Abstract
Oxidised regenerated cellulose/collagen matrix (ORC/collagen matrix) modifies wound microenvironments by binding and inactivating excess levels of proteases such as elastase, plasmin and gelatinases in wound exudates. To compare levels of the gelatinases matrix metalloproteinase 2 (MMP‐2), elastase and plasmin in wound exudates collected from chronic venous insufficiency patients with venous leg ulcers treated with either an ORC/collagen matrix or a standard control therapy. During a 12‐week treatment period, wound exudate samples were obtained from a control group of 10 patients treated with a hydrocolloid dressing and a treatment group of 17 patients treated with a combination of ORC/collagen matrix and hydrocolloid dressing. On admission and days 5, 14 and every subsequent 14th day, ulcers were photographed to determine healing rate and changes in ulcer appearance, and MMP‐2 concentration and the gelatinase, elastase and plasmin activities were analysed from wound exudates. The patients treated with ORC/collagen matrix showed a significant decrease in elastase, plasmin and gelastinase activity as compared with the control group, with no significant difference in the MMP‐2 concentrations between the two groups. The results show a significant and immediate reduction in protease activity in wound exudates from venous leg ulcers treated with ORC/collagen.
Keywords: Chronic venous ulcers, Elastase, Gelatinase, MMP‐2, Plasmin, Wound healing
Introduction
Chronic venous insufficiency (CVI) is a functional disorder of the venous system of the lower limb. The basis of the pathology is venous hypertension caused by valvular insufficiency and reflux, which can lead to venous outflow obstruction (1). Epifascial, subfascial and transfascial forms of CVI can occur independently (2); however, in practice, CVI usually results from a combination of these forms (3). It is the consistent venous hypertension, which is the initiating factor in causing alterations in the microcirculation and which ultimately leads to skin changes and venous ulceration (4). The precise mechanism for the development of venous leg ulcers is still uncertain. A recent hypothesis suggests that leukocytes become trapped in the capillaries and attach to the endothelium, where they become activated and release proteolytic enzymes and free radicals (5). These in turn have destructive effects on lipid membranes and proteins as well as on many connective tissue compounds 4, 5. This theory is supported by the increased expression of matrix metalloproteinases (MMPs) observed in the periulcer skin 4, 5.
In general, two consistent findings concerning the pathogenesis of chronic wounds have been reported: chronic inflammation and an imbalance in protease/protease‐inhibitor levels (5). Normal wound healing progresses by controlling the balance between the repair processes that lead to new tissue formation and the destructive processes that are necessary to remove damaged tissue. Within this complex environment, there are many regulating factors that precisely control the biological processes necessary to achieve normal wound repair (6). An alteration in any of these physiological processes can lead to a non healing state and the formation of a chronic wound (6). Chronic wounds fail to follow the normal pattern of wound repair, which involves inflammation, granulation and reepithelisation. Instead, they remain in a persistent inflammatory state characterised by ongoing proteolysis and degradation, as indicated by increased levels of neutrophil elastase and gelatinases (7). The resulting degradation of matrix molecules, such as fibronectin, laminin and various collagens (7), hinders cell adhesion, preventing the formation of new tissue and reepithelisation in chronic wounds.
Elastase is clearly one of the most destructive enzymes in the human body and has been well characterised in non healing wounds (8). Elastase has a broad specificity, preferentially cleaving bonds that are carboxy‐terminal to valine and to a lesser extent alanine (9). Plasmin is another serine protease, whose primary substrate is fibrinogen/fibrin and as such plays an essential role in haemolysis. It is converted from plasminogen through enzymatic cleavage by plasminogen activator, which also belongs to the family of serine proteases (5). Plasmin participates in a variety of pericellular proteolytic events, such as cell migration and angiogenesis (10). It activates MMPs and growth factors such as transforming growth factor‐β11, 12 and degrades vascular endothelial growth factor, which is important for angiogenesis (13). An excessive concentration of both elastase and plasmin in chronic non healing wounds is thought to degrade growth factors, extracellular matrix proteins such as fibronectin, and endogenous protease inhibitors, all of which are important for wound healing 8, 13, 14.
