Abstract
Prevention and treatment of bacterial colonised/infected wounds are critical. Many commercially available silver dressings claim broad‐spectrum bactericidal activity over days and are indicated for serious conditions including burns and ulcers. However, there is no peer‐reviewed literature available for many newer dressings. This study compared the activity of some of these dressings. Six silver‐containing dressings were compared using log reduction, silver release and corrected zone of inhibition assays. Only the nanocrystalline silver dressing was bactericidal against Staphylococcus aureus, and the only other dressing that produced any log reduction was a silver collagen matrix dressing. These two dressings and a silver alginate dressing produced zones of inhibition, although the collagen matrix and alginate dressings had decreasing zone sizes over time, and the latter liquefied after five transfers. The remaining dressings (two ionic silver foam dressings and a silver sulphate dressing) did not produce zones of inhibition. For the foam, alginate and collagen matrix dressings, antimicrobial activity was related to silver release. The silver sulphate dressing released large quantities of silver, but only through the dressing edges, as the wound‐contacting surface appeared to be hydrophobic. The results of this study emphasise the importance of confirming product claims regarding silver dressing efficacy.
Keywords: Advanced wound dressing, Bactericidal, Bacteriostatic, Silver
INTRODUCTION
Chronic and acute wounds are often heavily colonised or infected by bacteria (1) which interfere with wound healing processes by inducing an inflammatory host response 2, 3. This makes prevention and treatment of bacterial colonisation a critical part of wound care. Traditional methods of controlling bacterial colonisation involve the use of topical antimicrobial agents such as silver nitrate and silver sulphadiazine, as well as a variety of other antiseptics, such as chlorhexidine, quaternary ammonium compounds and povidone‐iodine. However, over the past decade, a large number of advanced silver‐containing dressings have become commercially available. The majority of these dressings are marketed as effective against a broad range of bacteria over multiple days and are indicated for a variety of serious conditions including partial thickness burns, ulcers of various aetiologies, donor and graft sites, traumatic and surgical wounds, dermatologic disorders and skin tears. However, there are little data available in the peer‐reviewed literature regarding these dressings, particularly those which have been released recently.
The purpose of this study was to compare the activity of some recently available silver‐containing dressings. Six dressings were tested – a silver sulphate dressing, a silver alginate dressing, a silver collagen matrix dressing, a nanocrystalline silver dressing and two ionic silver foam dressings.
MATERIALS AND METHODS
Materials
All dressings used were obtained free from the companies which produce them. All dressings were stored as indicated on the packaging and tested within their expiry dates. Unless mentioned, all other materials were purchased from Fisher Scientific Canada, Inc.
Dressings tested in this study were as follows:
-
•
A silver sulphate (Ag2SO4) dressing (Mepilex® Ag Antimicrobial Soft Silicone Foam Dressing, Molnlycke Health Care LLC, Sweden). Product information indicates that this dressing inactivates a wide range of bacteria, including Staphylococcus aureus, within 30 minutes; provides a rapid sustained silver release; can be worn for 7 days and does not stain. The dressing is indicated for low to moderately exuding wounds such as partial thickness burns, leg ulcers, foot ulcers and pressure ulcers (4).
-
•
A silver alginate dressing (Algicell™ Ag, Derma Sciences, Inc., Ontario, Canada). Product information indicates that this dressing contains 1.4% ionic silver; has a kill rate of 99.99%, 99.91% and 97.46% at days 1, 3 and 5 of challenge, respectively, for S. aureus and behaves as a bacterial barrier with controlled sustained silver release. The dressing is indicated for diabetic foot ulcers, leg ulcers, pressure ulcers, donor sites and traumatic and surgical wounds (5).
-
•
Two ionic silver foam dressings (Poly‐ Mem® Silver Non‐Adhesive Pads, and PolyMem® Silver Shapes, Ferris Mfg. Corp., Burr Ridge, IL). Product information indicates that both of these dressings kill 99.9% of bacteria tested (including S. aureus), do not stain skin and can be worn for 3 days. These dressings are indicated for first‐ and second‐degree burns, leg ulcers, pressure ulcers, diabetic ulcers, venous ulcers, donor and graft sites, traumatic and acute wounds, surgical wounds, dermatologic disorders and skin tears (6).
-
•
A silver collagen matrix dressing with calcium alginate and ethylenediaminetetraacetic acid (EDTA) (Biostep™ Ag, Smith and Nephew, Inc., Largo, FL). The silver is in the form of silver chloride. Product information for this dressing indicates that it has antibacterial activity, targets and deactivates excess matrix metalloproteinases and has a 6‐day wear. The silver in the dressing is intended to prevent colonisation of the dressing (7). The dressing is indicated for management of full‐thickness and partial‐thickness acute and chronic wounds including pressure ulcers, diabetic ulcers, mixed vascular etiology ulcers, venous ulcers, first‐ and second‐degree burns, donor or graft sites, abrasions, dehisced surgical wounds and traumatic wounds (7).
-
•
A nanocrystalline silver dressing (Acticoat™, Smith and Nephew, Inc.). Product information for this dressing indicates that it kills bacteria in vitro in 30 minutes, acts as an antibacterial barrier for up to 3 days, provides sustained silver release and is effective against over 150 microorganisms including Gram‐positive and Gram‐negative bacteria (including S. aureus), antibiotic‐resistant bacteria, yeast and mold. The product is indicated for reducing infection in partial‐ and full‐thickness wounds including pressure ulcers, venous ulcers, diabetic ulcers, surgical wounds, first‐ and second‐degree burns and graft and dermal substitute recipient sites (8).
