An incorrect version of the above mentioned article was erroneously published due to an administrative error on the part of the authors.
The correct version presents the level of statistical significance of the results, and encompasses a clarification with regards to interpretation of the results.
The authors regret and apologize for this error. The correct version of this article follows.
Changes in cardiac pumping efficiency and intra‐thoracic organ volume during negative pressure wound therapy of sternotomy wounds, assessment using magnetic resonance imaging
Christian Torbrand, Martin Ugander, Henrik Engblom, Göran K Olivecrona, Olof Gålne, Håkan Arheden, Richard Ingemansson, Malin Malmsjö
ABSTRACT
Knowledge on the effects of negative pressure wound therapy (NPWT) on the intra‐thoracic organs is limited. The present study was performed to investigate the effects of NPWT on the volume of the intra‐thoracic organs, using magnetic resonance imaging (MRI), in a porcine sternotomy wound model. Six pigs underwent median sternotomy followed by NPWT at −75, −125 and −175 mmHg. Six pigs were not sternotomised. MR images covering the thorax and heart were acquired. The volumes of the thoracic cavity, lungs, wound fluid and heart were then determined. The volumes of the thoracic cavity and intra‐thoracic organs increased after sternotomy and decreased upon NPWT application. The total heart volume variation, which is inversely related to cardiac pumping efficiency, was higher after sternotomy and decreased during NPWT. NPWT did not result in the evacuation of wound fluid from the bottom of the wound. NPWT largely closes and restores the thoracic cavity. Cardiac pumping efficiency returns to pre‐sternotomy levels during NPWT. This may contribute to the clinical benefits of NPWT over open‐chest care, including the stabilizing effects and the reduced need for mechanical ventilation.
Key words: Experimental surgery • Negative pressure wound therapy • Post‐sternotomy mediastinitis • Sternotomy wound • Wound healing
Authors: C Torbrand, Department of Ophthalmology, Lund University Hospital, Lund, Sweden; M Ugander, Department of Clinical Physiology, Lund University Hospital, Lund, Sweden; H Engblom, Department of Clinical Physiology, Lund University Hospital, Lund, Sweden; GK Olivecrona, Department of Cardiology, Lund University Hospital, Lund, Sweden; O Gålne, Department of Ophthalmology, Lund University Hospital, Lund, Sweden; H Arheden, Department of Clinical Physiology, Lund University Hospital, Lund, Sweden; R Ingemansson, Department of Cardiothoracic Surgery, Lund University Hospital, Lund, Sweden; M Malmsjö, MD, PhD, Department of Ophthalmology, Lund University Hospital, Lund, Sweden and Vascular Research, Lund University, BMC A13, SE‐221 84 Lund, Sweden
Address for correspondence: M Malmsjö, MD, PhD, Vascular Research, Lund University, BMC A13, SE‐221 84 Lund, Sweden E‐mail: malin.malmsjo@med.lu.se
INTRODUCTION
Cardiac surgery is complicated by post‐sternotomy mediastinitis in 1–5% of all procedures and is a life‐threatening complication. The reported early mortality in post‐sternotomy mediastinitis following coronary bypass graft surgery is between 8% and 25%. Traditional treatment of post‐sternotomy mediastinitis includes surgical debridement, drainage, irrigation and reconstruction using pectoral muscle flaps or omentum transposition. Post‐sternotomy mediastinitis can also be treated by negative pressure wound therapy (NPWT) based on the principle of applying sub‐atmospheric pressure by controlled suction through a porous dressing. NPWT has resulted in reduced mortality in post‐sternotomy mediastinitis (1).
Scientific evidence regarding the mechanisms by which NPWT promotes wound healing has started to emerge. The suction force created by NPWT has been found to alter the micro‐vascular wound edge blood flow and stimulate granulation tissue formation and the removal of bacteria (2). However, knowledge of the effects of NPWT on the intra‐thoracic organs is still limited (2). The organs in the mediastinum are vital and the heart and lung function need to be taken into consideration during NPWT. Studies on the lung function have shown better ventilation during NPWT than during conventional open‐chest care 3, 4, presumably because of the sternum‐stabilising effect and the reduced need for mechanical ventilation. Conversely, a decrease in cardiac output has been reported during NPWT when compared with the open‐chest situation 5, 6. However, the effect of NPWT on cardiac output has hitherto only been studied in sternotomised pigs and no comparison has yet been made with non‐sternotomised pigs (intact chest).
