ABSTRACT
The use of paraffin‐impregnated gauze for burns and skin graft donor sites is commonly associated with wound adherence with consequent pain and trauma upon removal. This prospective clinical study was performed to evaluate a new class of lipido‐colloid dressings (Urgotul™) in promoting healing and in reducing tissue adherence. In a 6‐month period, 25 consecutive patients were recruited. Two separate burn or donor sites on each patient were dressed with tulle‐gras (TG) or Urgotul™ and covered with standard secondary dressings. Objective assessment of wounds by two reviewers, and patients' subjective assessments were recorded. Twenty‐three (92%) patients were followed up for a mean of 3 months. Mean time to complete epithelialisation was 9·6 and 11·9 days for the Urgotul™ and TG sites respectively (P < 0·05). Bleeding was seen in 52% of Urgotul™ sites compared with 100% of the TG sites at first dressing change (P < 0·05). Patients reported ‘moderate pain’ during dressing change in 22% and 57% in the Urgotul™ and TG groups respectively (P < 0·05), with 35% of TG sites being ‘very painful’ requiring extra analgesia. We found that compared with TG, Urgotul™ was associated with faster epithelialisation, less pain and trauma (bleeding) during dressing changes.
Keywords: Burns, donors sites, dressings, hydrocolloid, lipido‐colloid, Urgotul™
INTRODUCTION
The treatment of partial thickness burns has been the subject of debate and research for many years. It is generally agreed that early excision and grafting of deep burns reduce morbidity and mortality, whereas early excision of partial thickness burns remains contentious. Removal of non viable tissue reduces the risk of wound infection and burn conversion, however, surgical debridement down to viable tissue causes significant blood loss and sacrifice of healthy tissue (1). In recent times, modern dressing materials enable conservative treatment, preserving viable tissue with no increased risk of infection (2).
In burns, which require excision and skin grafting, the donor site created after harvesting a split‐thickness skin graft (SSG) becomes an additional wound. Any delay in healing of the donor site is a complication, which can sometimes cause the patient more inconvenience than the condition for which the graft was initially indicated, at times requiring later skin grafting.
Greasy, neutral or impregnated dressings, e.g. tulle gras have been used for many years to treat acute skin lesions (injuries, burns, etc.). The aim is to create and maintain a local environment conducive to the healing process based on the concept of healing in a moist environment (3). However, in actual practice, these greasy dressings often dry out and require frequent dressing changes, and they almost always adhere to wounds causing wounds to bleed upon their removal. This makes wound care painful and disruptive to the healing process. An improved form of dressing was required, one which could provide an ideal moist healing environment and at the same time overcome many of the traditional problems of adherence, trauma and pain associated with conventional adherent dressings.
Urgotul™ is a lipido‐colloid wound dressing impregnated with hydrocolloid particles dispersed in a petroleum jelly matrix, wherein on contact with wound exudate, the hydrocolloid particles would absorb water, swell, and liquefy to form a moist gel layer, thereby maintaining a moist and warm environment at the same time preventing external bacterial colonisation. It was launched in 2000 by the Urgo laboratories in Dijon, France, as an improved alternative to both conventional and modern dressings characterised by its atraumatic and painless removal 4, 5.
We have conducted a prospective randomised study to evaluate the efficacy of Urgotul™ compared with tulle gras, a conventional dressing material, in the treatment of partial thickness burns and SSG donor sites in terms of rate of healing, discomfort to patients during dressing change, ease of dressing removal and amount of bleeding relating to wound adherence upon dressing removal.
METHODS
Materials
Tulle gras
We used tulle‐gras (TG) dressing (Jelonet, Smith and Nephew Ltd, Hull, UK) as a comparative material. TG is made from sterilised open‐weave bleach cloth impregnated with soft paraffin. During the First World War, Lumière first introduced TG dressings for local treatment of gunshot wounds, and during the Second World War, it was advocated by Wallace of Edinburgh in the Oxford War Manuals for the treatment of burns wounds (Wallace, 1941). Its popularity continued thereafter as a well‐established covering designed to prevent adhesions to wounds, but in practice, when wound exudate dries out, it causes the dressing to strongly adhere to the wound causing pain, trauma and bleeding on removal (6). Another observation was that of granulation tissue growing through the open weave of the fabric, and on removal, precious newly epithelialised skin would be torn off along with the dressing. This again caused pain to the patient and trauma to the wound bed.
Urgotul™
A sterile, EC, class IIB medical device developed and produced by Urgo laboratories in Dijon, France (7). A new generation dressing utilising the concept of lipido‐colloid technology, it is a bilaminar non occlusive, thin sheet consisting of a 100% polyester net with non deformable filaments, impregnated with hydrocolloid particles dispersed in a petroleum jelly matrix (8) (Figure 1).
