of clinical dermatology, 3rd edition. London: Churchill Livingstone Publications, 2002: 178–83. [Google Scholar]
- 9. Lumley JSP. The skin. In: Lumley JSP, editor. Hamilton bailey's physical signs: demonstrations of physical signs in clinical surgery, 18th edition. Oxford: Butterworth‐Heinemann Publications, 1997: 140–1. [Google Scholar]
- 10. Standkard C, Cruse C, Wells K, Karl R. Chronic pressure ulcer carcinomas. Ann Plast Surg 1993;30: 274–7. [DOI] [PubMed] [Google Scholar]
- 11. Browse NL. The skin. In: Browse NL, editor. An introduction to the symptoms and signs of surgical disease, 3rd edition. London: Arnold publications, 2001: 66–9. [Google Scholar]
- 12. Ozek C, Celik N, Bilkay U, Akalin T, Erdem O, Cagdas A. Marjolin's ulcer of the scalp: report of five cases and review of the literature. J Burn Care Rehabil 2001;22: 65–9.DOI: 10.1097/00004630-200101000-00013 [DOI] [PubMed] [Google Scholar]
- 13. Johnston WH, Miller TA, Frileck SP. Atypical pseudoepitheliomatous hyperplasia and squamous cell carcinoma in chronic cutaneous sinuses and fistulas. Plast Reconstr Surg 1980;66(3):395–400. [PubMed] [Google Scholar]
- 14. Adanali G, Senen D, Tuncel A, Ibrahimoglu D, Erdogan B. Squamous cell carcinoma developing in a pilonidal sinus. Plast Reconstr Surg 2002;110(5):1367–8.DOI: 10.1097/00006534-200210000-00035 [DOI] [PubMed] [Google Scholar]
- 15. Furukawa H, Yamamoto Y, Minakawa H, Sugihara T. Squamous cell carcinoma in chronic lymphedema: case report and review of the literature. Dermatol Surg 2002;28: 951–3.DOI: 10.1046/j.1524-4725.2002.02075.x [DOI] [PubMed] [Google Scholar]
- 16. Altunay IK, Gokdemir G, Kurt A, Kayaoglu S. Hidradenitis suppurativa and squamous cell carcinoma. Dermatol Surg 2002;28(1):88–90.DOI: 10.1046/j.1524-4725.2002.01090.x [DOI] [PubMed] [Google Scholar]
- 17. Lapins J, Ye W, Nyren O, Emtestam L. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol 2001;137(6):730–4. [PubMed] [Google Scholar]
- 18. Kontochristopoulos G, Kyriakis K, Symeonidou S, Katsiboulas V, Aroni K, Panteleos D, Katsambas A. Squamous cell carcinoma in chronic trophic ulcers of leprosy patients. J Eur Acad Dermatol Venereol 2000;14(3):230–1.DOI: 10.1046/j.1468-3083.2000.00061-8.x [DOI] [PubMed] [Google Scholar]
- 19. Imtiaz KE, Khaleeli AA. Squamous cell carcinoma developing in necrobiosis lipoidica. Diabet Med 2001;18(4):325–8.DOI: 10.1046/j.1464-5491.2001.00452.x [DOI] [PubMed] [Google Scholar]
- 20. Mello LF, Barcelos MG, Meohas W, Pinto LW, Melo PA, Nogueira Neto NC, Smith J. Chronic ulceration of the leg following extensive scarring due to a snake bite complicated by squamous cell carcinoma. Skeletal Radiol 2000;29(5):298–301.DOI: 10.1007/s002560050613 [DOI] [PubMed] [Google Scholar]
- 21. Garrett AB. Multiple squamous cell carcinomas in lesions of discoid lupus erythematosus. Cutis 1985;36(4) (313–4):316. [PubMed] [Google Scholar]
- 22. Heinz C, Fanihagh F, Steuhl KP. Squamous cell carcinoma of the conjunctiva in patients with atopic eczema. Cornea 2003;22(2):135–7. [DOI] [PubMed] [Google Scholar]
- 23. Powell J, Robson A, Cranston D, Wojnarowska F, Turner R. High incidence of lichen sclerosus in patients with squamous cell carcinoma of the penis. Br J Dermatol 2001;145(1):85–9.DOI: 10.1046/j.1365-2133.2001.04287.x [DOI] [PubMed] [Google Scholar]
- 24. Yang D, Morrison BD, Vandongen YK, Singh A, Stacey MC. Malignancy in chronic leg ulcers. Med J Aust 1996;164(12):718–20. [DOI] [PubMed] [Google Scholar]
- 25. Voisard JJ, Lazareth I, Baviera E, Priollet P. Leg ulcers and cancer: 6 case reports. J Mal Vasc 2001;26(2):85–91. [PubMed] [Google Scholar]