MMPs belong to the family of zinc‐dependent neutral endopeptidases that are collectively capable of degrading all matrix components. To date, 24 different vertebrate MMPs have been identified of which 23 have been found in humans’(15). Important members of the human MMP family include the fibroblast‐type (16) and the neutrophil‐type 17, 18 interstitial collagenases, three distinct stromelysins 19, 20, 21, 22, the 72‐ and 92‐kDa gelatinases 23, 24 and PUMP‐1 (MMP‐7) (25). Because of their ability to degrade the type IV collagen, which is found in basement membranes, widespread interest has focussed on the gelatinases (A and B), originally named for their potent hydrolytic action on gelatin. Because basement membranes are a barrier for migrating cells, several investigators suggested that gelatinases could facilitate tumour invasion, metastasis and angiogenesis 7, 26. Gelatinase A (72 kDa) is secreted by proliferating skin fibroblasts, H‐ras‐transformed bronchial epithelial cells and SV40‐transformed lung fibroblasts (25). Gelatinase B (92 kDa) resembles the 72‐kDa enzyme in primary structure and substrate specificity, but it has an additional 54‐residue proline‐rich domain and is secreted by keratinocytes, inflammatory cells and SV40‐transformed lung fibroblasts (24).
The MMPs and their natural inhibitors – the tissue inhibitors of metalloproteinases (TIMPs) – play an important role in diseases, which are characterised by an unbalanced degradation of the extracellular matrix. MMP‐1, MMP‐8 (interstitial collagenases), MMP‐2 (72‐kd type IV collagenase, gelatinase A) and MMP‐9 (92‐kd type IV collagenase, gelatinase B) have been implicated in various aspects of tissue maintenance and wound repair 25, 26. These MMPs are thought to be indispensable for normal wound repair as they are involved in angiogenesis, embryogenesis, organ and nerve development (26). Thus, many studies have examined the levels of individual MMPs in both acute and chronic wounds; MMP‐2 is threefold increased in wounds of patients with a diabetic foot syndrome (DFS) (27) and tenfold increased in pressure ulcers (25). Furthermore, gelatinase activity is higher in CVI ulcers than in acute wounds (28).
Oxidised regenerated cellulose (ORC)/collagen matrix is a device composed of oxidised regenerated cellulose and collagen and has been shown to reduce elastase, plasmin and metalloproteinase activity in chronic wound exudates of diabetic patients in vitro 29, 30. Clinically, ORC/collagen matrix has been reported to accelerate the healing rate in venous leg ulcers compared with controls, in a randomised controlled trial (31).
In our study, we have assessed the MMP‐2 concentration and the gelastinase, elastase and plasmin activities in wound exudate obtained from chronic venous ulcers during treatment with ORC/collagen matrix in comparison with controls. Moreover, we have analysed the healing rate between ORC/collagen matrix‐treated patients versus control group.
Methods
Patients
The study protocol was approved by the local ethical board before initiation of the study, and all patients gave their informed written consent to participate. Wound exudates were collected from 27 patients randomly divided into two groups; 17 patients (average age 68 ± 9 years) were treated with ORC/collagen matrix and a hydrocolloid secondary dressings, the remaining 10 control patients (average age 66 ± 10 years) were treated solely with the hydrocolloid dressing. Wound exudates were collected from all patients upon admission (day 0) and days 5, 14 and then at 14‐day intervals and were collected in accordance with the local ethical committee guidelines. The majority of patients enrolled were elderly (average age 63 ± 8 years) and female with chronic wounds present for at least 30 days prior to study enrolment, but not longer than 3 months. All patients included in this trial had no systemic inflammatory diseases or malignant tumours.
Chronic wound exudate collection and elution
The wound exudate was collected by absorption onto a piece of RELEASE® (Johnson & Johnson Medical Ltd, North Yorkshire, UK) dressing, which was placed directly on the venous ulcer. The RELEASE dressing was cut to the size of the wound, then placed in contact with the ulcer bed, covered with BIOCLUSIVE® (Johnson & Johnson Medical Ltd) and left for 6 hours. The dressing was then removed and frozen at −80°C until study completion. Samples from a total of 27 patients were collected in this study.
Protein
Using the Bradford assay, total protein levels were determined for all samples, as calculated from a standard curve obtained with bovine serum albumin (32).