Bactericidal efficacy – log reduction assay
Log reductions were used to determine the ability of commercial silver dressings to kill bacteria in 30 minutes, using the methods of Gallant‐Behm et al. (9) All tests were performed in triplicate. Briefly, S. aureus ATCC 25923 [three colonies of a Mueller‐Hinton agar (MHA) plate or 1 ml of a 4‐ to 5‐hour growth culture grown as described below] was used to inoculate 100 ml of tryptic soy broth (TSB) and was grown overnight at 37°C and 0.409 g. One milliliter of this culture was used to inoculate another flask of TSB (100 ml) and was grown under the same conditions for 4–5 hours to ensure the bacteria were in log‐phase growth [∼108 colony‐forming units (CFU)/ml]. Using aseptic technique, dressings were cut into various sizes, with dressings that expand considerably upon saturation being cut to smaller sizes than those that do not. The nanocrystalline silver and silver alginate dressings were cut into 2.54 × 2.54 cm pieces. Silver collagen matrix dressings were cut into 2.54 × 1.27 cm pieces. Silver sulphate dressings and both ionic silver foam dressings were cut into 1.27 × 1.27 cm squares. The dressing pieces were placed on thin sheets of plastic (aseptically cut into 3.8 × 3.8 square cm pieces) in the inverted lid of a sterile Petri dish. Experimental dressing pieces were then moistened with the required moistening volume of sterile distilled water. To determine the moistening volume, the saturation volume of each dressing was determined by a simple water holding test in which the dressings were weighed, submerged in water for 2 minutes, carefully removed (without squeezing), allowed to drip for 10 seconds and then re‐weighed (Table 1). Then the inoculum volume was subtracted from 90% of the saturation volume to obtain the moistening volume. Control dressing pieces were moistened with the moistening volume of STS, a detergent which inactivates silver (6.0% [w/v] NaCl, 1% [v/v] polysorbate 20 and 0.1% [w/v] sodium thioglycolate for S. aureus (10)). Both control and experimental dressing pieces were then inoculated with 75 µl of inoculum per 1.27 cm square. A second sheet of plastic was then laid on top of the dressings to contain the inoculum, followed by the Petri dish base placed upright to ensure good contact between the bacteria and the dressings. The inoculated dressings were then incubated at 37°C for 30 minutes. After removal of the dressings from the incubator, they were placed in STS in order to achieve a 1:10 dilution of the original inoculum. The dressings in STS were then vigorously vortexed, and the resulting solutions containing the recovered bacteria were serially diluted in phosphate‐buffered saline (pH 7.0, containing 8.5 g/l NaCl, 0.61g/l KH2PO4 and 0.96 g/l K2HPO4). Three 20 µl drops from each dilution were plated on MHA. The plates were then incubated at 37°C and after 24 and 48 hours the numbers of bacterial colonies were counted. The counts generated from the experimental pieces of dressings were used to calculate the surviving number of CFU, whereas the counts generated from the control pieces of dressings were used to calculate the numbers of bacterial CFU in the original inoculants that were not trapped in the dressings. The log10 of the starting numbers and surviving numbers of bacteria were then determined. Log reductions were then calculated as the difference between the log of the initial number of bacteria and the log of the final surviving number of bacteria.
Table 1.
Dressing saturation volumes
| Dressing description | Average saturation volume (µl/cm2, ±SD, n = 3) |
|---|---|
| Silver sulphate dressing | 808 ± 43 |
| Non adhesive ionic silver foam dressing | 594 ± 43 |
| Shaped ionic silver foam dressing | 172 ± 66 |
| Silver collagen matrix dressing | 176 ± 5 |
| Silver alginate dressing | 151 ± 11 |
| Nanocrystalline silver dressing | 53 ± 1 |
Silver dissolution assay
The release of silver from each dressing was determined with static dissolution tests using methods similar to those of Wright et al. (11) except that corrections were made for dressing saturation volume and dressing breakage. Briefly, one 2.54 × 2.54 cm piece of dressing per 5 ml of sterile distilled water plus the dressing saturation volume was placed in a sealed vial wrapped in aluminium foil to prevent silver precipitation. The vial was then incubated at 37°C for 24 hours. Afterwards, the dressings were aseptically removed from the vials, allowed to drip into the vial for 10 seconds and then disposed off. The remaining solutions were immediately filtered with a 70‐µm filter if pieces of the dressings had broken off in solution (both of the ionic silver foam dressings, the silver collagen matrix dressings and the silver alginate dressings) in order to prevent further leaching of silver from the pieces after the 24‐hour time point and to facilitate atomic absorption spectroscopy (AAS). The solutions were then acidified in 9% nitric acid/0.9% tartaric acid to ensure that all silver released remained in solution. The solutions were then submitted for total silver analysis by AAS. For AAS, a Varian 220 FS double‐beam Atomic Absorption Spectrophotometer (Varian Inc., Palo Alto, CA) was used, with the following instrument parameters: an Ag hollow cathode lamp with a wavelength of 328.1 nm and a lean air‐acetylene flame. A calibration plot was generated using silver standards of 0.5, 1.0, 3.0 and 5.0 ppm, prepared from a silver standard stock solution of 1000 ppm. If dressings had released more than 5 ppm into solution, the solutions were diluted as necessary with distilled water until they were in the linear range for silver analysis (0.1–5 ppm).
Bacteriostatic longevity: day‐to‐day corrected zone of inhibition assay
The bacteriostatic longevity of the dressings was assessed using day‐to‐day transfer corrected zone of inhibition (CZOI) assays. The method used for this procedure, a modified form of the Kirby–Bauer assay, is similar to that of Wright et al. (11), with modifications as described below. Briefly, 100 µl of S. aureus taken from an overnight culture was spread onto MHA plates, and silver dressing pieces pre‐moistened with their saturation volume of distilled water were then placed onto the centre of the plates. The original dressing placement was traced onto the bottom of the Petri plate to correct for dressings which shrank over time (as with the silver collagen matrix dressing). The plates were incubated overnight at 37°C and then the zones of bacterial inhibition and dressing widths (or tracings as appropriate) were measured in two perpendicular directions. The CZOI was calculated by subtracting the dressing width from the zone width, and the results for the two directions were averaged. After zone measurement, the dressings were then transferred to new bacteria‐seeded MHA plates, and this procedure was repeated for a total of 9 days. During this period, if all three dressing pieces of an experimental group ceased to produce any zone of inhibition, they were eliminated from the procedure. The shaped ionic silver foam dressing was not tested in this protocol (see Discussion).