- 26. Philips TJ, Salman SM, Rogers GS. Non‐healing leg ulcers: a manifestation of basal cell carcinoma. J Am Acad Dermatol 1991;25(1 Part 1):47–9. [DOI] [PubMed] [Google Scholar]
- 27. Schwarze HP, Loche F, Gorguet MC, Kuchta J, Bazex J. Basal cell carcinoma associated with chronic venous leg ulcer. Int J Dermatol 2000;39: 75–80.DOI: 10.1046/j.1365-4362.2000.00842.x [DOI] [PubMed] [Google Scholar]
- 28. Granel F, Barbaud A, Schmutz JL. Basal and squamous cell carcinoma associated with chronic venous leg ulcer. Int J Dermatol 2001;40: 539.DOI: 10.1046/j.1365-4362.2001.01094.x [DOI] [PubMed] [Google Scholar]
- 29. Smith J, Mello LF, Nogueira Neto NC, Meohas W, Pinto LW, Campos VA, Barcellos MG, Fiod NJ, Rezende JF, Cabral CE. Malignancy in chronic ulcers and scars of the leg (Marjolin's ulcer. a study of 21 patients. Skeletal Radiol 2001;30(6):331–7.DOI: 10.1007/s002560100355 [DOI] [PubMed] [Google Scholar]
- 30. Harrist TJ, Schapiro B, Quinn TR, Clark WH. The skin (Chap 24). In: Rubin E, Farber JL, editors. Pathology, 3rd edition. Philadelphia: Lippincott‐Raven Publishers, 1999: 1293–4. [Google Scholar]
- 31. Fleming KA. The skin (Chap 28). In: McGee JO, Isaacson PG, Wright NA, editors. Oxford textbook of pathology, Oxford: Oxford University Press, 1992: 2169–71. [Google Scholar]
- 32. Arons MS, Rodin AE, Lynch JB, Lewis SR, Blocker TG. Scar tissue carcinoma. Part II: an experimental study with special reference to burn scar carcinoma. Ann Surg 1966;163: 445–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fleming MD, Hunt JL, Purdue GF, Sandstad J. Marjolin's ulcer: a review and re‐evaluation of a difficult problem. J Burn Care Rehabil 1990;11: 460–9. [PubMed] [Google Scholar]
- 34. Hill BB, Sloan DA, Lee EY, McGrath PC, Kenady DE. Marjolin's ulcer of the foot caused by non‐burn trauma. South Med J 1996;89: 707–10. [DOI] [PubMed] [Google Scholar]
- 35. Ozek C, Cankayali R, Bilkay U, Guner U, Gundogan H, Songur E, Akin Y, Cagdas A. Marjolin's ulcers arising in burn scars. J Burn Care Rehabil 2001;22(6):384–9.DOI: 10.1097/00004630-200111000-00006 [DOI] [PubMed] [Google Scholar]
- 36. Reynolds PL, Strayer SM. Treatment of skin malignancies. The Journal of Family Practice 2003;52(6):456–64. [PubMed] [Google Scholar]
- 37. Stal S, Spira M. Basal and squamous cell carcinoma of the skin (Chap 11). In: Aston JS, Beasley RW, Thorne CNM, editors. Grabb and Smith's plastic surgery, 5th edition Philadelphia: Lippincott‐Raven Publishers, 1997: 117–9. [Google Scholar]
- 38. Nguyen TH, Ho DQ. Nonmelanoma skin cancer. Curr Treat Options Oncol 2002;3(3):193–203. [DOI] [PubMed] [Google Scholar]
- 39. Hochman M, Lang P. Skin cancer of the head and neck. Med Clin North Am 1999;83: 261–82. [DOI] [PubMed] [Google Scholar]
- 40. Sheen YS, Sheen MC, Sheu HM, Yang SF, Wang YW. Squamous cell carcinoma of the big toe successfully treated by intra‐arterial infusion with methotrexate. Dermatol Surg 2003;29(9):982–3.DOI: 10.1046/j.1524-4725.2003.29267.x [DOI] [PubMed] [Google Scholar]
- 41. Thomas DJ, King AR, Peat BG. Excision margins for nonmelanotic skin cancer. Plast Reconstr Surg 2003;112(1):57–63.DOI: 10.1097/01.PRS.0000067479.77859.31 [DOI] [PubMed] [Google Scholar]
- 42. Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol 1992;26(6):976–90. [DOI] [PubMed] [Google Scholar]
- 43. Ashinoff R. Mohs micrographic surgery (Chap 12). In: Aston JS, Beasley RW, Thorne CNM, editors. Grabb and Smith's plastic surgery, 5th edition. Philadelphia: Lippincott‐Raven Publishers, 1997: 121–5. [Google Scholar]