The aim of the present study was to examine the effects of NPWT on the cardiac pumping efficiency and the volumes of the intra‐thoracic organs. Magnetic resonance imaging (MRI) was performed on non‐sternotomised pigs and sternotomised pigs before and after the application of NPWT at −75, −125 and −175 mmHg. Thereafter, the volumes of the thoracic cavity, heart, lungs and wound fluid were measured. Furthermore, the effects of NPWT on total heart volume variation (THVV), which is inversely related to cardiac pumping efficiency, were examined.
MATERIALS AND METHODS
Animals
An uninfected porcine sternotomy wound model was used in the present study. Twelve domestic landrace pigs of both genders, with a mean body weight of 50 kg, were fasted overnight with free access to water. The study was approved by the Ethics Committee for Animal Research, Lund University, Sweden. The investigation complied with the ‘Guide for the Care and Use of Laboratory Animals' as recommended by the U.S. National Institutes of Health (1996). The pigs were anaesthetised as described previously 6, 7.
Surgical procedure
Six pigs underwent sternotomy and NPWT while six pigs were not sternotomised. A midline sternotomy was performed and the pericardium and pleurae were opened. The sternotomy wound was prepared for NPWT. A polyurethane foam dressing was placed between the sternal edges and two non‐collapsible drainage tubes were inserted into the foam. The open wound was then sealed with a transparent adhesive drape. The drainage tubes were connected to a vacuum source (VAC® pump unit, KCI, Copenhagen, Denmark), which was set to deliver a continuous negative pressure of −75, −125 or −175 mmHg.
Magnetic resonance imaging
After surgery, the pigs were transported from the operating room to the MRI scanner. MRI was first performed at baseline (0 mmHg). A negative pressure was then applied and MRI was performed when the target pressure had been reached. This procedure was repeated for each negative pressure (−75, −125 and −175 mmHg). In order to eliminate time effects, the sequence of application of the three different negative pressures was varied between the animals using a 3 by 3 Latin square design.
MRI was conducted using a 1·5T system (Philips Medical Systems, Best, the Netherlands) with a five‐element cardiac coil and the pig in the supine position. All imaging was performed using steady‐state free precession sequences. A survey scan was acquired including sagittal images. Typical imaging parameters were: spatial resolution 1·8 × 1·8 mm, slice thickness 10 mm.
All subsequent images were acquired during ventilator‐controlled end expiratory apnea at the functional residual lung capacity. Images of the thoracic anatomy were acquired in the transverse and sagittal planes, covering the entire thoracic cavity. Typical imaging parameters were: spatial resolution 1·1–1·5 × 1·1–1·5 mm, slice thickness 5–8 mm.
The heart was imaged in the short‐axis, and two‐, three‐ and four‐chamber long‐axis planes. Typical imaging parameters were: spatial resolution 1·4 × 1·4 mm, slice thickness 8 mm, retrospective electrocardiogram (ECG) triggering, temporal resolution 30 ms.
MRI analysis
All images were evaluated using freely available software (Segment 1·699 http://segment.heiberg.se,13). Intra‐thoracic volume measurements were performed in transverse images with simultaneous viewing of sagittal images and image plane inter‐sections for orientation. The right lung, left lung, intra‐thoracic wound fluid and total thoracic areas were manually delineated by defining dedicated regions of interest in all transverse images (Figure 1). For the non‐sternotomised pigs, the intra‐thoracic volumes had been imaged with ECG triggering. Quantification was performed in the first time frame at 0 ms in relation to the R‐wave of the ECG.
Figure 1.