Statistics
One‐way analysis of variance tests with Tukey–Kramer multiple comparisons post‐tests were performed for all assays in which more than two dressings were compared (log reductions, absorptive capacity, silver release and CZOI assays up to day 6). For days 7–9 in the CZOI assays, where only the nanocrystalline silver dressing and the silver collagen matrix dressing were still active, they were compared using unpaired t‐tests with Welch corrections. All statistical analyses were performed using Graphpad InStat Version 3.06 (GraphPad Software, San Diego, CA; © 2003, www.graphpad.com).
RESULTS
Table 1 shows the dressing saturation volumes for each commercially available silver dressing tested. There were significant differences in absorptive capacity between dressings (P < 0.0001). The silver sulphate dressing had a significantly higher absorptive capacity than all other dressings tested (P < 0.001), while the non adhesive ionic silver foam dressing had a significantly higher absorptive capacity than all the remaining dressings (P < 0.001). The only other significant differences were that the silver collagen matrix dressing, and the shaped ionic silver foam dressing had significantly higher absorptive capacities than the nanocrystalline silver dressing (P < 0.05).
Table 2 shows the log reductions measured for each commercially available silver dressing tested. There were significant differences in the activity of the various silver‐containing dressings (P < 0.0001). The nanocrystalline silver dressing produced significantly higher log reductions than all other dressings tested (P < 0.001). It was also the only dressing to produce a total kill, and the only dressing that was bactericidal, where the definition of bactericidal is a dressing capable of producing a log reduction greater than three (9). The silver collagen matrix dressing produced significantly higher log reductions than the remaining four dressings (P < 0.001). The remaining four dressings showed no significant differences from one another, and all produced no positive log reductions.
Table 2.
Bactericidal efficacy
| Dressing description | Average log reduction (±SD, n = 3) |
|---|---|
| Silver sulphate dressing | −0.61 ± 0.08 |
| Non adhesive ionic silver foam dressing | −0.03 ± 0.01 |
| Shaped ionic silver foam dressing | −0.33 ± 0.08 |
| Silver alginate dressing | −0.08 ± 0.30 |
| Silver collagen matrix dressing | 1.18 ± 0.29 |
| Nanocrystalline silver dressing | >3.46 ± 0.00 |
Table 3 shows the silver release from the commercially available silver‐containing dressings into distilled water after a 24‐hour period. There were significant differences between groups (P < 0.0001). The silver sulphate dressing released significantly more silver than all other dressings (P < 0.001), and the nanocrystalline silver dressing released significantly more silver than the remaining dressings (P < 0.001). The other dressings were not significantly different from one another.
Table 3.
24‐Hour silver release
| Dressing description | Average silver release (ppm ± SD) | Average silver release (mg/cm2± SD) |
|---|---|---|
| Silver sulphate | 318.63 ± 15.72 | 0.69808 ± 0.03124 (n = 3) |
| Non adhesive ionic silver foam | 0.10 ± 0.02 | 0.00014 ± 0.00004 (n = 3) |
| Shaped ionic silver foam | 0.13 ± 0.11 | 0.00009 ± 0.00007 (n = 2) |
| Silver alginate | 2.58 ± 1.58 | 0.00401 ± 0.00245 (n = 2) |
| Silver collagen matrix | 0.26 ± 0.11 | 0.00040 ± 0.00018 (n = 2) |
| Nanocrystalline silver | 92.87 ± 5.88 | 0.14398 ± 0.00911 (n = 3) |
The CZOIs over a 9‐day period are shown in Figure 1 for the nanocrystalline silver dressing (n = 3), the silver collagen matrix dressing (n = 2) and the silver alginate dressing (n = 3). The silver sulphate dressing (n = 3) and non adhesive ionic silver foam dressing (n = 2) did not generate any zones of inhibition on the first day of testing and had S. aureus growing under the dressings. Therefore, no transfers were performed for these dressings. The nanocrystalline silver dressing generated consistent zone sizes until the experiment was terminated after 9 days. The silver collagen matrix dressing zone sizes began to decrease after day 7 (P < 0.05) but were still present out to day 9. The silver alginate dressing zone size decreased after day 1 (P < 0.001). Smaller zones were generated by the silver alginate dressing out to day 6, by which point the dressing completely liquefied, making further transfers impossible. There were significant differences (P < 0.05) in zone sizes between dressings on all days up to day 6, with the silver alginate dressing having the largest zone on the first day, and the smallest zone on all subsequent days. On all days but days 4 and 5, the silver collagen dressing produced smaller zones than the nanocrystalline silver dressing, but there were no significant differences in zone sizes between these two dressings except on day 1.
Figure 1.
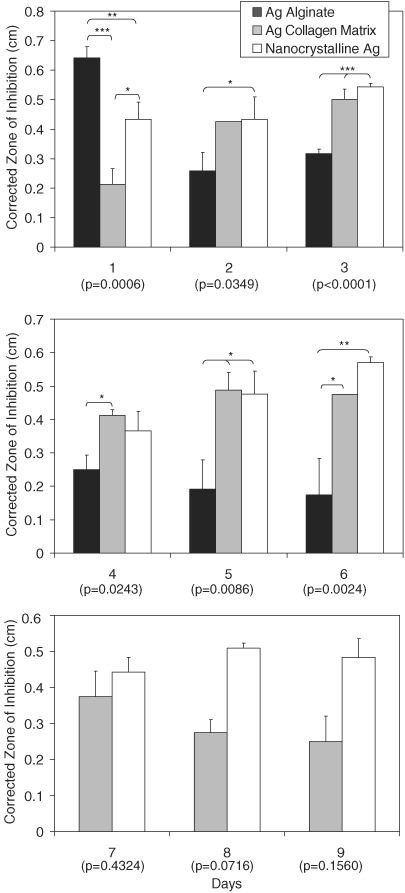
Corrected zone of inhibition of Staphylococcus aureus over a series of days for nanocrystalline silver dressings (n = 3), silver collagen matrix dressings (n = 2) and silver alginate dressings (n = 3). Error bars show standard deviation. P values from one‐way analysis of variance testing are written below each day on the x‐axis. When there were significant differences between groups (P < 0.05), Tukey–Kramer multiple comparisons post‐testing is indicated on the graph. *P < 0.05, **P < 0.01 and ***P < 0.001.