Figure 1.
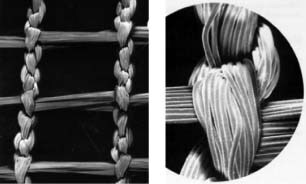
Structure of Ugotul™ filaments.
When the product comes into contact with wound exudate, the hydrocolloid particles hydrate and together with the petroleum jelly component form a lipido‐colloid interface. This prevents adherence of the dressings to the wound surface, while providing an optimal wound environment of moisture, protection and warmth.
Excess exudate drains through the constantly open mesh into a secondary absorbent dressing, thus avoiding any build‐up or maceration of the wound or surrounding skin. Newly formed granulation tissue is prevented from migrating through the dressing by the very small diameters of the mesh and in effect, prevents pain, bleeding and trauma on removal. This mesh is constricted of continuous filaments that do not migrate into the wound, in contrast, TG fibres are often disrupted and incorporated into the wound, exacerbating wound adherence and eliciting a foreign body reaction (Figure 2).
Figure 2.

Structure of TG showing unraveling and dislodgement of gauze threads.
Study protocol
This is a prospective randomised control study with Institutional Review Board (IRB)‐approved study protocol. The following inclusion criteria were used: partial thickness burns and donor sites after SSG harvesting. The exclusion criteria were: deep dermal and full thickness burns requiring excision, infected burns, total body surface area (TBSA) burns of more than 30% requiring admission to the Intensive Care Unit, known sensitivity or allergy to TG or Urgotul™ and patient refusal.
Informed consent would be obtained from patients meeting inclusion criteria and included as research subjects. The patient would serve as his own control, and have two separate sites of similar burn depth or one confluent burn area divided by an imaginary line (3, 4) or SSG sites randomised to one of the materials for comparison (Figure 5).
Figure 3.
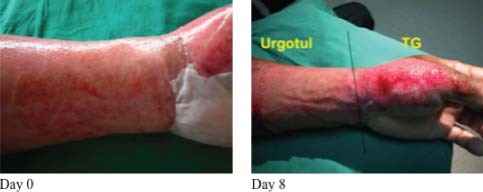
At Day 8, the area dressed with Ugotul™ shows faster epithelialisation and less bleeding on removal of dressings.
Figure 4.
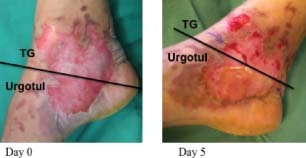
The area dressed with Ugotul™ showed faster epithelialisation and less bleeding on removal of dressings at Day 5.
Figure 5.
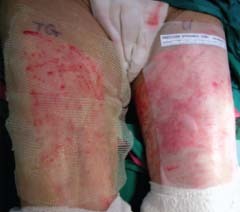
SSG donor sites dressed with TG (right thigh) and Ugotul™ (left thigh).
The partial thickness burn wounds selected for comparison of dressing material were of similar burn depth as assessed by two blinded observers. All SSG donor sites were of equal depth as all skin grafts were harvested with a fixed selected depth on the standardised electrical dermatome.
Wounds were cleansed according to local protocol with only physiological saline. Application of Urgotul™ or TG placed onto the wound was covered with standard secondary dressings (gauze and bandage). Dressings were changed every 4–5 days, i.e. 5rd, 10th 14th days, etc…)
Assessment of healing was performed by two blinded observers at each dressing change with weekly photographs taken for later planimetric assessment.
Participation in the study concluded upon reaching the study endpoint, which was complete healing of wounds.
Additional data collected
Further evaluation was performed and documented at each dressing change to provide qualitative information. The additional following data were collected:
-
•
Ease of dressing removal
At each dressing change, nurses were asked to grade the removal of dressing as ‘very easy’, ‘easy’ or ‘difficult’.
-
•
Bleeding on dressing change
At each dressing change, two blinded observers graded the amount of bleeding present after the dressing material was removed as ‘none’, ‘minimal’, ‘moderate’ or ‘important’, the latter being bleeding requiring momentary application of pressure causing the subject further discomfort.
-
•
Pain reported by patients on dressing removal
Patients were asked to grade their pain at the first dressing change as ‘minimal’, ‘moderate’ or ‘important’, the latter being intolerant pain requiring extra analgesia on top of their current analgesia regime.
Statistical analysis
Statistical analyses were performed using the SPSS statistical software (version 16·0). Univariate analysis was performed by chi‐square tests or by Fisher's exact probability test for the comparison of proportion between the two groups. A P‐value of less than 0·05 was taken as statistically significant. For comparison of the mean between the two groups, the Wilcoxon test was used.