Protease activity assay
MMP‐2 assay
MMP‐2 concentration was determined using a ‘sandwich’ ELISA (Calbiochem™, QIA 63). The assay was performed according to the manufacturer’s guidelines. In brief, samples were incubated for 2 hours with a biotinylated detector monoclonal antibody during which time any MMP‐2/biotinylated antibody complex present binds to the capture antibody. Unbound material was washed away and horseradish peroxidase‐conjugated streptavidin was added, which binds to the biotin label on the detector antibody. The horseradish peroxidase catalysed the conversion of the chromogenic substrate tetra‐methylbenzidine from a colourless to a blue solution (or yellow after the addition of stopping reagent), and the intensity is proportional to the amount of MMP‐2 present in the sample. The coloured intensity was quantified by using a spectrophotometer.
Gelatinase activity
The Gelatinase Activity Assay Kit from Chemicon™ (ECM 700) was used to determine gelatinase activity. The manufacturer’s protocol was followed; in brief, we rehydrated the biotin‐binding plate by adding 200 μl of diluted assay buffer to each well and let it incubate at room temperature for 1 minute. We then added 100 μl of the MMP/substrate mixture to the biotin‐binding plate and incubated it at 37°C for 30 minutes. After washing the sample (five times), streptavidin‐enzyme conjugate (1:3000 dilution) was pipetted to each well. The wells were washed again (five times) and incubated with substrate solution. After an additional incubation step (20 minutes at room temperature), a final stop solution was added and the optical density was then measured by using a photometer.
Elastase assay
Elastase activity in the wound exudate samples was measured spectroflourimetrically using a peptide substrate activity assay (Enzchek, E 12056, Molecular Probes™, Leiden, the Netherlands). The substrate comprises a short peptide synthesised to mimic the appropriate enzyme cleavage site and it contains a fluorescent group, which is released upon hydrolysis. The resulting increase in fluorescence was monitored with a fluorescence microplate reader. The activity was expressed either as units per minute or as corrected for total protein. Each sample was tested three times and the average value calculated. To confirm the identity of the protease responsible for substrate digestion, a selective inhibitor of elastase, N‐methoxysuccinyl‐Ala‐Ala‐Pro‐Val‐chloromethylletone, was used.
Plasmin activity
Plasmin activity was also measured using a peptide substrate hydrolysis method. Wound exudates samples were incubated in 100 mM Tris buffer, pH 8·0, 0·13 M dithiobis, containing 20 mM Z‐Lys‐S‐Benzyl (prepared in distilled water) (SB 002, Enzyme Systems Products™, Livermore, CA, USA). The rate of hydrolysis of Z‐Lys‐SBzl was monitored spectrophotometrically by allowing the released benzyl mercaptan to react directly with dithiobis. The increase in absorbance at 412 nm, because of formation of the carboxy‐nitrophenoxide, was measured and the rate of change in absorbance determined. Each sample was tested three times and the average value calculated.
Ulcer assessment
All ulcers were photographed on admission and at each wound exudates collection time point to provide a visual record of any changes in appearance of the ulcer and to determine healing rate. The surface area of all ulcers was measured by planimetry (Pharma Med Concept™, Düren, Germany).
Statistical analysis
Statistical analysis was performed using analysis of variance and Bonferroni’s post hoc test. The level of significance was considered to be P < 0·05.
Results
MMP‐2 concentration
There were no significant differences in MMP‐2 concentration between patients treated with ORC/collagen matrix and the control group (Figure 1).
Figure 1.
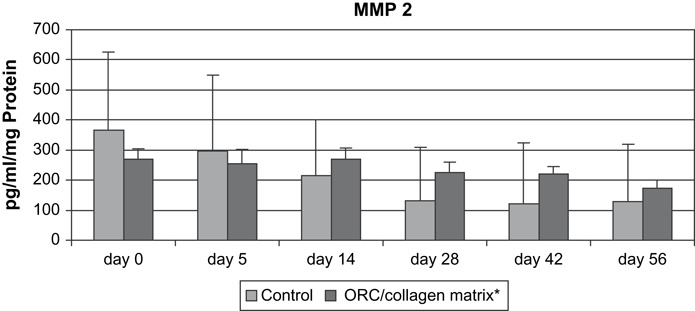
MMP‐2 (pg/ml/mg protein) in wound exudates of patients with chronic venous insufficiency.
Gelatinase activity
In patients treated with ORC/collagen matrix, gelatinase activities were significantly reduced on days 5, 14, 28 and 42 compared with day 0 (Figure 2).
Figure 2.

Gelatinase activity (%/ml/mg protein) in wound exudates of patients with chronic venous insufficiency. #P < 0·05 versus day 0.