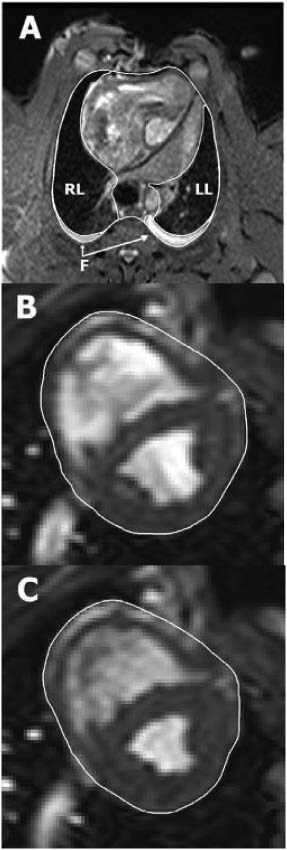
Illustration of manual delineation of organ volumes. Image (A) shows how the intra‐thoracic structures were outlined in a transverse magnetic resonance (MR) image through the thorax of a pig with an open sternotomy wound. The right lung (RL), left lung (LL) and intra‐thoracic fluid (F) were delineated in each image throughout the entire thorax, allowing the volume of each structure to be quantified. Images (B) and (C) show short‐axis MR images of the same animal's heart in end diastole and end systole, respectively. The pericardial border of the heart was manually delineated in all short‐axis slices covering the entire volume within the pericardium. The total heart volume in end diastole and end systole, as well as the total heart volume variation throughout the cardiac cycle, was then quantified.
The THV was measured by manually delineating the pericardial border of the heart in short‐axis images (Figure 1) in both end diastole and end systole, as previously described (7). Long‐axis images and image plane inter‐sections were viewed simultaneously for orientation. The THVV, expressed in percent, was calculated according to the following formula:
THVV(%) = [(THV in end diastole – THV in end systole)/THV in end diastole] × 100.
The non‐sternotomised pigs were different animals than the sternotomised pigs. In order to determine whether or not the pigs were similar in size, the distance between ten vertebrae was measured in a mid‐sagittal survey MRI image in all pigs.
Calculations and statistics
The experiments were performed on six sternotomised and six non‐sternotomised pigs. Statistical analysis was performed using Kruskal‐Wallis test with Dunn's post‐test for multiple comparisons. Significance was defined as P < 0·05. The results are presented as mean values ± the standard error on the mean.
RESULTS
Intra‐thoracic organ volume
The volume of the thoracic cavity and the intra‐thoracic organs was greater in the sternotomised pigs than in the non‐sternotomised pigs, P = 0·0414 and P = 0·0217, respectively (2, 3). The lung volume was larger in the sternotomised pigs, P = 0·1523 for the right lung and P = 0·2810 for the left lung (2, 3). Upon the application of NPWT, the volume of the total thoracic cavity and intra‐thoracic organs was reduced, P = 0·0192 and P = 0·0188, respectively, when comparing 0 mmHg with −125 mmHg (Figure 3). The lung volume was decreased when NPWT was applied, P = 0·0850 for the right lung and P = 0·0520 for the left lung, compared with 0 mmHg (2, 3).
Figure 2.
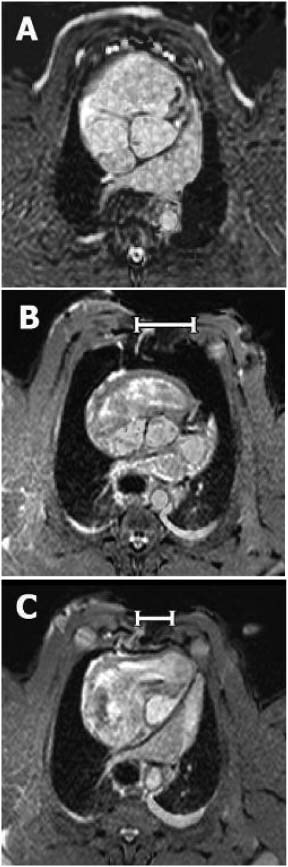
Representative transverse MR images at the level of the aortic root selected from a stack of 50 contiguous 5‐mm thick sections covering the entire thorax. The images were obtained from: (A) a non‐sternotomised pig and (B) a sternotomised pig before (0 mmHg) and (C) after the application of −125 mmHg. Note the differences in the size, shape and position of the intra‐thoracic organs induced by sternotomy and topical negative pressure. In particular, note how negative pressure wound therapy draws the two sternal edges closer to each other (white bars), thereby decreasing the circumference of the thorax, making it similar to that of the non‐sternotomised pig.
Figure 3.
