Abstract
The aim of this paper was to provide a literature synthesis on current wound care practices for the management of chronic wounds in palliative care and end‐of‐life patients, focusing on the control of wound‐related symptoms for comfort and improved quality of life. These wounds included pressure ulcers, venous and arterial leg ulcers, diabetic ulcers and fungating malignant wounds. Wound‐related symptoms included pain, exudate, malodour, infection, bleeding, dressing comfort and negative psychological and social functioning. Best care wound practices were formulated for each wound type to ease suffering based on the literature review. Although symptom management strategies for comfort may work in tandem with healing interventions, it is important to recognise when efforts towards wound closure may become unrealistic or burdensome for the patient at end of life. Thus, unique aspects of palliative wound care feature clinical indicators for early recognition of delayed healing, quality of life measurement tools related to chronic wounds, and comfort care strategies that align with patient wishes and realistic expectations for wound improvement.
Keywords: Chronic wounds, Comfort, Palliative care, Quality of life, Wounds
INTRODUCTION
The aim of this paper is to provide a literature review of current wound care practices for the management of chronic wounds in palliative care and end‐of‐life patients, focusing on the control of wound‐related symptoms for comfort and improved quality of life (QOL). These wounds include pressure ulcers, venous or arterial leg ulcers, diabetic ulcers and fungating malignant wounds. Specific recommendations for palliative wound management are given based on this literature synthesis.
Symptom control in palliative care aims to prevent and relieve suffering through effective management of pain and other distressful symptoms related to the chronic disease or life‐threatening illness to enhance QOL (1). In the USA, palliative care can be initiated during active treatment of the disease and continued through the disease spectrum to include comfort care. Hospice care is strictly comfort care delivered to patients with an estimated 6 months prognosis. Through an interdisciplinary team approach, the patient and family are supported to address patient‐centered goals, needs and wishes during the illness in conjunction with other life supporting therapies or during the dying process with comfort care (2). For some palliative care patients with wounds, treatment of the underlying condition will result in full or partial wound healing using best practice wound care (3). However, many of the wounds that palliative care patients tend to develop are often a result of the advanced life‐limiting disease that has weakened the healing process preventing normal wound closure despite treatment 4, 5, 6, 7, 8. Focus of wound care then becomes centred on what strategies will provide the patient the most comfort in controlling symptoms, such as pain, exudate, odour, infection, bleeding, dressing comfort and reducing the negative impact on psychological and social functioning 4, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21.
Chronic wounds described in the literature review are wounds that usually have a history of at least 3‐ to 6‐months' duration without showing signs of improvement (60–70% healthy granulation tissue) or a response to treatment 5, 9, 22, 23. Chronic wounds affect an estimated 5–7 million patients and cost more than 30 billion dollars annually in the USA 23, 24, 25, 26, 27. Significant to the burden are the indirect costs to the patient and family relating to loss of productivity, employment, caregiver stress and QOL 8, 17, 25, 28, 29, 30.
SEARCH STRATEGY
A literature review was conducted of these studies from 1992 to 2008, using Medline, CINAHL, PubMed and Cochrane Systematic Review databases. The Nebraska Medical Center rating system for level and quality of evidence was used for article selection (Figure 1). There is limited evidence on the most common types of wounds and uniform care in palliative patients 6, 10, 11, 31. Thus, some of the care practices are based on expert opinion or case studies blended with aspects of best wound care practices that support the patient's goal and QOL 9, 15, 16, 32.
Figure 1.

Rating system for level and quality of evidence.
LITERATURE SYNTHESIS
Quality of life
For some disease states such as fungating malignancies, a complete cure or healing may not be achieved, so that assessing the QOL is an essential measure for the palliative care patient with a wound 8, 28, 33. With this in mind, outcomes for care are measured in terms of the extent to which this goal for best QOL is achieved for the patient and family 34, 35.
It is difficult to measure QOL as an outcome when there is ‘no gold standard’ (34, p. 1190). Complicating the definition further, QOL is recognised as a ‘multidimensional construct' (36, p. 29). The World Health Organization defines QOL as the individuals' perceptions of life shaped by their cultural belief system relative to their goals and interests (2). In palliative care, the patient defines QOL as what is most meaningful in the following domains: physical, social, psychological, cultural and spiritual (1).
One of the first instruments developed for measuring QOL was the QOL Index. This index was tested randomly on 349 haemodialysis patients and showed internal reliability and validity 17, 33, 36. This index measures satisfaction or dissatisfaction in areas that are important to the individual: health and functioning, socio‐economic, psychological and spiritual and family (36). The values are scored together for each satisfaction and importance response with higher scores of each showing a positive QOL (36).
The QOL Index has been used for palliative care patients to identify areas that the patient needs support or intervention to relieve suffering and thus, improve well‐being. For a palliative care patient living with a chronic wound, it is important that the patient's QOL experiences related specifically to the wound be assessed regularly to guide clinical interventions (33).
An instrument that is sensitive to the concerns of the patient and can measure the impact of chronic wounds on QOL is the Cardiff Wound Impact Schedule (CWIS) (33). This questionnaire was tested in a three‐step process on patients with leg ulcers and diabetic foot ulcers measuring three domains of QOL: physical symptoms and daily living, social life and well‐being. A few of the stress items included disturbed sleep, pain, social restrictions and dressing discomfort. Significant correlation at P < 0·001 was found between experience and stress (33). There were no significant differences in scores with the two wound types with high internal consistency, reproducibility and validity 17, 33. This instrument is also notable in that it is sensitive specifically to the distress caused by the chronic wound regardless of the etiology or state of healing (33). Identifying specific stressors or concerns of the patient forms the basis for patient involvement in his/her cares to guide wound management for an optimal realistic outcome.