Elastase activity
Elastase activity in wound exudates collected from patients treated with ORC/collagen matrix were decreased at all time points when compared with day 0 and with the corresponding activity measured in the control group (Figure 3).
Figure 3.
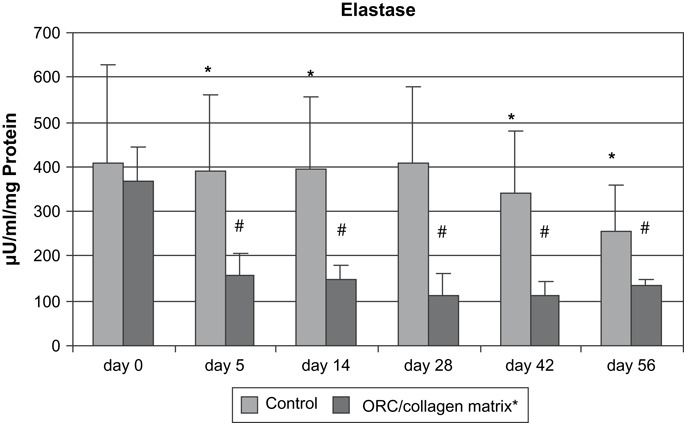
Elastase activity (μU/ml/mg protein) in wound exudates of patients with chronic venous insufficiency. *P < 0·05 versus oxidised regenerated cellulose/collagen matrix and #P < 0·05 versus day 0.
Plasmin activity
No significant differences in plasmin activity were found between patients treated with ORC/collagen matrix and the control group. In addition, no significant differences were found between the day 0 and the later time points (Figure 4).
Figure 4.

Plasmin activity (μU/ml/mg protein) in wound exudates of patients with chronic venous insufficiency.
Healing rate
In comparison with an equivalent number of control patients, the wound size of ORC/collagen matrix‐treated patients with chronic venous ulcer showed no significant differences during the 12 weeks’ of this research study.
Discussion
In this study, we compared the MMP‐2 concentration and activities of gelatinase, elastase and plasmin in wound exudate derived from chronic venous ulcers treated with either an ORC/collagen matrix or a control treatment (a hydrocolloid dressing). Our results were similar to those mentioned in the literature. The reduction in gelatinase, elastase and plasmin activity during treatment with ORC/collagen matrix confirms its ability to rebalance the wound environment in vivo.
High protease activity may arise as a result of one or any combination of the following factors: an increase in the expression of the protease, an increase in the extracellular activation of latent proteases or a reduction in the level of endogenous protease inhibitors. If cellular proteolysis is excessive or poorly regulated, it can lead to generalised tissue destruction, a characteristic of many pathological inflammatory conditions including non healing chronic wounds (33). This hypothesis is supported by several studies in which human chronic wound tissue and fluids have been analysed (11, 34). The combined use of collagen and oxidised regenerated cellulose matrix specifically inhibits the action of these proteases without affecting the activity of endogenous growth factors (29). Our study provides additional support to this mechanism of action, as we found a significant decrease in the activity of elastase and plasmin after 5 days of treatment with ORC/collagen matrix.
In various fibroproliferative diseases, we have shown a significantly lower MMP‐to‐TIMP ratio in serum and tissue compared with control patients 35, 36, 37. A determination of the concentration of the MMPs was therefore also a primary focus of this study. In addition to their importance in development of fibroproliferative diseases, MMPs play a critical role in the physiologic processes of wound healing and are multifunctional; required at different times and in different concentrations 11, 38. Epithelial regeneration requires, for example, the production of MMP‐1 by the epidermal cells to facilitate migration from the wound edges (39). Proteases are also important for the degradation of denatured matrix proteins after tissue trauma and later for the remodelling of the initial neodermis, which leads to scar formation (38). Different MMPs are important for the activation of latent growth factors and for the changing of procollagen molecules in tropocollagen molecules. In contrast to this, it seems that a permanent increase in the concentration of MMPs has a negative effect on wound healing.
In our study, patients treated with ORC/collagen matrix and the control group resulted in no significant changes in MMP‐2 concentration. In contrast, in patients treated with ORC/collagen matrix, gelatinase activity was significantly reduced on days 5, 14, 28 and 42 compared with day 0.