- 44. Kirsner RS, Spencer J, Falanga V, Garland LE, Kerdel FA. Squamous cell carcinoma arising in osteomyelitis and chronic wounds. Treatment with Mohs micrographic surgery vs amputation. Dermatol Surg 1996;22(12):1015–8.DOI: 10.1016/S1076-0512(97)00011-3 [DOI] [PubMed] [Google Scholar]
- 45. Finizio L, Vidali C, Calacione R, Beorchia A, Trevisan G. What is the current role of radiation therapy in the treatment of skin carcinomas? Tumori 2002;88(1):48–52. [PubMed] [Google Scholar]
- 46. Yaparpalvi R, Mahadevia PS, Gorla GR, Beirler JJ. Radiation therapy for the salvage of unresectable subungual squamous cell carcinoma. Dermatol Surg 2003;29(3):294–6.DOI: 10.1046/j.1524-4725.2003.29065.x [DOI] [PubMed] [Google Scholar]
- 47. Veness MJ, Palme CE, Smith M, Cakir B, Morgan GJ, Kalnins I. Cutaneous head and neck squamous cell carcinoma metastasis to cervical lymph nodes (nonparotid): a better outcome with surgery and adjuvant radiotherapy. Laryngoscope 2003;113(10):1827–33.DOI: 10.1097/00005537-200310000-00031 [DOI] [PubMed] [Google Scholar]
- 48. Paletta FX. Squamous cell carcinoma of the skin. Clin Plast Surg 1980;7: 313–36. [PubMed] [Google Scholar]
- 49. Garner KL, Rodney WM. Basal and squamous cell carcinoma. Prim Care 2000;27(2):447–58. [DOI] [PubMed] [Google Scholar]
- 50. Alam M, Ratner D. Primary care: cutaneous squamous cell carcinoma. N Engl J Med 2001;344: 975–83.DOI: 10.1056/NEJM200103293441306 [DOI] [PubMed] [Google Scholar]
- 51. Martin H, Strong E, Spiro RH. Radiation‐induced skin cancer of the head and neck. Cancer 1970;25: 61–71. [DOI] [PubMed] [Google Scholar]
- 52. Joseph MG, Zulueta WP, Kennedy PJ. Squamous cell carcinoma of the skin of the trunk and limbs: the incidence of metastases and their outcome. Aust N Z J Surg 1992;62(9):697–701. [DOI] [PubMed] [Google Scholar]
- 53. Friedman HI, Cooper PH, Wanebo HJ. Prognostic and therapeutic use of microstaging of cutaneous squamous cell carcinoma of the trunk and extremities. Cancer 1985;56(5):1099–105. [DOI] [PubMed] [Google Scholar]
- 54. Ames FC, Hickey RC. Metastasis from squamous cell skin cancer of the extremities. South Med J 1982;75(8):920–3. [DOI] [PubMed] [Google Scholar]
- 55. Baldursson BT, Hedblad MA, Beitner H, Lindelof B. Squamous cell carcinoma complicating chronic venous leg ulceration: a study of the histopathology, course and survival in 25 patients. Br J Dermatol 1999;140(6):1148–52. [DOI] [PubMed] [Google Scholar]
- 56. Berkwits L, Yarkony G, Lewis V. Marjolin's ulcer complicating a pressure ulcer: case report and literature review. Arch Phys Med Rehabil 1986;11: 831–3. [PubMed] [Google Scholar]
- 57. Chaloner D, Noirit J. Treatment and healing rates in a community leg ulcer clinic. Br J Nurs 1997;6(5):250–2. [DOI] [PubMed] [Google Scholar]
- 58. Quioc V. Cost effectiveness in leg ulcer management in the community. Br J Community Nurs 2001;6(6):276–82. [DOI] [PubMed] [Google Scholar]
- 59. Walsh R. Improving diagnosis of malignant leg ulcers in the community. Br J Nurs 2002;11(9):604–13. [DOI] [PubMed] [Google Scholar]
- 60. Ellison DA, Hayes L, Lane C, Tracey A, McCollum CN. Evaluating the cost and efficacy of leg ulcer care provided in two large UK health authorities. J Wound Care 2002;11(2):47–51. [DOI] [PubMed] [Google Scholar]
- 61. Moffatt CJ, Franks PJ, Oldroyd M, Bosanquet N, Brown P, Greenhalgh RM, McCollum CN. Community clinics for leg ulcers and impact on healing. BMJ 1992 5;305(6866):1389–92. [DOI] [PMC free article] [PubMed] [Google Scholar]