Patients with chronic wounds suffer a variety of adverse stressors negatively impacting their daily lives (33). These stressors include pain, exudate leakage, restricted mobility, poor hygiene, feelings of disgust or shame because of disfigurement or malodour, sleep disturbance, loss of sexuality, dissatisfaction with treatments, loss of control, social isolation, dependency, residency relocation, anger and lack of confidence in the healthcare provider because of failure to heal 8, 12, 17, 19, 20.
Mudge et al. (37) found in a qualitative interview study cultural differences between France, Canada and the UK regarding chronic leg ulcer pain experiences. This study found that the French group described concern with body image, the British group with taking multiple medications for pain and the Canadians with finances (37). All groups, however, feared infection (37). The authors highlighted that the cultural groups focused on the distress of the symptoms rather than delayed wound healing.
In a separate but similar qualitative study that focused on symptom distress, researchers found that 12 women with fungating breast cancer wounds related greater freedom and an improved sense of femininity and sexuality when the correct wound care product was used to absorb exudates and control odour (8). Clinical concern was not centred on wound closure but symptom management to improve the women's sense of well‐being (8). These studies show that it is important that information assessed be used to inform decisions with the patient with attention to the patient's descriptive experiences to guide cares that support well‐being 8, 12, 17, 19.
Wound chronicity
Chronic wounds ‘fail to progress through an orderly and linear sequence’ of cellular healing processes: homeostasis, inflammation, proliferation, remodelling, contraction and maturation of a scar for functional integrity (9, p. 1162; 38, 39). Normal healing is usually complete within 2–12 weeks 9, 24. The underlying cause of delayed healing is multi‐factorial but may include advanced disease or organ dysfunction, older age, physical inactivity, compromised mobility, infection, lower limb arterial insufficiency, diabetic neuropathy, fungating malignancies and malnutrition 5, 7, 11, 16, 32, 40, 41, 42, 43.
Clinicians need to be knowledgeable with early recognition of the non healing wound and the factors contributing to the chronicity to identify realistic outcomes that support QOL as defined by the patient 4, 19, 25, 44. A challenge in palliative wound management then is identifying early when a wound is slow to heal despite best wound practices 25, 45. Although symptom management strategies for comfort may work in tandem with healing interventions, it is also important to recognise when efforts towards wound closure may become unrealistic or burdensome for the patient, so that management shifts to meeting the patient's priority for comfort 4, 32, 40. Therefore, appropriate wound care management for the palliative patient lies in recognising patient concerns and assessing for factors that contribute to wound chronicity for informed decision‐making for realistic goals 4, 25.
In the 2008 position document, The European Wound Management Association (EWMA) published a schematic diagram of the multiple factors that interact to show the complexity of chronic wound management (25) (Figure 2). Notably, this diagram includes assessing goals and patient concerns in addition to the risk factors, wound bed characteristics, underlying medical condition, prognosis and clinician skill and knowledge for appropriate wound care management that promotes an improved QOL 9, 25.
Figure 2.
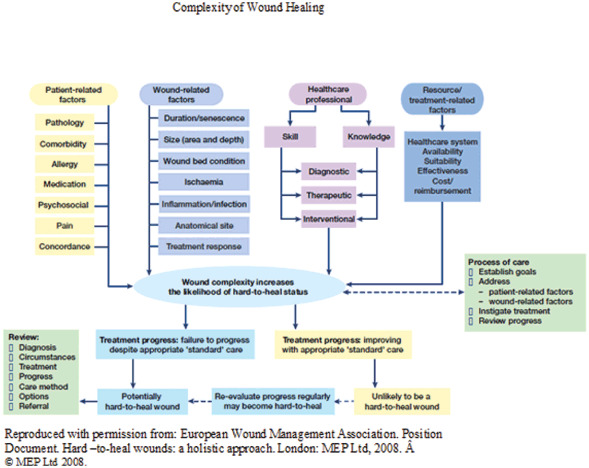
Complexity of wound healing. Reproduced with permission from Ref. (25).
Clinical risk factors of chronic wounds
Several researchers have conducted studies to help identify clinical indicators that may signal delayed wound healing in the context of the basic etiology of the ulcer. These indicators would signal the need to alter the treatment plan or change the goals consistent with the patient's wishes 4, 22, 25. Sheehan et al. (46) found that assessing uncomplicated diabetic foot ulcer healing at 4 weeks was an important clinical indicator for early identification of non healing wounds with standard interventions. In a systemic review of guidelines for the treatment of diabetic ulcers, Steed, et al. (2006) reported that patients who fail to show a reduction in ulcer size by 40% after 4 weeks of treatment should be re‐evaluated for other treatment strategies, patient education and decision‐making (47). Margolis et al. (48) found that in a cohort study of more than 31 000 patients that ulcer size (>2 cm2), duration (>2 months) and ulcer depth predicted healing outcomes: if patients had all three adverse parameters, diabetic neuropathic foot ulcers had only a 22% chance of healing by 20 weeks (25). Treece et al. (49)stratified and validated risks for diabetic foot ulcer healing with use of size, sepsis, arteriopathy and denervation (SAD) system in a prospective single‐centre cohort study. The researchers found significant associations with ulcer healing with three of these categories independently contributing to the outcome‐size, sepsis and arteriopathy (49). In a multi‐site, 15‐month long study, van Rijswijk (50) found that patients with full‐thickness pressure ulcers that did not show a reduction in size of 45% after 2 weeks or 77% after 4 weeks should be re‐evaluated for alternative treatment strategies. In a separate study van Rijswijk and Polansky explored outcomes and variables as predictors for time to healing of stage III and stage IV pressure ulcers: poor nutritional status at baseline and percent reduction in ulcer area after 2 weeks had significant effect on healing (51). Both studies confirm importance of wound measurement and regular assessment for reevaluation of treatment plan.