Lobmann et al. showed that gelatinase activity plays a crucial role in the wound healing by showing that MMP‐2 was significantly increased compared with average concentrations in biopsies of traumatic wounds 17, 40. In an animal wound model, Wall et al. showed that during the early stages of wound healing, the diabetes mouse possesses significantly reduced protein levels of MMP‐2 (41). Experimental studies of Tarlton et al. emphasised the involvement and influence of MMP‐9 in wound healing and hypothesised the role of MMP‐9 and neutrophil elastase as prognostic indicators for wound healing (42).
An accelerated healing in both venous leg and diabetic foot ulcers was shown in clinical trials with ORC/collagen matrix 31, 43. For example, some investigators have postulated that the resultant effect on clinical outcome was related to the ability of the ORC/collagen matrix to act as an alternate substrate for MMPs and cause a reduction in protease activity 27, 29, 30, 31, 32, 43. Other advantages in using ORC/collagen matrix include its easy application and the finding that a lower infection rate was observed in the ORC/collagen matrix‐treated group.
Different studies have confirmed the hypothesis that chronic wounds are conditioned by an increasing concentration and activity of MMPs. Wysocki et al. showed that wound exudate from chronic wounds contains higher levels of MMPs compared with acute wounds (44). Bullen et al. showed a significant decrease in TIMPs and an increase of gelatinases in CVI, an effect we also showed in this study (34). Tarnuzzer and Schultz found a significant increase in MMP‐2 and MMP‐9 in wound exudate of patients with chronic wounds compared with acute wounds (45). Similar results were found by Yager et al. in wound exudates from five patients with pressure ulcers. They showed that the levels of MMP‐2 and MMP‐9 were elevated more than tenfold and 25‐fold, respectively, in fluids from pressure ulcers compared with fluids from mastectomy wounds (46). Our findings confirm these results in a larger patient group. In addition to the increase in proteases, a lower concentration of TIMPs was found by Weckroth in the wound exudate of patients with CVI (28). Saito et al. analysed 110 biopsies taken from the lower calf and lower thigh in 73 patients with CVI. They showed that a higher synthesis of MMP‐2 in dermal fibroblasts and leukocytes leads to a pro‐ulcer‐forming environment (47). Tengrove et al. showed in wound exudates from 15 patients with a venous leg ulcer that elevated levels of MMP activity decreased significantly after 2 weeks, when the ulcers began to heal, as evidenced by a reduction in wound size (48). This is similar to our results; however, we followed the ulcers for a total of 12 weeks. Nwomeh et al. also showed that significant higher levels of MMP‐1 and MMP‐8 and lower levels of TIMP‐1 characterised chronic non healing ulcers when compared with healing wounds (6). Although levels of both MMP‐1 and MMP‐8 varied greatly in these chronic ulcers, MMP‐8 was found to be the predominant collagenase presented in these wounds.
In our study, we analysed the wound exudate of patients with CVI for a longer period of time during treatment with a hydrocolloid dressing and ORC/collagen matrix. Our results provide supporting evidence that ORC/collagen matrix leads to a more physiologic wound healing environment by decreasing protease activity.
Previous studies have reported the importance of MMPs, elastase and plasmin in the development of chronic wounds 7, 49. Herrick et al. and Hoffmann et al. showed elevated concentrations in the wound exudate of non healing wounds (50). In our study, we were able to confirm these findings. Furthermore, we showed a significant reduction of elastase and gelatinase activity after only 5 days of treatment with ORC/collagen matrix. However, although significant changes in plasmin activity could not be observed, there was a trend to reduced activity. This may reflect the fact that these wounds were ‘stuck’ in the inflammatory phase of healing and that elastase and gelatinase were the predominant proteases present in the wound. In previous in vitro studies, ORC/collagen is capable of reducing elastase, gelatinase and plasmin levels; however, its effect in vivo was not reported.
According to recent studies, high elastase activity may lead to degradation of fibronectin (51) and growth factors 8, 46 in chronic wounds. The persistence of high elastase levels in these wounds suggests that the wound is still in the inflammatory phase of wound repair even though all wounds had been presented for at least 30 days. Plasmin is the major fibrinolytic enzyme. Its role in chronic wounds remains unclear. It is possible that reduced plasmin activity could contribute to fibrosis, which has been reported in leg ulcers (40). However, previous studies from Palolathi et al. have suggested that plasmin activity is increased in chronic wound exudate (49). The mechanisms responsible for the increased levels of plasmin in the chronic wound environment remain unclear. Previous studies have shown that epidermal keratinocytes can regulate plasmin activation through the expression of critical mediators, including urokinase‐type plasminogen activator and its receptor (52).