DISCUSSION
In 30 minutes, only the nanocrystalline silver dressing was bactericidal against S. aureus, contrary to the product information for some of the other dressings. This corresponded to release of greater concentrations of active silver compared with most of the other dressings tested. Interestingly, the silver released by the nanocrystalline silver was about 6% of traditional silver treatments which release Ag+ only. Silver nitrate is provided at 2.95–5.91 mg/cm2/day, while silver sulphadiazine is provided at 1.21 mg/cm2/day in burn treatment 12, 13. The nanocrystalline silver dressing also showed an inhibitory effect for over 9 days of in vitro challenges with S. aureus, indicating sustained release of active silver. These results are in good agreement with those of previous studies 9, 14, 15. Gallant‐Behm et al. (9) found that of eight silver‐containing dressings tested, only nanocrystalline silver dressings produced bactericidal activity, which was related to its rapid sustained release of active silver. Thomas and McCubbin 14, 15 gave nanocrystalline silver dressings the highest score when comparing 10 silver‐containing dressings, and attributed its antimicrobial activity to the rapid release of active silver. Wright et al. (16) found that relative to silver nitrate, silver sulphadiazine and mafenide acetate, nanocrystalline silver provided the fastest, broadest spectrum fungicidal activity. Yin et al. (17) compared these same dressings against five wound pathogens and came to the same conclusions. Another study by Wright et al. (11), comparing the nanocrystalline silver dressing to a controlled release silver film dressing, found that the nanocrystalline silver showed more rapid bactericidal activity against a broader spectrum of organisms. Wright et al. (18) compared nanocrystalline silver dressing with polyhexamethylene biguanide (PHMB), and they both showed potent in vitro bactericidal activity. However, in a porcine full‐thickness infected wound model, PHMB reduced the bacterial population initially, but could not keep it in check to the same degree as the nanocrystalline silver dressing (18). The PHMB‐containing dressing was also highly inflammatory in the wound bed, delaying healing (18), whereas nanocrystalline silver shows anti‐inflammatory/pro‐healing activity (19). The antimicrobial efficacy of nanocrystalline silver dressings showed in these studies has been corroborated by other in vivo and clinical studies 20, 21, 22, 23. This suggests that the species of silver released, as well as the total silver released, appear to be important factors for activity, because the dressing showed good antimicrobial activity despite releasing 6% of the amount of silver provided by traditional silver treatments. Based on the product information, nanocrystalline silver dressings were the only dressings in this study to release species other than ionic silver (Ag+), also releasing metallic silver (Ag(0), likely in cluster form) and a higher oxidation state species (24). This dressing, which is designed to be a porous moisture wicking wound interface, has a low absorptive capacity – significantly lower than the other dressings except the silver alginate dressing – because the only absorptive material is the middle gauze layer, and therefore it requires a secondary dressing for clinical use. It did not shed dressing components during any of the assays.
Despite the fact that the silver sulphate dressing released high quantities of silver compared with the other dressings (about 30% of the amount provided by traditional silver‐containing burn treatments), it was not able to generate any log reduction in 30 minutes, or any CZOI, with bacteria growing under the wound‐contacting surface of the dressing, suggesting it had no bacteriostatic activity. Both the top surface and the wound‐contacting surface of the dressing were hydrophobic – they both repelled water droplets – preventing fluid from entering, or silver from being released from, the dressing through these surfaces. Silver could, however, be released by cut dressing edges (Figure 2). Thus, during the log reduction assay, when bacteria were placed on the wound‐contacting surface, they did not contact silver within the dressing. Similarly, when the wound‐contacting surface was placed down onto the agar seeded with S. aureus, no liquid transfer occurred between the plate and the dressing, no silver was released onto the plate and therefore no CZOI was produced. The silver released into solution during the silver dissolution assay was released through the edges of the dressing – the only portion of the dressing which allowed for fluid exchange. This was confirmed by cutting a silver sulphate dressing into strips, moistening the strips to their saturation volume and placing them onto plates seeded with S. aureus such that the dressings contacted the plate cut edge down. The plates were then incubated overnight at 37°C, and in this orientation, the dressing was able to produce CZOIs. Unfortunately, this orientation is not clinically relevant, as the edges/interiors of the dressing are unlikely to contact the wound. In its current configuration, minimal silver would be released into the wound and likely no bacteria would be taken into the dressing because of no fluid exchange. The saturation volume measured is not clinically relevant either, because the dressing was submerged and fluid could enter through the edges. The absorption capacity in a wound environment for this dressing configuration appears to be essentially zero. Overall, although the dressing contains a large quantity of soluble silver, in its current configuration, it has neither wound fluid absorption capacity nor antimicrobial activity. Product information for the dressing confirms that its wound‐contacting surface is hydrophobic, despite its claims of absorbency, as it indicates that ‘The [wound‐contacting] layer seals the wound edges, preventing the exudate to [sic] leak onto the surrounding skin, thus minimizing the risk of maceration’.(4)
Figure 2.
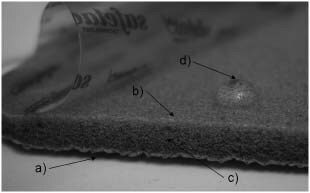
Dressing configuration for the silver sulfate dressing. The top surface of the dressing (a) and the wound‐contacting adhesive surface of the dressing (b) both appear to be hydrophobic, but fluid exchange can occur via the dressing edges (c), allowing silver release when the dressing is submerged. A water droplet placed on the wound‐contacting surface (d) shows its hydrophobicity.