In an in vitro study, Stephens et al. (43) isolated anaerobic microflora from the deep tissue of 18 patients with refractory chronic venous leg ulcers and studied the effects of these organisms on extracellular matrix proteolysis and cellular wound healing responses. The researchers found significant inhibition of fibroblast and keratinocyte cell growth by the bacteria, which would delay re‐epithelialisation and contribute to chronic inflammation (43). Bacterial loads in excess of 105 organisms per gram of tissue can delay wound healing, or if the patient has osteomyelitis 9, 38, 52, 53. In the guidelines for venous, diabetic and pressure ulcers, The Wound Healing Society published recommendations that ulcers having bacterial loads of 106 or more per gram of tissue or any tissue level of beta haemolytic streptococci will show delayed healing 27, 47, 54.
Delayed healing can also be expected with fungating malignant wounds because of microbial bioburden, necrotic tissue and foreign substances, causing pain, exudate and odour from bacterial lipid metabolism 9, 43, 45. Unless the underlying cancer can be palliated with radiation or chemotherapy, wound healing is not expected because of the overall poor prognosis (16).
In two separate retrospective non experimental studies with pressure ulcers, clinicians found that patients who have a high disease burden at end of life show skin failure 5, 6. Brown (5) found that 51 patients out of 74 or 68·7% acquiring nosocomial full thickness stage III and stage IV ulcers had a 180‐day mortality rate and that none of the ulcers healed. Brown (5) ascertained from this data that patients did not die from the ulcers, but rather that the disease burden resulted in immobility, malnutrition and decreased tissue perfusion that allowed for skin atrophy. Similarly, Galvin's (6) 2‐year retrospective audit study of 542 palliative care unit admissions found that despite a pressure ulcer and skin care protocol, the incidence of pressure ulcer damage was not reduced. The majority of ulcers was sacral and occurred as a result of the tumour or degenerative condition at end of life (6).
Langemo and Brown reviewed that ‘gross examination of muscle mass, subcutaneous tissue thickness, wound granulation and tissue necrosis' in the context of the disease prognosis is the current standard for setting realistic goals with the patient for wound healing at the end of life (55, p. 208). The non healing wound would be maintained comfortably for the patient and stabilised to prevent infection or complications (44).
Other intrinsic and extrinsic factors that can delay healing are malnutrition or protein deficiencies, tobacco and alcohol use, immunosuppressive medications, systemic steroids, non steroidal anti‐inflammatories, hypothermia, stress, infection, frailty, anaemia, poor glycaemic control, prothrombotic conditions, inadequate tissue oxygenation and pressure 5, 6, 38, 42, 56, 57.
Risk assessment tools
Accurate assessment of pressure ulcer risk is part of the holistic care of the patient and can help inform decision between the clinician and the patient. If possible, prevention of pressure ulcer formation is an important component of holistic care in palliative care to promote comfort and dignity 7, 40. Because of the lack of research evidence found in the systematic review, Pancorbo‐Hidalgo et al. (58)could not conclude that use of risk assessment scales in clinical practice decrease pressure ulcer incidence. Nevertheless, the best practice guideline of using a valid, reliable risk assessment screening tool ensures systematic and uniform evaluation of clinical indicators to guide intervention and if possible prevent ulcer formation 52, 56.
The Braden and Norton scales are supported by the [Agency for Healthcare Policy and Research (AHCPR)] and the National Pressure Ulcer Advisory Panel (NPUAP) for identifying risk factors for pressure ulcers 52, 56, 59. In a systematic review of literature in 2006 of risk assessment scales for pressure ulcer prevention, the authors found the Braden scale to be the best risk tool for sensitivity, inter‐rater reliability, specificity and validity (58). With the Braden scale, risk factors include skin moisture, mobility, activity, friction or shearing, nutrition and sensory/perception (60). Measures to prevent pressure ulcers could then be planned to include pressure support systems, positioning, encouraging activity if possible, skin hygiene and nutritional interventions (7). Regarding nutrition, wound experts note a lack of research evidence for the palliative care patient to support intervention to reduce the risk of ulcers (7). However, the clinical practice guidelines outlined by AHCPR encourage dietary intake and supplementation as tolerated by the malnourished patient with wounds (52).
There is also a lack of evidence concerning the validity of existing risk tools for palliative care patients 7, 40. In an effort to address the vulnerability of the terminally ill for skin breakdown, Chaplin developed the Hunters Hill Marie Curie Center pressure sore risk assessment tool (the Hunters Hill tool) which was piloted on 291 patients in a 41‐bedded specialist palliative care unit (40). This tool retained various risk factors of other tools but also considered the skin condition. Seven risk factors were identified: sensation, mobility, moisture, activity in bed, nutrition/weight change, skin condition and friction/shear (40). Specific medical conditions of the terminally ill were enumerated with the risk factors, such as dyspnoea, extreme fatigue, muscle atrophy, cachexia, reduced subcutaneous tissue and dehydration (40). The risks were scored weekly or with significant changes from a minimum score of 7 to a maximum score of 28 relating a very high risk (40). Notably, Chaplin related the importance of recognising the comfort of the patient over the prevention practice. For example, using a specialised bed to prevent pressure sores may result in ‘immobility, respiratory infection or social isolation for the patient' (40, p. 30). Interestingly, to measure the validity of the tool, the researcher used comparative analysis of professional judgement of palliative care nurses for patients at risk for pressure ulcer development and the numerical scores over an 18‐month period: validity of the tool depended upon its application (40). With this data, thresholds were established for low, medium, high and very high risks (40). More research is required to test this tool for inter‐rater reliability and validity by others in palliative care settings.
Another pressure sore risk tool was developed for the hospice patient in Sweden called the Hospice Pressure Ulcer Risk Assessment Scale (HoRT) (7). It identified three primary factors that contribute to pressure ulcer development at the end of life: physical activity (graded 1–4, where 4 indicates full function and 1 indicates very deteriorated or no function), mobility and age (below 75 years or older) (7). HoRT compared favourably to the Norton and Braden scales for accuracy (7). As with the Hunter Hill tool, more testing is needed to validate the HoRT tool for general use. Both of these studies related that small sample sizes, short duration of study time because of terminal conditions and ethical considerations limited effective analysis in palliative care populations 7, 40.