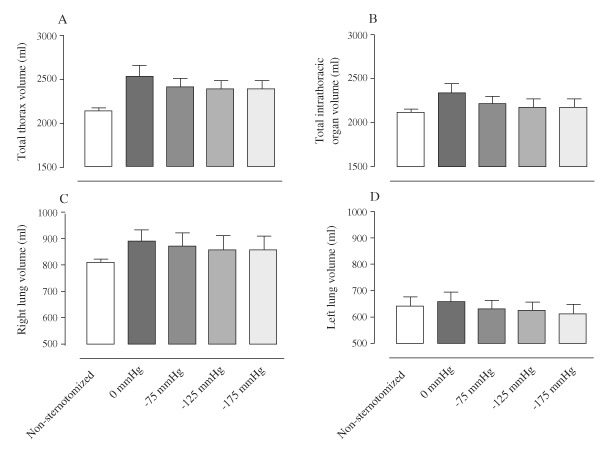
Volumes of: (A) the thoracic cavity, (B) the intra‐thoracic organs, (C) right lung and (D) left lung, measured using MRI, in non‐sternotomised pigs and in sternotomised pigs before (0 mmHg) and after the application of negative pressure wound therapy of −75, −125 and −175 mmHg. The results are presented as mean values ± standard error on the mean. Note how the volumes of the thorax and intra‐thoracic organs decrease upon the application of negative pressure, most prominently upon the change from 0 to −75 mmHg. Further increases in negative pressure only result in minor reduction in volume.
Cardiac pumping efficiency
The THVV, which reflects cardiac pumping efficiency (7), increased following sternotomy, P = 0·1854 (Figure 4). When NPWT was applied, the THVV decreased, P = 0·1568 when comparing 0 mmHg with −125 mmHg (Figure 4).
Figure 4.
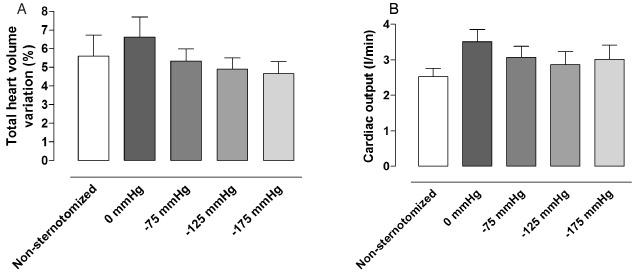
The (A) total heart volume variation and (B) cardiac output in non‐sternotomised pigs and sternotomised pigs before and after the application of negative pressure wound therapy (NPWT) of −75, −125 and −175 mmHg. The results are presented as mean values ± standard error on the mean. Note that after sternotomy, the cardiac pumping effciency appears to decrease (increase in total heart volume variation), and cardiac output increases, and these measures return to control levels upon application of NPWT.
Wound fluid
Wound fluid had accumulated in the bottom of the thoracic cavity, in the right and left pleura because these were opened during the surgical procedure. The application of negative pressure did not decrease the amount of free fluid at the bottom of the thoracic cavity, P > 0·30 when comparing 0 mmHg with −125 mmHg (Figure 5).
Figure 5.
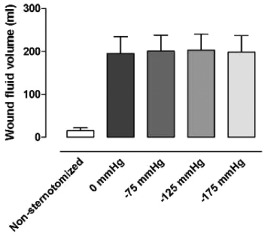
The intra‐thoracic wound fluid volume, measured using MRI, in non‐sternotomised pigs and in sternotomised pigs before and after the application of negative pressure wound therapy (NPWT) at −75, −125 and −175 mmHg. The results are presented as mean values ± standard error on the mean. Note that NPWT does not result in the evacuation of fluid from the bottom of the wound.
Pig size
In order to determine whether the non‐sternotomised pigs and the sternotomised pigs were of similar size, the distance between ten vertebrae was measured. This distance was 232 ± 2 mm in the sternotomised pigs and 231 ± 3 mm in the non‐sternotomised pigs (P > 0·30), indicating similar pig size.
DISCUSSION
Intra‐thoracic organ volume
We found that the total volume of the intra‐thoracic organs increases upon sternotomy. We speculate that this reflects changes in the quantity of blood that collects in these organs as a result of a change in the pressure conditions. An alternative explanation of the increased lung volume may be that sternotomy results in increased end expiratory volumes of air in the lungs.