RESULTS
25 patients were recruited over a 6‐month period. Two dropped out (lost to follow up) and 23 patients (92%) completed the study. There was a total of 46 study wounds (24 partial thickness burns and 22 SSG donor sites), 23 dressed with Urgotul™ and 23 dressed with TG.
The mean age of the subjects was 44 years old (minimum: 23, maximum: 65). The mean overall size of the burns sustained was 12% TBSA, with a minimum of 2·5% and maximum of 14·5%. The average area dressed with Urgotul™ and TG was 118 and 112 cm2, respectively.
Typical examples of treated partial thickness burns and SSG donor sites with TG and Urgotul™ are shown (see 3, 4, 6)
Figure 6.
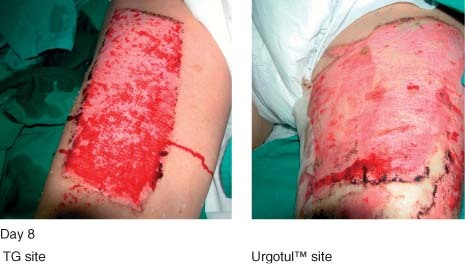
Less trauma to donor site during dressing change with Ugotul™ (left thigh) as evident by less bleeding.
There were no adverse events, none of the subjects developed an allergenic reaction to the dressings or had to undergo excision and further grafting. Two wounds (one dressed with Urgotul™ and one with TG) developed superficial infection that resolved with antimicrobial therapy.
-
•
Healing time
Twenty‐three patients were followed up for a mean of 3 months. Mean time to complete epithelialisation was 9·6 and 11·9 days for the Urgotul™ and TG sites respectively (P < 0·05) (see Table 1).
Table 1.
Mean time from application of dressing to complete epithelialisation
| Mean time to complete epithelialisation (days) | Range | P‐value (Wilcoxon test) | |
|---|---|---|---|
| Urgotul™ | 9·6 | 7–14 | P < 0·05 |
| Tulle gras (TG) | 11·9 | 7–21 |
-
•
Ease of dressing change
During dressing changes, nurses reported ‘very easy to use’ with 100% of Urgotul™ dressed wounds. Eighty‐seven percent of TG‐dressed wounds were ‘easy to use’ and 13% ‘difficult to use’ (see Figure 7).
Figure 7.
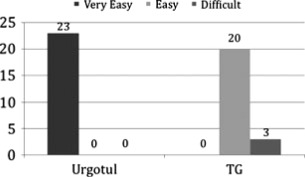
Ease of dressing removal. Hundred percent of the Urgotul™‐dressed wounds were reported as ‘very easy’ to change versus 87% and 13% reported as ‘easy’ and ‘difficult’ to change respectively in the TG‐dressed wounds.
Material adherence to the wound leading to bleeding was seen in 100% of TG sites compared with 52% of Urgotul™ sites at first dressing change (P < 0·05). Forty‐eight percent of Urgotul™ sites had no bleeding, 43% versus 13% of Urgotul™ and TG‐dressed wounds respectively had ‘minimal’ bleeding, and 17% of TG‐dressed wounds had ‘important’ bleeding. None of the Urgotul™ dressed wounds had ‘important bleeding’ (see Figure 8).
Figure 8.
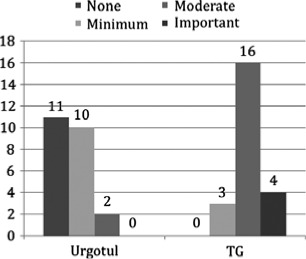
Bleeding on dressing removal. Forty‐eight percent of the Urgotul™‐dressed wounds showed no bleeding, 43% versus 13% of Urgotul™‐dressed and TG‐dressed wounds respectively noted minimal bleeding during first dressing change. Seventeen percent of TG‐dressed wounds had ‘important’ bleeding. None of the Urgotul™ dressed wounds had ‘important bleeding’.
-
•
Reported pain by patients
With respect to results on dressing comfort, ‘minimal pain’ was reported more frequently with Urgotul™ than TG, i.e. 65% versus 26% in the Urgotul™ and TG‐dressed wounds respectively (P < 0·05). Twenty‐two percent versus 57% in the Urgotul™ and TG‐dressed wounds gave patients ‘moderate pain’ and 35% of the TG‐dressed wounds were ‘very painful’ on dressing change. None of the Urgotul™‐dressed wounds were reported as ‘very painful’ (see Figure 9).
Figure 9.
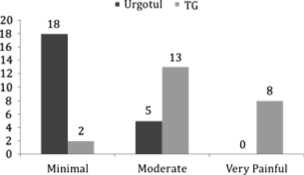
Pain reported by patients at first dressing removal. Seventy‐eight percent versus 9% (P < 0·05) of the Urgotul™ and TG‐dressed wounds respectively gave patients minimal pain during dressing change. Thirty‐five percent of the TG‐dressed wounds were ‘very painful’ on dressing change. None of the Urgotul™‐dressed wounds were reported as ‘very painful’.