Assessment of chronic wounds for targeting interventions
In an effort to standardise the assessment of chronic wounds by clinicians and increase knowledge to guide discussions with patients, wound care experts in 2002 introduced the TIME acronym which was further developed by the EWMA in a position document 57, 61. This TIME model offers a comprehensive approach to monitor certain wound parameters in addition to the risk factors that can help identify patients with non healing wounds. Goals can then be addressed with appropriate tailored interventions. The TIME framework includes the following parameters: Tissue (non viable or deficient), Infection or inflammation, Moisture (balance or imbalance), and Edge of wound (non advancing or undermined) 57, 61. This clinical tool provides guidance in monitoring the wound and targeting the interventions. For example, appropriate wound bed preparation for a diabetic ulcer could entail patient assessment for underlying cause, debridement, moisture and bacterial balance and adequate oxygenation (47).
Expanding on the TIME tool, a group of wound healing experts outlined guidelines with another original mnemonic, MEASURE, for assessing chronic wounds: Measure (length, width, depth and area), Exudate (quantity and quality‐odor), Appearance (wound bed), Suffering (pain), Undermining, Re‐evaluate (wound treatment effectiveness) and Edge (condition of edge and surrounding skin) (45). Assessing these parameters for clinical outcomes for controlling exudate, minimising or eliminating odour, preventing infection and relieving pain offers goals for improving QOL and alternative end points if wound healing is not achievable 22, 62. This outline is noteworthy also because it incorporates a patient‐centered approach with inclusion of pain assessment to identify suffering, a key concern of palliative care 1, 3.
Both the TIME and MEASURE guidelines were developed as conceptual tools to inform the medical community on the benefits of wound bed preparation for a systematic approach for proper chronic wound management and for developing best practice principles. These tools offer an understanding as to why a wound is not healing based on the underlying wound abnormality and whether a certain treatment is effective 39, 45. It remains a challenge on how effectively these two tools are applied reliably by clinicians 39, 45, 57.
There are several tools or scales that are considered reliable and valid for assessing wound healing. For pressure ulcer healing, the Bates‐Jensen Wound Assessment Tool (BWAT) formerly known as the Pressure Sore Status Tool (PSST) and the NPUAP Pressure Ulcer Scale for Healing (PUSH) provide valid measurement 10, 63 (Figure 3). The BWAT is time consuming and detailed, making this tool impractical for most clinical uses, but beneficial for research purposes 15, 63. The PUSH scale was developed for easier clinical application, sensitivity to wound changes and inter‐reliability (63). In a prospective study of nursing home residents with predominantly stage II pressure ulcers, the PUSH scores significantly decreased over time among the healed ulcers and compared favourably with the then PSST scale used for confirmation (63). Although this study was well controlled for the small sample size (32 ulcers) and for variance, the only PUSH item that showed significant change was the wound size (length × width). Exudate and tissue type did not show change possibly because of predominately stage II ulcers and multiple types of treatment interventions for the control of symptoms (63). Further studies on patients with full‐thickness wounds and disease burden would be beneficial.
Figure 3.
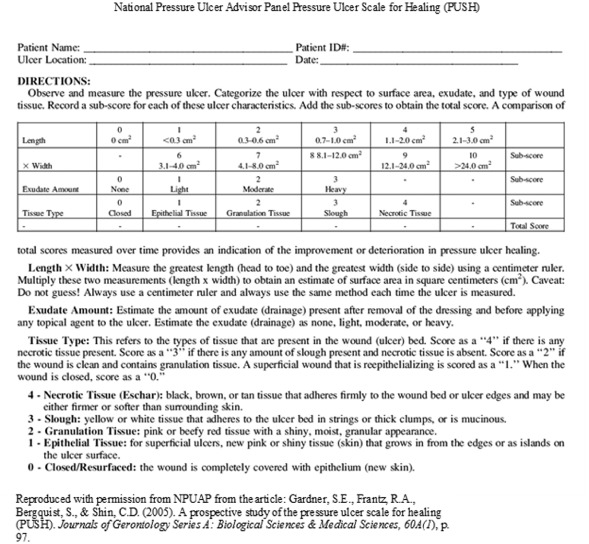
National pressure ulcer advisor panel pressure ulcer scale for healing (PUSH). Reproduced with permission from Ref. (63).
As with all tools used, wound assessment data must be used in conjunction with clinical judgement of patient risk factors and the overall goals of the patient to evaluate appropriate outcomes in wound management (64). Although there is no official system for assessing malignant fungating wounds, two assessment tools for measuring risks and patient perceptions have been created but not yet validated for generalised applicability for the palliative care experience: the Treatment Evaluation by LE Roux's (TELER) method and the Wound Symptoms Self‐Assessment Chart (WoSSAC) 15, 65, 66. Unlike previous measurement tools, these two tools emphasise the importance of specific QOL measures for optimal symptom control expressed by the individual rather than objective measurement of wound progression. In fact, Grocott (65) related in her longitudinal case study the various limitations in objectively measuring fungating wounds with ultrasound, photographs and weight of dressings to determine exudate volume as these dressing products required multiple fits confounding the control of variables. Furthermore, because of infiltration of the tumour into the skin and adjacent structures, necrotic tissue caused malodour requiring subjective measurement (65). Clinical note‐taking with a statistical method of measurement (TELER) and a self‐report questionnaire (WoSSAC) allow the patient to relate changes in the wound subjectively and document the distress with daily life 65, 66, 67. Both tools provide indicators on tracking progress towards or away from the patient‐centered treatment goals for symptom management, using an ordinal scale of codes (TELER) or a 5‐point Likert scale (WoSSAC). The primary difference is that the TELER is recorded by the clinician based on a qualitative consensus agreement with the patient through dialogue and the WoSSAC is completed by the patient 66, 67. There is potential for bias with the TELER method as the researcher records (66). Also, Browne et al. 66, 67indicated that they had to modify definitions of the TELER indicator codes to gain consensus with the patients, which could challenge ensuring a robust system if other researchers did the same for future study. Nevertheless, these two palliative care tools recognise the expertise of the patient who lives with the chronic wound and the need to include symptom distress in assessing wound management for optimal care.