Upon application of NPWT, the volume of the thoracic cavity decreased. NPWT contracts the wound and draws the two sternal edges together, thereby decreasing the circumference of the thorax. Previous studies have reported similar findings, that is, soft tissue wound contraction and ‘macro‐deformation’ upon NPWT (8). During NPWT, air is evacuated from the thorax, decreasing the total intra‐thoracic volume. NPWT thus largely restores the macroscopic anatomical conditions in the thorax, which may explain the clinical benefits of NPWT over open‐chest care, including the stabilizing effects and the reduced need for mechanical ventilation 3, 4.
The volume of the intra‐thoracic organs decreases upon the application of NPWT, but does not return to pre‐sternotomy levels. This may be explained by the fact that the NPWT foam acts as wound filler that separates the sternal edges, resulting in a larger thoracic cavity during NPWT than before sternotomy. Also, the open‐heart surgery may have resulted in interstitial oedema, which may account for the organs being slightly enlarged.
Cardiac pumping efficiency
The THVV, which may be regarded as a measure which inversely relates to heart pumping efficiency (7), increased after sternotomy. Upon the application of NPWT, the THVV decreased to values that are similar to those in the non‐sternotomised pigs. A retrospective analysis of two previous studies suggests similar changes in cardiac output 6, 9. Cardiac output is higher after sternotomy and then return to pre‐sternotomy levels during NPWT. These findings are in accordance with previous reports. Cardiac output has been shown to increase upon sternotomy (10). The results suggest that this is a result of opening the thorax and pericardium, but the exact mechanism is unclear. Other reports show a decrease in cardiac output when the sternotomy wound is resealed during NPWT 5, 6. Many have been concerned regarding the findings that cardiac output decreases during NPWT, and suggestions have been made of invasive monitoring of haemodynamics. However, the results from the present study are reassuring and indicate that cardiac pumping efficiency returns to pre‐sternotomy levels during NPWT.
Wound fluid
Wound fluid is produced during surgery and there will thus be more fluid at the bottom of the thoracic cavity in the sternotomised pigs than in the non‐sternotomised pigs. In the sternotomised pigs, NPWT did not decrease the amount of wound fluid in the bottom of the thoracic cavity. We have found that the negative pressure is not transduced to the bottom of the thoracic cavity (11), which may explain why the wound fluid is not evacuated. However, in the clinical situation the patient is mobilised. The wound fluid may then move around in the thoracic cavity and eventually come in contact with the foam and negative pressure and thereby be sucked out. However, drainage of the wound fluid from the thoracic cavity may not be crucial for resolving the mediastinitis. We believe that one of the most important issues in mediastinitis is the infection in the bone of the sternum. The NPWT dressing is in contact with the entire wound surface and the sternum bone is exposed to the negative pressure. We have shown previously that NPWT affects the fluid in the sternum (12). Presumably, fluid is drawn from the surrounding tissue to the sternum and into the vacuum source. This ‘endogenous drainage’ may be one possible mechanism by which osteitis is resolved by NPWT in post‐sternotomy mediastinitis.
Limitations of the study
The measurements in the sternotomised pigs were compared with those in the non‐sternotomised pigs. We determined that the two groups of pigs were of similar size (vertebra distance). It is, therefore, unlikely the limitation of having different pigs in the two groups have affected the conclusions drawn from the study.
Conclusions
NPWT largely closes and restores the thoracic cavity. This may contribute to the clinical benefits of NPWT over open‐chest care, including the stabilizing effects and the reduced need for mechanical ventilation. The volume of the intra‐thoracic organs increased after sternotomy and decreased during NPWT. Similarly, cardiac output increased and cardiac pumping efficiency decreased upon sternotomy and then returned to pre‐sternotomy values when the thorax was resealed by NPWT. NPWT does not result in the evacuation of wound fluid from the bottom of the wound, presumably because the negative pressure is not transduced to the bottom of the wound. However, NPWT is in full contact with the sternal bone edge, which may be crucial in resolving the bone infection in mediastinitis.