There are no studies concerning the silver sulphate dressing in the peer‐reviewed literature, to the knowledge of the authors. A handful of case studies 25, 26, 27, 28, 29 and one open non comparative multicentre investigation (30) have been presented as posters, which did not show direct measures of antimicrobial activity. One poster on an open non comparative single‐centre study did include microbiological culture swabs and concluded that the dressing showed antimicrobial efficacy. However, their tabulated data showed that 6 of 11 patients initially colonised with S. aureus, and 9 of 11 patients initially colonised with Pseudomonas aeruginosa, had little or no reduction of these species after 30 days treatment (31). Another poster on an in vitro study indicated that the dressing was bactericidal against various wound pathogens within 30 minutes to 3 hours and could be challenged daily for 7 days and retain its activity (32). However, for all tests they put 1 g of dressing in 50 ml of media plus bacteria. One gram of this dressing has an area of 5.9 cm2, and thus, in their assay, the dressing was instantly exposed to approximately 8.5 g/cm2 of fluid. Because wound exudate is classified as mild (0.25 g/cm2/24 hours), moderate (0.5 g/cm2/24 hours) or heavy (1.0 g/cm2/ 24 hours) (33), the method used above would instantly expose the dressings to 8.5 times the 24‐hour fluid volume of highly exudative wounds, suggesting that the results of their study may not be indicative of the clinical efficacy of the dressings (34). Furthermore, because this study was performed with the dressings submerged, this would allow for contact between bacteria and silver through the absorbent edges of the dressing, explaining the discrepancy between that study (32) and the current study.
The silver collagen matrix dressing was not bactericidal in half an hour. It was bacteriostatic for 9 days. The silver released was significantly lower than that of the nanocrystalline silver dressing, releasing 0.02% of the Ag+ provided by traditional silver‐containing burn treatments. This suggests at least a partial explanation for its lack of bactericidal efficacy. To the knowledge of the authors, there are no studies concerning the silver collagen matrix dressing in the peer‐reviewed literature. There are a handful of case studies which have been presented as posters 35, 36, 37, 38, 39, which do not focus on the antimicrobial activity of the dressing. In the CZOI assay, the dressing edges left behind material from the fifth transfer on, and also left material in solution during the total silver assay, suggesting that material could be left behind in a wound bed, which could result in generation of a foreign body response, depending on dressing bioabsorbability. The absorptive capacity of the silver collagen matrix dressing was mid‐range. Overall, although the silver collagen matrix dressing did show bacteriostatic longevity, it did not appear to be the best product examined in this study in terms of its antimicrobial activity, its absorptive capacity or its use. However, it is only indicated for prevention of bioburden in the dressings, unlike the other materials tested in this study, which should be taken into consideration.
The only other dressing showing any antimicrobial activity in vitro was the silver alginate dressing. Although the dressing was unable to generate any log reduction in 30 minutes against S. aureus, it was able to generate CZOIs for 6 days. The zone size was much higher on the first day than on subsequent days, suggesting an initial dump of silver, followed by a lower sustained release. Thus, the dressing was not bactericidal in this study, but it was bacteriostatic. There are no studies concerning the silver alginate dressing to the knowledge of the authors – their product claims, which do not match the results of this study, are based on data the company has on file. The results of this study may have serious consequences, as studies have indicated that when an antimicrobial agent is provided in concentrations such that bacterial inhibition occurs but the bacteria are not actually killed, selection for resistant organisms occurs 40, 41. The antimicrobial activity of this dressing appears to be related to its silver release, which was significantly lower than that of the only bactericidal dressing, but was higher than some dressings which were unable to generate bacteriostatic activity. The silver alginate dressing released approximately 0.2% of the Ag+ released by traditional silver‐containing burn treatments. Its absorptive capacity was low and highly variable. As well, portions of the dressing flaked off during the total silver assay, and fibres were left behind after each transfer in the CZOI assay. Furthermore, the dressings began to fall apart at the third transfer, and after the fifth transfer, they liquefied completely. This suggests that – contrary to claims in the product information indicating that the dressing does not leave any silver coated nylon thread residue in the wound and remains intact, facilitating ease of removal (5)– fibres could fall off in the wound, potentially causing a foreign body response and/or delayed wound healing. Furthermore, the dressings may become more difficult to remove with time, as they may start to break apart or liquefy. It is anticipated that the dressing breakdown observed could occur more quickly in the relatively harsh and dynamic conditions of the wound environment. It is uncertain what the wound environment would then be exposed to in terms of liquefaction products.
The product information for the shaped ionic silver foam dressing and the non adhesive ionic silver foam dressing indicates that the dressings have the same silver technology (6), and therefore they should have equal performance. This was confirmed in this study, where both dressings performed poorly, with log reductions of zero. As well, the dressings released about the same level of silver into the solution during a 24‐hour period, at approximately 0.005% of the Ag+ released by traditional silver‐containing burn treatments. The shaped foam dressing had a significantly lower absorptive capacity than the non adhesive silver foam, possibly because of the adhesive added to the shaped dressings. The non adhesive ionic silver foam dressing was not able to generate CZOIs (bacteria grew under the wound‐contacting surface), and therefore, the shaped ionic silver foam dressing is not expected to generate CZOIs either. Thus, neither of the ionic silver foam dressings showed bactericidal or bacteriostatic activity against S. aureus, corroborating the results of previous studies: There are a variety of case studies concerning the ionic silver foam dressings which have been presented as posters 42, 43, 44, 45, 46, 47, 48, 49, 50, which do not show direct antimicrobial measures, but there are also two in vitro studies in the peer reviewed literature 51, 52 that tested an ionic silver foam dressings, along with a variety of other silver containing dressings, for antimicrobial activity using methods similar to those of Taherinejad et al. (32), described above. Both studies concluded that the ionic silver foam dressing was ineffective against P. aeruginosa and only marginally effective against S. aureus 51, 52. One of the above studies also tested the nanocrystalline silver dressing and concluded that it had a broad spectrum of bactericidal activity (52). The clinical relevance of the antimicrobial testing performed in these studies remains uncertain because of the methods used (34), however, the results of both those studies and this study indicate that the lack of activity corresponded to low silver release, as the silver released in this study was barely detectable using AAS. As well, although these dressings have a fairly high absorptive capacity, the dressings appeared to shed pieces, both into solution during the silver dissolution assay and onto plates during the CZOI assay, suggesting that in a clinical situation, dressings pieces could be left in the wound. Thus, the ionic silver foam dressings showed the worst performance overall, with the shaped dressing being inferior to the non adhesive dressing.