Wound pain
In a systematic review of 37 studies describing the negative impact of leg ulcers on daily life of patients, Persoon et al. (17) found that pain was the first and most dominant distressful experience, disturbing sleep, mobility, socialisation, mood, grooming and relationships. In addition, descriptive studies suggested under‐treatment and reporting of pain by nursing or medical staff 10, 12, 17, 19, 20, 68. These patients with chronic wound pain verbalised feeling ‘out of control’, pessimism about healing and ignorance about the leg ulcer (12, 20). Clearly, to alleviate suffering it is important to have a thorough pain assessment confirming the patient's subjective experience.
In chronic wound pain, there is a prolonged inflammatory response stimulating the local afferent skin receptors (nociceptive) or peripheral nerve endings with increased sensitivity or hyperalgesia (69). Prolonged damage can cause neuropathic pain. Allodynia can develop with repeated noxious stimulation of the nerves so that any stimuli can be painful to the patient, making pain difficult to control (69). With this in mind, clinicians should use proper compression bandaging technique to reduce allodynia pain in patients with venous leg ulcers (9).
To assist clinicians in managing pain, Krasner (70)developed an original holistic approach to assess and manage chronic wound pain. These pain processes are further described by Krasner as three types: cyclic (periodic discomfort), non cyclic (single incident) and chronic (persistent discomfort). This model is placed in the context of wound bed assessment, preparation and aetiology so that the wound and pain are treated at the same time for optimal QOL (71). The clinician also assessed the timing of the wound pain to identify the stimulus for pain intervention: off loading the diabetic ulcer or decreasing oedema in the venous ulcer for improved oxygen transport to healing tissues with compression bandages or choosing the appropriate dressing for gentle removal (71). On‐going assessment and team communication are essential for effective management (71).
Several recognised, validated pain measurement scales are available for assessing the patient's perspective of pain: Visual Analogue Scale (VAS), Numeric Box Scale, The Faces Rating Scale (FRS), and the Face Legs Activity Cry and Consolability (FLACC) scale 71, 72. In a systematic review of literature, de Laat et al. (10) found that The McGill pain questionnaire (MPQ), VAS and FRS were valid and reliable to diagnose pressure ulcer pain. Although the McGill questionnaire measures sensitivity to the overall wound pain experience and is able to associate pain with affective distress, it is lengthy and difficult to complete for acutely ill or terminally ill patients in the clinical setting 10, 73. For patients who cannot self‐report pain intensity, the FLACC pain tool is valid and reliable (72). With pain measurement, it is important also to assess the patient for socio‐cultural issues related to pain (74).
To reduce pain, local wound care involves assessing for the underlying cause. In an international survey of 11 countries, wound practitioners rated dressing removal to be the time of greatest pain for the wound patient (74). Pain with dressing removal has been substantiated by various researchers and experts with case studies to highlight the problem 32, 41, 68, 69, 75, 76. Factors that contribute to pain with dressing changes are dried out dressings, packed gauze, products that adhere, adhesive dressings and cleansing (74). Exposing wounds to air with the dressing removal can be painful for the terminally ill and should be covered with a moist dressing during changes (32). For palliative management of wounds to avoid pain, an ideal dressing would offer non bulky comfort sized to the wound, gentle adherence, cost‐effectiveness, a moist wound healing environment, minimisation of shear, friction and pressure, impermeability to bacteria, long wear time, absorbency of excess exudate to prevent skin excoriation and ease of dressing use by patient or caregiver 11, 15, 18, 27, 54. Importantly, the patient and caregiver are active participants in management decisions and product choices (77).
Appropriate dressing prevents strike‐through and exudate leakage which can increase bioburden and thus, infection which, in turn, increases pain (77). To prevent pain from peri‐wound maceration, skin can be protected by applying a barrier, such as zinc oxide paste or a liquid film‐forming acrylate 38, 78. Hydrocolloids should be used with caution as this type of dressing has strong adhesion and can tear fragile peri‐wound skin upon removal 15, 18. Using a permeable non adherent contact layer with a secondary absorbent dressing, such as calcium alginates, hydrogels, hydrofibres, foam dressings or soft silicones, is recommended by World Union of Wound Healing Societies (WUWHS) to handle leakage and reduce pain (77).
There is limited research evidence available to promote one dressing product over another for wound care management (79). It is hoped that with more research conclusions can be drawn to help the clinician choose the appropriate dressing. Therefore, the following dressing products described are only a few examples—with limited critique—that have been beneficial for wound care management.
One composite dressing product that has limited study is the Versiva by ConvaTec (Skillman, NJ, USA). It has been promoted to reduce wound pain and handle exudate leakage. It combines three layers of action into one product: hydrocolloid, hydrofibre and a top layer of polyurethane (gelling) foam‐film (80). In a multi‐centre, non randomised study that assessed performance of five different ConvaTec products on healing of venous leg ulcers, researchers found that healing or marked improvement was observed in 82% of leg ulcers within 5 weeks of using Versiva under the compression bandage. The product also offered a long wear time of 5 days which was cost effective, reducing the number of times the compression therapy needed to be reapplied. Exudate was absorbed well and peri‐wound skin was protected with easy removal. Pain was also reduced, although it is not known if this was because of the soothing gel or the compression therapy (80). There were other limitations to the study because of possible company bias, non randomisation and non comparison and a small sample size (n = 11) (80). To date, there has been no conclusive evidence that supports one dressing type under compression bandaging that affects ulcer healing (81).
Another dressing product that has shown promise but without sufficient research is the product, Mepitel (Mölnycke Health Care, Goteborg, Sweden). In a systematic review of randomised control trials (RCTs) and case studies, White and Morris (21) found support for this lipidocolloid soft polymer silicone dressing for the management of traumatic wounds, skin tears and chronic painful draining wounds. This dressing used as a first layer allows wound exudates to pass through its netting to a secondary absorbent dressing, allowing for fewer dressing changes.