In summary, we have shown that ORC/collagen matrix treatment reduces gelatinase and elastase activity in wound exudates of patients with CVI. Thereby, rebalancing the hostile chronic wound microenvironment and facilitating wound repair.
Acknowledgments
This study was supported by an unrestricted research grant from Ethicon GmbH. The authors would like to thank Cecilia Welling, PhD, MMSI‐consultancy, Germany, for her help in preparing the manuscript.
Both authors contributed equally to this work.
References
- 1. Sandor T. Pathomechanism of chronic venous insufficiency and leg ulcer. Acta Physiol Hung 2004;91:131–45. [DOI] [PubMed] [Google Scholar]
- 2. McDaniel HB, Marston WA, Farber MA, Mendes RR, Owens LV, Young ML, Daniel PF, Keagy BA. Recurrence of chronic venous ulcers on the basis of clinical, etiologic, anatomic, and pathophysiologic criteria and air plethysmography. J Vasc Surg 2002;35:723–8. [DOI] [PubMed] [Google Scholar]
- 3. Sieggreen MY, Kline RA. Recognizing and managing venous leg ulcers. Adv Skin Wound Care 2004;17:302–11; quiz 312–3. [DOI] [PubMed] [Google Scholar]
- 4. Borzini P, Mazzucco L, Panizza R, Rivara G. Regarding “Randomized trial and local biological effect of autologous platelets used as adjuvant therapy for chronic venous leg ulcers”. J Vasc Surg 2004;39:1146–7; Author reply 1147. [DOI] [PubMed] [Google Scholar]
- 5. Li W, Chong S, Huang E, Tuan T. Plasminogen activator/plasmin system: a major player in wound healing? Wound Repair Regen 2003;11:239–47. [DOI] [PubMed] [Google Scholar]
- 6. Nwomeh BC, Yager DR, Cohen IK. Physiology of the chronic wound. Clin Plast Surg 1998;25:341–56. [PubMed] [Google Scholar]
- 7. Agren MS, Eaglstein WH, Ferguson MW, Harding KG, Moore K, Saarialho‐Kere UK, Schultz GS. Causes and effects of the chronic inflammation in venous leg ulcers. Acta Derm Venereol 2000;210:3–17. [PubMed] [Google Scholar]
- 8. Yager DR, Chen SM, Ward SI, Olutye OO, Diegelmann RF, Cohen IK. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen 1997;5:23–32. [DOI] [PubMed] [Google Scholar]
- 9. Owen CA, Campbell EJ. The cell biology of leukocyte‐mediated proteolysis. J Leukoc Biol 1999;65:137–50. [DOI] [PubMed] [Google Scholar]
- 10. Chapman HA. Plasminogen activators, integrins, and the coordinated regulation of cell adhesion and migration. Curr Opin Cell Biol 1997;9:714–24. [DOI] [PubMed] [Google Scholar]
- 11. Parks WC. Matrix metalloproteinases in repair. Wound Repair Regen 1999;7:423–32. [DOI] [PubMed] [Google Scholar]
- 12. Rifkin DB, Gleizes PE, Harpel J, Nunes I, Munger J, Mazzieri R, Noguera I. Plasminogen/plasminogen activator and growth factor activation. Ciba Found Symp 1997;212:105–15. [DOI] [PubMed] [Google Scholar]
- 13. Lauer G, Sollberg S, Cole M, Flamme I, Stuerzebecher J, Mann K, Krieg T, Eming S. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Invest Dermatol 2000;115:1–12. [DOI] [PubMed] [Google Scholar]
- 14. Grinell F, Zhu M. Identification of neutrophil elastase as the proteinase in burn wound fluid responsible for degradation of fibronectin. J Invest Dermatol 1994;103:155–61. [DOI] [PubMed] [Google Scholar]
- 15. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 2003;92:827–39. [DOI] [PubMed] [Google Scholar]
- 16. Goldberg GI, Wilhelm SM, Kronberger A, Bauer A, Grant GA, Eisen AZ. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation‐induced rat protein. J Biol Chem 1986;261:6600–5. [PubMed] [Google Scholar]
- 17. Hasty KA, Pourmotabbed TF, Goldberg GI, Thompson JP, Spinella DG, Stevens RM, Mairardi CL. Human neutrophil collagenase. A distinct gene product with homology to other matrix metalloproteinases. J Biol Chem 1990;265:11421–4. [PubMed] [Google Scholar]