This study did not examine the benefits or disadvantages related to other physical or chemical components of the dressings (e.g. the glycerol and cleansing agents present in the ionic foam dressings, or the EDTA and carboxymethylcellulose present in the silver collagen matrix dressing), but these components should also be taken into consideration when deciding on the appropriateness of a dressing for a clinical situation.
Although caution must be exercised when extrapolating the results of in vitro studies to the clinical environment (15), important differences were detected in the antimicrobial efficacy of the dressings tested, with the nanocrystalline silver dressing having stronger antimicrobial activity than any of the other dressings. The silver alginate and silver collagen matrix dressings have some bacteriostatic activity, but this may not be sufficient support for immunocompromised patients indicated for treatment with these dressings. Furthermore, exposure of bacteria to silver concentrations which are bacteriostatic but not bactericidal creates a high‐risk situation in terms of development of silver‐resistant bacteria 40, 41. The silver sulphate dressing does not appear to have clinically relevant antimicrobial activity in its current configuration because of the hydrophobicity of the wound‐contacting surface, while the ionic silver foam dressings do not appear to contain sufficient silver for antimicrobial activity. The latter three dressings, which were the only dressings in the study described as non staining, were the dressings that showed neither bactericidal nor bacteriostatic activity, suggesting that non staining Ag+‐containing products may not release enough silver to be effective.
The results of this study emphasise the importance of confirming claims made in product information through simple in vitro efficacy tests. If a dressing does not show efficacy in the relatively benign environment of a Petri plate, it is unlikely to do so in the wound environment. Finally, this study corroborates the conclusions of others that silver release, log reduction and day‐to‐day transfer CZOI assays should be used in conjunction to analyse silver‐containing dressings 9, 17. Sufficient information about the antimicrobial activity of the dressings examined in this study, and more specifically why certain dressings were active while others were not, could not have been gained from running only one of the above assays.
ACKNOWLEDGEMENTS
Shiraz Merali, from the Chemical & Materials Engineering department at the University of Alberta, provided assistance with the AAS. The following sources of funding were provided: Natural Sciences and Engineering Research Council of Canada (NSERC) Undergraduate Student Research Award (MHC); NSERC Canada Research Chair (REB); NSERC CGS‐D2, Alberta Ingenuity Graduate Scholarship in Nanotechnology and Killam Memorial Scholarship (PLN). None of the authors have conflicts of interest.
REFERENCES
- 1. Bowler P. The anaerobic and aerobic microbiology of wounds: a review. Wounds 1998;10:170–8. [Google Scholar]
- 2. Robson MC. Wound infection: a failure of wound healing caused by an imbalance of bacteria. Surg Clin N Am 1997;77:637–50. [DOI] [PubMed] [Google Scholar]
- 3. Heggers JP, Haydon S, Ko F. Pseudomonas aeruginosa exotoxin A: its role in retardation of wound healing. J Burn Care Rehab 1992;13:512–8. [PubMed] [Google Scholar]
- 4. Mepilex Ag (homepage on the Internet) . Sweden: Molnlycke Health Care LLC; © 2009. Mepilex Ag: the effective antimicrobial absorbent foam dressing; 2 pages. URL http://www.molnlycke.com/Files/Wound_Care/Productsheets/MepilexAg_PS.pdf [accessed on 3 February 2009].
- 5. Product Literature (homepage on the Internet) . Ontario, Canada: Derma Sciences Inc.; © 2006. ALGICELL™Ag Silver Alginate Brochure; 4 pages. URL http://dermasciencesinc.com/editor/uploads/files/DermaSciences_ALGICELL_Ag.pdf [accessed on 3 February 2009].
- 6. Brochures and Literature (homepage on the Internet) . IL, USA: Ferris Mfg. Corp.; © 2008. PolyMem®Silver: the world's only QuadraFoam®silver dressings; 4 pages. URL http://www.polymem.com/Silver.pdf?line_id=5 [accessed on 3 February 2009].
- 7. Biostep™ Ag Collagen Matrix Dressing Downloadable Materials (homepage on the Internet). Largo, Florida, USA: Smith and Nephew, Inc.; © 2008. Biostep Ag Product Insert; 4 pages. URL http://global.smith‐nephew.com/cps/rde/xbcr/smithnephewls/Biostep_Ag_Patient_Insert_PI_02273‐A.pdf [accessed on 3 February 2009].
- 8. Acticoat™ product range (homepage on the Internet) . Largo, FL, USA: Smith and Nephew, Inc.; © 2008. Acticoat™product information; 2 pages. URL http://wound.smith‐nephew.com/ca_en/Product.asp?NodeId=2722&Tab=1&Hide=True [accessed on 3 February 2009].