Wound cleansing has been identified as a source of wound pain (74). Best practice guidelines recommend warm potable water, mild wound cleansing agent or saline for simple cleansing of wounds (52). The NPUAP also recommends avoiding hot water and excessive rubbing (56). Palliative care experts recommend gentle cleansing of the wound, avoiding cold fluids and antiseptics and not to wipe across the wound during dressing changes (82). Alvarez et al. (9) and Hollinworth (82) stress the importance of talking with the patient to identify anxiety, pacing the procedure according to the patient's preference, offering ‘time‐out' to the patient and assessing the pain before, during and after the dressing change to minimise trauma.
The use of antiseptic agents for wound cleansing is debatable. Antiseptic agents such as povidone iodine, iodophor, Dakin's solution, acetic acid and hydrogen peroxide are not recommended by the AHCPR for wound cleansing because of toxicity studies in the laboratory (52). However, povidone iodine has proved useful in palliative wound care as a topical antiseptic for wet gangrene to decrease bacterial burden 9, 57. Also, in an international consensus document, wound care experts support limited use of antiseptic agents to reduce high bacterial loads in wounds to aid healing or to prevent wound infection (83). Clinicians should use these agents with caution and know the indications and risks for safe practice. Furthermore, antiseptic agents need to be used in context with a management plan that is multi‐disciplinary and addresses the underlying cause of the infection so that the patient can fight the infection (83). Agents that show limited research efficacy in reducing bioburden are silver and cadexomer iodine (83). In a retrospective study of 11 patients with chronic critical limb ischaemia, Williams (84) found that topical cadexomer iodine in the form of microbeads prevented wet gangrene in all patients, delayed amputations in five and limb salvage therapy in four. More research is needed on larger sample sizes. Microbeads release iodine into the wound bed at levels not toxic to the cells to reduce bacteria 38, 84.
Topical wound pain control can involve debridement to reduce necrotic tissue and bacterial burden 9, 38, 57, 85. With a secondary advantage of also preventing wound infection, debridement may be surgical or sharp, autolytic, enzymatic, mechanical or biological (larval therapy) 9, 38, 57, 85. In a systematic review of new and experimental treatments of diabetic foot ulcers, the authors found that maggot debridement significantly decreased offensive odour and pain and has been recommended as a last resort for gangrene and osteomyelitis cases to prevent amputation (24). Patient consent to maggot treatment would be a priority in the goals of care discussion. Topical application of silver sulfadiazine 1% is used by the Calvary Hospital in New York to protect against infection in diabetic ulcer wounds of palliative care patients following debridement (9). However, debridement is not recommended for fungating malignant wounds because of bleeding risk or ischaemic arterial wounds because of desiccation and the poor potential to granulate 38, 79. In a systematic review of literature, McDonald and Lesage (15) found that for ischaemic arterial wounds the distal perfusion prior to debridement should be an ankle–brachial index of greater than 0·5, toe pressure greater than 50 mm Hg, and transcutaneous oxygen saturation greater than 30% for best outcome.
In a systematic review restricted to six RCTs, Briggs and Nelson (75) found that use of eutectic mixture of local anaesthetic cream (EMLA 5% Astrazeneca, Wilmington, DE, USA), which consists of lidocaine and prilocaine, significantly reduced the pain intensity during and after sharp debridement of leg ulcers as determined by VAS versus placebo. A thick layer of EMLA 5% cream applied to the ulcer 30–45 minutes prior to debridement and covered with plastic film produced a mean reduction of 20·65 mm in pain score with a 95% confidence interval and no adverse effects on ulcer size of healing 69, 75, 86. de Laat et al. (10) found a need for further research on the effects of EMLA cream to relieve pressure ulcer pain. Because EMLA cream has a pH of 9·4 and can penetrate damaged skin, daily use of this anaesthetic cream to control pain is not recommended (87).
Adjuvant therapies for fungating wounds are surgery, radiotherapy, hormone therapy or chemotherapy to reduce the tumour and pain 11, 16, 76. Goals of care discussions, however, are important to determine whether the benefits of treatment outweigh the burden in view of the prognosis. Radiation can cause radiotherapy skin reactions and chronic damage to connective tissue and vascular supply (16). Surgery can cause enterocutaneous fistulas producing corrosive drainage (16).
For topical local pain control of fungating wounds, the Palliative Care Institute and The Center of Curative and Palliative Wound Care at Calvary Hospital, Bronx, New York, have formulated topical lidocaine 2·75% in Balmex zinc oxide cream (9). This cream controls pain quickly and lasts up to 4 hours for relief of tumour pain per case study reports.
Other topical anaesthetic agents that show promise in controlling pain in ulcers are lidocaine patches, topical morphine and ibuprofen in non adhesive foam dressings, but more clinical trials are needed for conclusive evidence 69, 88. Systematically reviewed in the literature, Evans and Gray (69) found lidocaine patches applied to cover painful areas worn for a maximum of 12 hours daily controlled pain. Zeppetella et al. (88)studied topical morphine 10 mg/1 ml in 8 g of Intrasite gel applied to painful sacral ulcers once daily and covered with Tegaderm dressing in five hospice patients versus placebo of water mixed with Intrasite gel. Intrasite gel is a hydrogel used for debridement (88). Pain intensity scores were rated and compared twice daily using VAS. This pilot study found significant reduction in pain (P < 0·01) with no adverse effects (88). In a similar randomised control study on 18 patients with leg ulcers, Vernassiere et al. (87) did not find statistical significant pain control with topical use of morphine 10 mg mixed with 15 g of Intrasite gel. Further RCT studies are needed with larger patient samples to draw conclusion on the efficacy of topical morphine. Although isolated controlled studies have reported effective relief of pain with moist wound healing dressings containing ibuprofen, a systematic review of the literature has failed to substantiate effectiveness with RCT evidence (69). Evan's and Grey's review (2005) reported a lack of research for conclusive benefit in using aspirin, capsaicin or clonidine (69). If topical analgesia of ulcers could be achieved, then this would reduce the need for systemic opioid management, which exposes patients to opioid side effects such as constipation, sedation and nausea (87).