- 18. Devarajan P, Mookhtiar K, Van Wart HE, Berliner N. Structure and expression of the cDNA encoding human neutrophil collagenase. Blood 1991;7:2731–8. [PubMed] [Google Scholar]
- 19. Wilhelm SM, Collier IE, Kronberger A, Eisen AZ, Marmer BL, Grant GA, Bauer EA, Goldberg GI. Human skin fibroblast stromelysin: structure, glycosylation, substrate specificity, and differential expression in normal and tumorigenic cells. Proc Natl Acad Sci USA 1987;84:6725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saus J, Quinones S, Otani Y, Nagase H, Harris ED Jr, Kurkinen M. The complete primary structure of human matrix metalloproteinase‐3. Identity with stromelysin. J Biol Chem. 1988;263:6742–5. [PubMed] [Google Scholar]
- 21. Whitham SE, Murphy G, Angel P, Rahmsdorf HJ, Smith BJ, Lyons A, Harris TJ, Reynolds JJ, Herrlich P, Docherty AJP. Comparison of human stromelysin and collagenase by cloning and sequence analysis. Biochem J 1986;240:913–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Müller D, Quantin B, Gesnel MC, Millon‐Collard R, Abecassis J, Breathnach R. The collagenase gene family in humans consists of at least four members. Biochem J 1988;263:187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Collier IE, Wilhelm SM, Eisen AZ, Marmer BL, Grant GA, Seltzer JL, Kronberger A, He CS, Bauer EA, Goldberg GI. H‐ras oncogene‐transformed human bronchial epithelial cells (TBE‐1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem 1988;263:6579–87. [PubMed] [Google Scholar]
- 24. Wilhelm SM, Collier IE, Marmer BL, Eisen AZ, Grant, GA , Goldberg GI. SV40‐transformed human lung fibroblasts secrete a 92‐kDa type IV collagenase which is identical to that secreted by normal human macrophages [published erratum appears in J Biol Chem 1990 Dec 25;265(36):22570]. J Biol Chem 1989;264:17213–21. [PubMed] [Google Scholar]
- 25. Ravanti L, Kahari VM. Matrix metalloproteinases in wound repair. Int J Mol Med 2000;6:391. [PubMed] [Google Scholar]
- 26. Armstrong DG, Jude EB. The role of matrix metalloproteinases in wound healing. J Am Podiatr Med Assoc 2002;92:12–8. [DOI] [PubMed] [Google Scholar]
- 27. Lobmann R, Schultz G, Lehnert H. Proteases and the diabetic foot syndrome: mechanisms and therapeutic implications. Diabetes Care 2005;28:461–71. [DOI] [PubMed] [Google Scholar]
- 28. Weckroth M, Vaheri A, Lauharanta J, Sorsa T, Konttinen YT. Matrix metalloproteinases, gelatinase, and collagenase in chronic leg ulcers. J Invest Dermatol 1996;106:1119–24. [DOI] [PubMed] [Google Scholar]
- 29. Cullen B, Smith R, McCulloch E, Silcock D, Morrison L. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen 2002;10:16–25. [DOI] [PubMed] [Google Scholar]
- 30. Ghatnekar O, Willis M, Persson U. Cost effectiveness of treating deep diabetic foot ulcers with Promogran in four European countries. J Wound Care 2002;11:70–4. [DOI] [PubMed] [Google Scholar]
- 31. Vin F, Teot L, Meaume S. The healing properties of Promogran in venous leg ulcers. J Wound Care 2002;11:335–41. [DOI] [PubMed] [Google Scholar]
- 32. Bradford MM. A refined and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein‐dye binding. Anal Biochem 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- 33. Vaheri A, Stephens RW, Salonen EM, Pollanen J, Tapiovaara H. Plasminogen activation at the cell surface‐matrix interface. Cell Differ Dev 1990;32:255–62. [DOI] [PubMed] [Google Scholar]
- 34. Bullen EC, Longaker MT, Updike DL, Benton R, Ladin D, Hou Z, Howard EW. Tissue inhibitor of metalloproteinases‐1 is decreased and activated gelatinases are increased in chronic wounds. J Invest Dermatol 1995;104:236–40. [DOI] [PubMed] [Google Scholar]