REFERENCE
- 1. Torbrand C, Ugander M, Engblom H, Olivecrona GK, Gålne O, Arheden H, Ingemansson R, Malmsjö M. Changes in cardiac pumping efficiency and intra‐thoracic organ volume during negative pressure wound therapy of sternotomy wounds, assessment using magnetic resonance imaging. Int Wound J 2010;7:115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES
- 1. Sjogren J, Gustafsson R, Nilsson J, Malmsjo M, Ingemansson R. Clinical outcome after poststernotomy mediastinitis: vacuum‐assisted closure versus conventional treatment. Ann Thorac Surg 2005;79:2049–55. [DOI] [PubMed] [Google Scholar]
- 2. Malmsjo M, Ingemansson R, Sjogren J. Mechanisms governing the effects of vacuum‐assisted closure in cardiac surgery. Plast Reconstr Surg 2007;120:1266–75. [DOI] [PubMed] [Google Scholar]
- 3. Ramnarine IR, McLean A, Pollock JC. Vacuum‐assisted closure in the paediatric patient with post‐cardiotomy mediastinitis. Eur J Cardiothorac Surg 2002;22:1029–31. [DOI] [PubMed] [Google Scholar]
- 4. Kutschka I, Frauendorfer P, Harringer W. Vacuum assisted closure therapy improves early postoperative lung function in patients with large sternal wounds. Zentralbl Chir 2004;129 Suppl 1:S33–4. [DOI] [PubMed] [Google Scholar]
- 5. Conquest AM, Garofalo JH, Maziarz DM, Mendelson KG, Su Sun Y, Wooden WA, Meadows WM, Nifong W, Chitwood WR. Hemodynamic effects of the vacuum‐assisted closure device on open mediastinal wounds. J Surg Res 2003;115:209–13. [DOI] [PubMed] [Google Scholar]
- 6. Petzina R, Ugander M, Gustafsson L, Engblom H, Sjogren J, Hetzer R, Ingemansson R, Arheden H, Malmsjo M. Hemodynamic effects of vacuum‐assisted closure therapy in cardiac surgery: assessment using magnetic resonance imaging. J Thorac Cardiovasc Surg 2007;133:1154–62. [DOI] [PubMed] [Google Scholar]
- 7. Carlsson M, Cain P, Holmqvist C, Stahlberg F, Lundback S, Arheden H. Total heart volume variation throughout the cardiac cycle in humans. Am J Physiol Heart Circ Physiol 2004;287:H243–50. [DOI] [PubMed] [Google Scholar]
- 8. Morykwas MJ, Simpson J, Punger K, Argenta A, Kremers L, Argenta J. Vacuum‐assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg 2006;117 Suppl 7:121S–6S. [DOI] [PubMed] [Google Scholar]
- 9. Götberg M, Olivecrona G, Engblom H, Ugander M, Van Der Pals J, Heiberg E, Arheden H, Erlinge D. Rapid short‐duration hypothermia before reperfusion reduces myocardial infarct size and microvascular obstruction in pigs. BMC Cardiovascular Disorders. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reuter DA, Goresch T, Goepfert MS, Wildhirt SM, Kilger E, Goetz AE. Effects of mid‐line thoracotomy on the interaction between mechanical ventilation and cardiac filling during cardiac surgery. Br J Anaesth 2004;92:808–13. [DOI] [PubMed] [Google Scholar]
- 11. Malmsjo M, Petzina R, Ugander M, Engblom H, Gustafsson L, Mokhtari A, Hetzer R, Arheden H, Ingemansson R. Preventing heart injury during topical negative pressure therapy in cardiac surgery – assessment using real time MRI. J Thorac Cardiovasc Surg. In press. [DOI] [PubMed] [Google Scholar]
- 12. Petzina R, Ugander M, Gustafsson L, Engblom H, Hetzer R, Arheden H, Ingemansson R, Malmsjo M. Topical negative pressure therapy of a sternotomy wound increases sternal fluid content but does not affect internal mammary artery blood flow – assessment using MRI. J Thorac Cardiovasc Surg. In press. [DOI] [PubMed] [Google Scholar]
- 13. Heiberg E, Sjögren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and Validation of Segment – freely available software for cardiovascular image analysis. BMC Med Imaging 2010;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]