- 9. Gallant‐Behm CL, Yin HQ, Liu SJ, Heggers JP, Langford RE, Olson ME, Hart DA, Burrell RE. Comparison of in vitro disc diffusion and time kill‐kinetic assays for the evaluation of antimicrobial wound dressing efficacy. Wound Rep Reg 2005;13:412–21. [DOI] [PubMed] [Google Scholar]
- 10. Wright JB, Lam K, Burrell RE. Wound management in an era of increasing bacterial antibiotic resistance: a role for topical silver treatment. Am J Infect Control 1998;26:572–7. [DOI] [PubMed] [Google Scholar]
- 11. Wright JB, Hansen DL, Burrell RE. The comparative efficacy of two antimicrobial barrier dressings: in vitro examination of two controlled release silver dressings. Wounds 1998;10:179–88. [Google Scholar]
- 12. Moyer CA, Brentano L, Gravens DL, Margraf HW, Monafo WW. Treatment of large human burns with 0.5% silver nitrate solution. Arch Surg 1965;90:812–67. [DOI] [PubMed] [Google Scholar]
- 13. Monafo WW, West MA. Current treatment recommendations for topical burn therapy. Drugs 1990;40:364–73. [DOI] [PubMed] [Google Scholar]
- 14. Thomas S, McCubbin P. A comparison of the antimicrobial effects of four silver‐containing dressings on three organisms. J Wound Care 2003;12:101–8. [DOI] [PubMed] [Google Scholar]
- 15. Thomas S, McCubbin P. An in vitro analysis of the antimicrobial properties of 10 silver‐containing dressings. J Wound Care 2003;12:305–8. [DOI] [PubMed] [Google Scholar]
- 16. Wright JB, Lam K, Hansen D, Burrell RE. Efficacy of topical silver against fungal burn wound pathogens. Am J Infect Control 1999;27:344–50. [DOI] [PubMed] [Google Scholar]
- 17. Yin HQ, Langford R, Burrell RE. Comparative evaluation of the antimicrobial activity of ACTICOAT antimicrobial barrier dressing. J Burn Care Rehabil 1999;20:195–200. [DOI] [PubMed] [Google Scholar]
- 18. Wright JB, Lam K, Olson ME, Burrell RE. Is antimicrobial efficacy sufficient? A question concerning the benefits of new dressings. Wounds 2003;15:133–42. [Google Scholar]
- 19. Nadworny PL, Wang J, Tredget EE, Burrell RE. Anti‐inflammatory activity of nanocrystalline silver in a porcine contact dermatitis model. Nanomedicine 2008;4:241–51. [DOI] [PubMed] [Google Scholar]
- 20. Burrell RE, Heggers JP, Davis GJ, Wright JB. Efficacy of silver‐coated dressings as bacterial barriers in a rodent burn sepsis model. Wounds 1999;11:64–71. [Google Scholar]
- 21. Tredget EE, Shankowsky HA, Groeneveld A, Burrell RE. A matched‐pair, randomized study evaluating the efficacy and safety of Acticoat silver‐coated dressing for the treatment of burn wounds. J Burn Care Rehabil 1998;19:531–7. [DOI] [PubMed] [Google Scholar]
- 22. Doshi J, Karagama Y, Buckley D, Johnson I. Observational study of bone‐anchored hearing aid infection rates using different post‐operative dressings. J Laryngol Otol 2006;120:842–4. [DOI] [PubMed] [Google Scholar]
- 23. Rustogi R, Mill J, Fraser JF, Kimble RM. The use of Acticoat in neonatal burns. Burns 2005;31:878–82. [DOI] [PubMed] [Google Scholar]
- 24. Fan FF, Bard AJ. Chemical, electrochemical, gravimetric, and microscopic studies on antimicrobial silver films. J Phys Chem B 2002;106:279–87. [Google Scholar]
- 25. Blakely M, Weir D. The innovative use of Safetac soft silicone in conjunction with negative pressure wound therapy: three case studies. In: 20th Annual Symposium on Advanced Wound Care and the Wound Healing Society Meeting, 2007, Tampa, USA. URL http://www.molnlycke.net//Files/Wound_Care/Mepilex%20Ag%20PDFs/F6620%20Barrows%202008.pdf [accessed on 2 February 2009].
- 26. Meites H, Jett M, Gauthier S. The examination of antimicrobial soft silicone foam with regards to partial thickness burns. In: Third Congress of the World Union of Wound Healing Societies, 2008, Toronto, Canada. URL http://www.molnlycke.net//Files/Wound_Care/Mepilex%20Ag%20PDFs/F6620%20Meites%20et%20al%202008.pdf [accessed on 2 February 2009].
- 27. Nisbet TAS. Promoting complex wound healing through the utilization of an antimicrobial soft silicone foam dressing: solving the wound puzzle one piece at a time. In: 21st Annual Symposium on Advanced Wound Care and the Wound Healing Society Meeting, 2008, San Diego, USA. URL http://www.molnlycke.net//Files/Wound_Care/Mepilex%20Ag%20PDFs/F6620%20Nisbet%202008.pdf [accessed on 2 February 2009].
- 28. Barrows C. Enhancing patient outcomes – reducing the bottom dollar: the use of antimicrobial soft silicone foam dressings in home health. In: 21st Annual Symposium on Advanced Wound Care and the Wound Healing Society Meeting, 2008, San Diego, USA. URL http://www.molnlycke.net//Files/Wound_Care/Mepilex%20Ag%20PDFs/F6620%20Barrows%202008.pdf [accessed on 2 February 2009]. [DOI] [PubMed]
- 29. Timmins J. Management of a large haematoma with a new silver impregnated foam dressing. In: European Wound Management Association Conference, 2008, Lisbon, Portugal. URL http://www.molnlycke.net//Files/Wound_Care/Mepilex%20Ag%20PDFs/F6620%20Timmins%202008.pdf [accessed on 2 February 2009].
- 30. Schumann H, Apelqvist J, Schmidtchen A, Hansson C. Open, non‐comparative, multicentre investigation exploring the tolerance of an absorbent foam dressing containing silver used in chronic wounds. In: European Wound Management Association Conference, 2007, Glasgow, UK. URL http://www.molnlycke.net//Files/Wound_Care/Mepilex%20Ag%20PDFs/F6620%20Schumann%20et%20al%202008.pdf [accessed on 2 February 2009].
- 31. Durante CM. Chronic wounds and local malpractice: an antimicrobial silver soft silicone foam can help solving the problem. In: Third Congress of the World Union of Wound Healing Societies, 2008, Toronto, Ontario, Canada. URL http://www.molnlycke.net//Files/Wound_Care/Mepilex%20Ag%20PDFs/F6620%20Durante%202008.pdf [accessed on 2 February 2009].
- 32. Taherinejad F, Hamberg K. Antimicrobial effect of a silver‐containing foam dressing on a broad range of common wound pathogens. In: Third Congress of the World Union of Wound Healing Societies, 2008, Toronto, Ontario, Canada.
- 33. Varma AK, Bal A, Kumar H, Kesav R, Nair S. Efficacy of polyurethane foam dressing in debrided diabetic lower limb wounds. Wounds 2006;18:300–6. [Google Scholar]
- 34. Nadworny PL, Burrell RE. A review of assessment techniques for silver technology in wound care. Part 1: In vitro methods for assessing antimicrobial activity. J wound Technol 2008;1:6–13. [Google Scholar]
- 35. Allam RC, Partain R, Munson BA. A prospective, clinical in‐market evaluation to assess the performance of a new collagen matrix dressing on facilitating granulation and epidermal migration, in a variety of wound types. 2007. URL http://global.smith‐nephew.com/cps/rde/xbcr/smithnephewls/R._Allam_New_Collagen_Dressing_1.2_LOW.pdf [accessed on 3 February 2009].