- 35. Ulrich D, Hrynyschyn K, Pallua N. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in sera and tissue of patients with Dupuytren’s disease. Plast Reconstr Surg 2003;112:1279–86. [DOI] [PubMed] [Google Scholar]
- 36. Ulrich D, Lichtenegger F, Eblenkamp M, Repper D, Pallua N. Matrix metalloproteinases, tissue inhibitors of metalloproteinases, aminoterminal propeptide of procollagen type III, and hyaluronan in sera and tissue of patients with capsular contracture after augmentation with Trilucent breast implants. Plast Reconstr Surg 2004;114:229–36. [DOI] [PubMed] [Google Scholar]
- 37. Ulrich D, Noah EM, Von Heimburg D, Pallua N. TIMP‐1, MMP‐2, MMP‐9, and PIIINP as serum markers for skin fibrosis in patients following severe burn trauma. Plast Reconstr Surg 2003;111:1423–31. [DOI] [PubMed] [Google Scholar]
- 38. Barrick B, Campbell EJ, Owen CA. Leukocyte proteinases in wound healing: roles in physiologic and pathologic processes. Wound Repair Regen 1999;7:410–22. [DOI] [PubMed] [Google Scholar]
- 39. Parks WC. The production, role, and regulation of matrix metallproteases in the healing epidermis. Wounds 1995;7:23A. [Google Scholar]
- 40. Falanga V. Chronic wounds: pathophysiologic and experimental considerations. J Invest Dermatol 1993;100:721–5. [DOI] [PubMed] [Google Scholar]
- 41. Wall SJ, Bevan D, Thomas DW, Harding KG, Edwards DR, Murphy G. Differential expression of matrix metalloproteinases during impaired wound healing of the diabetes mouse. J Invest Dermatol 2002;119:91–8. [DOI] [PubMed] [Google Scholar]
- 42. Tarlton JF, Bailey AJ, Crawford E, Jones D, Moore K, Harding KD. Prognostic value of markers of collagen remodeling in venous ulcers. Wound Repair Regen 1999;7:347–55. [DOI] [PubMed] [Google Scholar]
- 43. Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg 2002;137:822–7. [DOI] [PubMed] [Google Scholar]
- 44. Wysocki AB, Staiano‐Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP‐2 and MMP‐9. J Invest Dermatol 1993;101:64–8. [DOI] [PubMed] [Google Scholar]
- 45. Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen 1996;4:321–5. [DOI] [PubMed] [Google Scholar]
- 46. Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluid from human pressure ulcers contain elevated mmp levels and activity compared to surgical wound fluid. J Invest Dermatol 1996;107:743–8. [DOI] [PubMed] [Google Scholar]
- 47. Saito S, Trovato MJ, You R, Lal BK, Fasehun F, Padberg FT Jr, Hobson RW 2nd, Duran WN, Pappas PJ. Role of matrix metalloproteinases 1, 2 and 9 and tissue inhibitor of matrix metalloproteinases 1 in chronic venous insufficiency. J Vasc Surg 2001;34:930–8. [DOI] [PubMed] [Google Scholar]
- 48. Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, Murphy G, Schultz G. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999;7:442–52. [DOI] [PubMed] [Google Scholar]
- 49. Palolahti M, Lauharanta J, Stephens RW, Kuusela P, Vaheri A. Proteolytic activity in leg ulcer exudate. Exp Dermatol 1993;2:29–37. [DOI] [PubMed] [Google Scholar]
- 50. Hoffmann R, Starkey S, Coad J. Wound fluid from venous leg ulcers degrades plasminogen and reduces plasmin generation by keratinocytes. J Invest Dermatol 1998;111:1140–4. [DOI] [PubMed] [Google Scholar]
- 51. Rao CN, Ladin DA, Liu YY, Chilukuri K, Hou ZZ, Woodley DT. α1‐Antitrypsin is degrade and non‐functional in chronic wounds but intact and functional in acute wounds: the inhibitor protects fibronectin from degradation by chronic wound fluid enzymes. J Invest Dermatol 1995;105:572–8. [DOI] [PubMed] [Google Scholar]
- 52. Schäfer BM, Stark HJ, Fusenig NE, Todd RF, Kramer MD. Differential expression of uPA, ist receptor (uPA‐R), and inhibitor type‐2 (PAI‐2) during differentiation of keratinocytes in an organotypic coculture system. Exp Cell Res 1995;220:415–23. [DOI] [PubMed] [Google Scholar]