Systemic medications for pain control may be ordered following the three‐step model described by the World Health Organization (89). Regular analgesia is determined by regular assessment of the pain intensity described by the patient for choosing drugs with different modes of action and as needed for breakthrough pain (89). Patients are assessed for side effects to prevent and control suffering (89). Appropriate pain medication administration should be timed accordingly to route at rest and prior to dressing cares to reduce suffering 15, 16, 18, 68, 76, 90. Non pharmacological interventions are also important in controlling pain by educating the patient and family to encourage participation. Some interventions are coping skills training, behavioural contracts, biofeedback, relaxation therapy, music, acupuncture, distraction, visual imagery, social and spiritual support, cold and warmth therapy, repositioning, pressure support, appropriate dressing choice, transcutaneous electrical nerve stimulation (TENS unit) and physical therapy exercise 1, 3, 9, 15, 18, 32, 56, 71, 76.
Wound odour and exudate
Odour and heavy exudates are two symptoms that are most distressing to patients, triggering anxiety with poor QOL 8, 65, 66, 67, 79. Draper (79) in a systematic review catalogued malodour distress as causing involuntary gagging, vomiting, decreased appetite, weight loss, social isolation and withdrawal. In addition, chronic wound fluid inhibits cell proliferation for healing (57).
Systemic or topical metronidazole has been effective for reducing odour. Small studies have shown metronidazole 0·75–0·8% gel or 1% cream applied directly to the wound once or twice daily or together with calcium alginate, hydrofibre, or foam dressings are significantly effective in controlling odour with patient satisfaction 9, 62, 79, 91. For palliative management, Institute for Clinical Systems Improvement recommends either topical metronidazole 0·75% gel or cream or crushed 500 mg tablets directly to wound for 7 days (3).
Draper (79) found in a systematic review of literature that activated charcoal dressings applied to fungating wounds significantly controlled odour if the dressing fit as a sealed unit and if the wound was maintained dry. If not sealed, the odour would escape. The charcoal dressings CarboFLEX (ConvaTec) and Clinisorb (CliniMed Ltd., London, UK) were found to meet these requirements (79). Antimicrobial dressings with activated charcoal such as Actisorb Silver (Johnson & Johnson Medicine Ltd., New Brunswick, NJ, USA) are also an option for controlling odour and also inactivating microorganisms (38). de Laat et al. (10) in a systematic literature review on pressure ulcers did not find research on the odour‐absorbing capacity of charcoal dressings. Other means of controlling odour based on case studies by experts are oral administration of chlorophyll tablets one tablet after meals and at bedtime and then advancing two tablets four times daily, kitty litter under the bed, commercial deodorisers and peppermint oils on dressings 3, 9, 76, 92.
AHCPR guidelines state that for effective management of wound exudate, the choice of dressing should control exudate without drying out the ulcer bed (52). Therefore, there are exudate measurement tools for the clinician to assess the amount of wound exudate to gauge choice of dressing for effective absorption. The PUSH tool defines exudate drainage after removal of the dressing and before applying any topical agents as none, light (covers less than 25% wound surface), moderate (covers 50–75%) and heavy (covers 75–100%) (93). The BWAT uses a metric measure guide divided into four 25% pie‐shaped quadrants to measure amount of exudate on the dressing: none (dry), scant (moist and not measureable), small (drainage <25% of dressing), moderate (saturated tissues with >25% to <75% of dressing) and large (tissues bathed in fluid with >75% of dressing) (94). Because accuracy with the tool is dependent upon the clinician's skill, adequate training is important (63).
Some dressing categories for exudate control based on the amount are the following 9, 38, 57: Film dressings are best for dry or minimal exudates as these are not absorbent. Hydrogels are for dry, sloughy wounds with small amounts of exudates and can provide a cooling relief for painful ulcers. Hydrocolloids are for mild‐to‐moderate exudating wounds that offer autolytic debridement; caution must be used with removal so friable skin is not stripped. Alginates are highly absorbent for moderate‐to‐heavy levels of exudates and may also be used for haemostatic control of bleeding wounds; care must be taken to cut the alginate to size the wound to prevent wicking onto peri‐wound skin (38). Foams are moderately absorbent and are non adhesive for ease of dressing removal.
Heavy exudate is common with fungating malignant wounds that can ulcerate and spread into the lymphatics denuding the skin or by tumour necrotic outgrowths or fistulas 11, 15, 16. Gross deformity can result, making it difficult to choose a correct dressing to fit the wound and absorb the fluid 11, 16. According to a systematic literature review, Adderley and Smith(95) could not find sufficient evidence to guide practice for care of fungating wounds because of lack of research. Current care has been suggested by wound care experts in this field for palliative management 11, 16, 79, 96. Treatments include gentle wound cleansing, applying alcohol‐free skin barrier films, choosing appropriate dressings to control exudate and odour, managing bacteria and controlling pain and bleeding 3, 15, 16, 38, 57, 79, 85.
Draper (79) found a lack of evidence to support the use of one type or brand of dressing for the care of fungating wounds. Optimal dressing choices include foam and alginates as these are non adhesive, vapor‐permeable absorbent dressings and ostomy appliances 3, 9, 15, 16, 38, 79. Dressings should have long wear time but should be changed when exudate strike‐through is present (15).