DISCUSSION
In the last decade, there has been an expansion in possibilities for treating burn wounds and SSG donor sites: a number of new materials and techniques and dressings containing active agents have become commercially available and even more are currently under development (9).
Hydrocolloid dressings are a family of wound management products which have been around for 25–30 years (9), e.g. Comfeel™, Granuflex™, Duoderm™, etc. are bilaminar dressings composed of an outer absorbent occlusive or non occlusive layer and inner layer containing gel‐forming agents such as sodium carboxymethylcellulose (NaCMC) (one of the main ingredients in hydrocolloid dressings) and gelatin. When the gel‐forming agent comes into contact with wound fluid, it forms a gel‐like substance, which prevents escape of water vapour, thus maintaining a moist wound environment, beneficial for the healing process. The wound fluid continually gets absorbed by the outer layer, thus reducing eschar formation at the wound bed. At the same time, the gel layer reduces wound adherence, reducing removal of newly epithelialised skin during dressing changes. And it is this ideal characteristic of low‐adherence which has hydrocolloid dressings favoured at the epithelialisation stage of acute wounds as cited by a recent Consensus Panel, for recommendations for chronic and acute wound dressings (2007) (10).
Urgotul™ is a newer generation dressing which has features like the family of hydrocolloid dressings mentioned above, but in addition to possessing hydrocolloid particles, the particles are impregnated in a petroleum jelly matrix, whereby on contact with wound exudate, the hydrocolloid particles hydrate and with the petroleum jelly component, forms a lipido‐colloid interface preventing adherence of the dressing to the underlying wound bed.
Our study has shown Urgotul™ to be more efficacious as a dressing for partial thickness burns and SSG donor sites compared with TG. It produces faster healing time: mean of 9·6 days for the Urgotul dressed wounds versus 11.3 days for the TG‐dressed wounds, less granulation tissue incorporated into dressings was observed and it offers a significant benefit during dressing change in terms of comfort experienced by patients, ease of use by nurses and less bleeding of wounds.
CONCLUSION
Urgotul™ is a highly promising dressing alternative to conventional dressings for partial thickness burns and skin graft donor sites and is well tolerated by patients. We compared it with the most commonly used dressing material (TG) by doctors all over the country managing partial thickness burn wounds, and look forward to its comparison with other families of dressings in the use of burns and SSG donor sites.
REFERENCES
- 1. Eldad A, Din A, Weinberg A, Neuman A, Lipton H, Ben‐Bassat H, Wexler MR. Cryopreserved cadaveric allografts for treatment of unexcised partial thickness flame burns: clinical experience with 12 patients. Burns 1997;23(7–8): 608–14. [DOI] [PubMed] [Google Scholar]
- 2. Hutchinson JJ, Lawrence JC. Wound infection under occlusive dressings. J Hosp Infect 1991;17(2) : 83–94. [DOI] [PubMed] [Google Scholar]
- 3. Hinman CD, Maibach H. Effect of Air Exposure and Occlusion on Experimental Human Skin Wounds. Nature 1963;200: 377–8. [DOI] [PubMed] [Google Scholar]
- 4. Benbow M, Iosson G. A clinical evaluation of Urgotul to treat acute and chronic wounds. Br J Nurs 2004;13(2): 105–9. [DOI] [PubMed] [Google Scholar]
- 5. Letouze A, Voinchet V, Hoecht B, Muenter KC, Vives R, Bohbot S. Using a new lipidocolloid dressing in paediatric wounds: results of French and German clinical studies. J Wound Care 2004;13(6): 221–5. [DOI] [PubMed] [Google Scholar]
- 6. Vloemans AF, Soesman AM, Suijker M, Kreis RW, Middelkoop E. A randomised clinical trial comparing a hydrocolloid‐derived dressing and glycerol preserved allograft skin in the management of partial thickness burns. Burns 2003;29(7): 702–10. [DOI] [PubMed] [Google Scholar]
- 7. Benbow M. Urgotul: alternative to conventional non‐adherence dressings. Br J Nurs 2002;11(2): 135–8. [DOI] [PubMed] [Google Scholar]
- 8. Hermans MH. Results of an internet survey on the treatment of partial thickness burns, full thickness burns, and donor sites. J Burn Care Res 2007;28(6): 835–47. [DOI] [PubMed] [Google Scholar]
- 9. Vaneau M, Chaby G, Guillot B, Martel P, Senet P, Teot L, Chosidow O. Consensus panel recommendations for chronic and acute wound dressings. Arch Dermatol 2007;143(10): 1291–4. [DOI] [PubMed] [Google Scholar]