- 36. Williams RL. Experience with novel porcine collagen matrix and silver used in long‐term dressings. 2007. URL http://global.smith‐nephew.com/cps/rde/xbcr/smithnephewls/R_Lewis‐Long_Term_Dressings.pdf [accessed on 3 February 2009].
- 37. Williams RL. Novel porcine collagen matrix used to stimulate wound closure in arrested wounds. 2007. URL http://global.smith‐nephew.com/cps/rde/xbcr/smithnephewls/R_Williams_Arrested_Wounds‐8x4.pdf [accessed on 3 February 2009].
- 38. Williams RL. Clinical experience with a novel porcine collagen matrix to facilitate final closure of granulating wounds originally treated with negative pressure wound therapy. 2007. URL http://global.smith‐nephew.com/cps/rde/xbcr/smithnephewls/R_Williams_Final_Closure‐8x4‐9_27.pdf.
- 39. Davis C, Dalbey C, Hill S. The successful use of a new adjunct therapy in a [sic] both acute and chronic wounds – a prospective, descriptive case series. 2007. URL http://global.smith‐nephew.com/cps/rde/xbcr/smithnephewls/S_Hill‐Succesful_use_of_adjunct‐8x4.pdf [accessed on 3 February 2009].
- 40. Zhao X, Drlica K. Restricting the selection of antibiotic‐resistant mutant bacteria: measurement and potential use of the mutant selection window. J Infect Dis 2002;185:561–5. [DOI] [PubMed] [Google Scholar]
- 41. Li X‐Z, Nikaido H, Williams KE. Silver‐resistant mutants of Escherichia coli display active efflux of Ag+ and are deficient in porins. J Bacteriol 1997;179:6127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Agathangelou C. Huge sacral pressure ulcer closed in four months using PolyMem Silver and PolyMem Wic Silver dressings. In: Third Congress of the World Union of Wound Healing Societies, 2008, Toronto, Canada. URL http://www.polymem.com/CaseStudies/MKL347_Huge_Sacral_PU_Healed_4_Months_Silver_REV0_0109.pdf??line_id=25 [accessed on 3 February 2009].
- 43. Hubbard M. Dramatic healing of three stalled venous hypertension ulcers in a bariatric patient using PolyMem Silver dressings under an Unna's boot. In: The WOCN Society's 39th Annual Conference, 2007, Salt Lake City, USA. URL http://www.polymem.com/CaseStudies/MKL296_3_Stalled_Venous_Wounds_in_Bariatric_Patient_Silver_Rev0_0108.pdf??line_id=24 [accessed on 3 February 2009].
- 44. Hubbard M. Pain relief and healing using PolyMem and PolyMem Silver dressings under compression for venous hypertension ulcers. In: 20th Annual Symposium on Advanced Wound Care, 2007, Tampa, USA. URL http://www.polymem.com/CaseStudies/MKL294_Venous_Hypertension_Pain_Relief_and_Healing_Compression_Hubbard_REV0_0308.pdf??line_id=22 [accessed on 3 February 2009].
- 45. Stamps J. Polymem Silver dressings successful in bringing quick closure to a chronic venous ulcer with an exposed tendon in a patient with arterial insufficiency. In: 20th Annual Symposium on Advanced Wound Care, 2007, Tampa, USA. URL http://www.polymem.com/CaseStudies/MKL285_Venous_Ulcer_with_Exposed_Tendon_Silver_REV3_1108.pdf??line_id=18 [accessed on 3 February 2009].
- 46. Thurs K. Chronic venous leg ulcer closed in only seven dressing changes using PolyMem Silver dressings. In: The WOCN Society's 39th Annual Conference, 2007, Salt Lake City, USA. URL http://www.polymem.com/CaseStudies/MKL283_Chronic_Leg_Ulcer_Silver_Thurs_REV2_1008.pdf??line_id=16 [accessed on 3 February 2009].
- 47. Caras J. Three malleolus wounds of two years' duration closed in four weeks using PolyMem Silver. In: 19th Annual Symposium on Advances in Skin and Wound Care, 2006, San Antonio, USA. URL: http://www.polymem.com/CaseStudies/MKL282_3_Malleolus_Wounds_Silver_REV2_0808.pdf??line_id=15 [accessed on 3 February 2009].
- 48. Benskin L. Maintaining tunnel tissue viability with PolyMem Silver QuadraFoam dressings during osteomyelitis treatment. In: 19th Annual Symposium on Advances in Skin and Wound Care, 2006, San Antonio, USA. URL http://www.polymem.com/CaseStudies/MKL215_Tunnel_Tissue_REV3_0808.pdf??line_id=10 [accessed on 3 February 2009].
- 49. Benskin L. Dramatic pain relief through the use of PolyMem QuadraFoam dressings on a deep axillary wound for entire course of healing. In: Third Congress of the World Union of Wound Healing Societies, 2008, Toronto, Canada. URL http://www.polymem.com/CaseStudies/MKL213_Axillary_Silver_REV3_0808.pdf??line_id=8 [accessed on 3 February 2009].
- 50. Benskin L. Activation of a stalled traumatic finger wound with PolyMem Silver QuadraFoam dressings. In: 19th Annual Symposium on Advances in Skin and Wound Care, 2006, San Antonio, USA. URL http://www.polymem.com/CaseStudies/MKL2090807_Stalled_Finger.pdf??line_id=7 [accessed on 3 February 2009].
- 51. Parsons D, Bowler PG, Myles V, Jones S. Silver antimicrobial dressings in wound management: a comparison of antibacterial, physical, and chemical characteristics. Wounds 2005;17:222–31. [Google Scholar]
- 52. Ip M, Lui SL, Poon VKM, Lung I, Burd A. Antimicrobial activities of silver dressings: an in vitro comparison. J Medical Microbiol 2006;55:59–63. [DOI] [PubMed] [Google Scholar]