In a systematic review of literature, Draper (79) cited that the dressing choice for fungating wounds should not only be based on the wound characteristics but also should have minimum bulk, prevent leakage, be comfortable and cosmetically acceptable to the patient. In a case study, Naylor (2001) found that a hydropolymer foam dressing‐Tielle Plus‐managed heavy exudate and malodour effectively for a patient who had a large malignant fungating breast and chest wall wound (112). This dressing was cosmetically acceptable and comfortable under clothing and provided proper seal on contours with its self‐adhesive border. Ultimately, the clinician should work with the patient in finding the appropriate comfortable dressing with maximum benefit for palliative management of the wound 11, 16.
There has been a limited amount of research showing some effectiveness with cadexomer iodine in venous and diabetic ulcers for the palliative treatment of heavily draining wounds by reducing the bioburden 9, 96.
Topical negative pressure wound therapy (NPWT) or vacuum‐assisted closure (VAC) devices have been used to handle heavy exudating or infected wounds: pressure ulcers, venous ulcers and diabetic ulcers (38). VAC devices for wound fluid management decrease the number of dressing changes, reducing the exudate burden as the ulcer heals. A multi‐center RCT of 342 patients with diabetic foot ulcers found significant ulcer closure with negative pressure (P = 0·007) 43·2% versus 28·9% with moist dressings and fewer amputations (4·1% versus 10·2% ) (97). However, a Cochrane systemic review of seven RCTs found no conclusive evidence supporting NPWT over saline gauze or hydrocolloid gel dressings for chronic wound healing (98). Additional high‐quality RCTs studies are required for conclusive evidence for healing chronic wounds. Furthermore, Alvarez et al. (9, p. 1183) states that ‘NPWT does not get used in the palliative care setting as CMS reimbursement policy mandates routine wound measurements that demonstrate wound closure, so that if the wound does not get smaller payment is denied’. NPWT is also contraindicated for the treatment of malignant cutaneous wounds, as these lesions could granulate (9).
Wound bleeding
Case studies for palliative management of wounds have found that bleeding may be controlled by careful removal of dressings moistened first with warmed normal saline, use of non adherent dressings, gentle pressure for 10–15 minutes at site, cauterisation or by applying gauze saturated with 1:1000 solution of epinephrine, topical low‐dose (100 units/ml) thromboplastin or 0·5–1% silver nitrate 3, 9, 15. If the fungating wound erodes major blood vessels, there is the risk of spontaneous haemorrhage (16). If haemorrhage can be anticipated, referral to vascular surgeon may be planned (15). For bleeding that is spontaneous and the patient is terminal, dark towels for absorption may lessen the anxiety of the patient and the family (15).
Wound infection
Controlling bacteria can decrease exudate, pain and odour to help stabilise a non healing wound 11, 15, 57. If antimicrobial management of a wound is necessary, a wound culture or tissue biopsy is recommended to isolate the organism, especially if febrile or cellulitic to determine the appropriate systemic antibiotic 15, 38, 57, 79. In a Cochrane intervention review of RCTs, O’Meara et al. (96)found no evidence to support the routine use of systemic antibiotics to promote healing by reducing bioburden in venous leg ulcers. The authors stated that the lack of research should not prevent the use of antibiotics when there is an infection (96). Other topical preparations such as mupirocin, ethacridine lactate, peroxide‐based preparations and povidone iodine could not be recommended for venous leg ulcer treatment because of insufficient evidence (96).
Silver impregnated dressings have been used for antimicrobial action in wounds. In Cochrane reviews of RCTs assessing the effectiveness of topical silver in the treatment of contaminated or infected wounds, the authors found significant less leakage as a secondary benefit in patients with leg ulcers or chronic wounds with a silver dressing in one trial (99). However, because of a lack of quality research, the benefits are not conclusive for silver as an effective treatment of chronic wounds 99, 100.
More research is also needed for evidence that honey treated with gamma irradiation is effective for treatment of wound infections, control of exudate and odour and aids healing 79, 101. However, honey is being used for palliative management of wounds: honey releases hydrogen peroxide at low concentrations which inhibits bacterial growth and debrides the wound (15).
Adjunctive therapies for wound healing
Advances in adjunctive therapies for chronic wound healing include electrical stimulation which activates fibroblasts and growth factors for granulation and cell migration, autologous surgical skin grafts, hyperbaric oxygen therapy (HBOT) for diabetic ulcers and oral pentoxifylline for venous leg ulcers 38, 57, 102, 103. There is weak evidence that ultrasound increases healing in venous leg ulcers and the healing action is not understood (104). New therapies are expensive and have had limited trials for evidence of efficacy (24). In a Cochrane review of four trials, authors found that HBOT used to treat diabetic foot ulcers significantly reduced the risk of amputation and may improve the chance of healing at 1 year (103). Because of side effects of breathing pure oxygen which may include toxicity to the brain and lungs and barotraumas to ears, lungs and sinuses, the American Academy of Dermatology advocates confirming peri‐wound hypoxia by transcutaneous oxygen measurement first before trialing HBOT (38). This treatment is also costly (38). For the adjunctive therapies to be considered for the palliative care patient, it would be important to consider prognosis, cost, risk and likelihood of healing in the context of patient's wishes for comfort.
Jull et al. (102)concluded from 11 trials that oral pentoxifylline (Trental, Aventis Pharmaceutical Company, Bridgewater, NJ, USA) 400 mg tablet taken three times daily increases chances of healing venous ulcers in conjunction with compression bandages by improving circulation to the tissues. There was also evidence that healing improved without the compression bandages (102). For palliative care patients who have ischaemic arterial ulcers and who do not have heart failure, Pletal (cilostazol, Otsuka Pharmaceutical Company, Princeton, NJ, USA) has been used to treat claudication pain as well as endovascular intervention for plaque excision (9). In a retrospective study of 37 elderly patients with critical leg ischaemia, Amato et al. (105) evaluated the effects of percutaneous transluminal angioplasty (PTA) on wound healing and reduction in pain with a procedural success rate of 84·2%; 23 (85·2%) patients were re‐occluded within 1 year, but complete or partial wound healing occurred in 80% and rest pain improvement in 57%; overall limb salvage was 74%, avoiding amputation. These patients were poor surgical candidates and aged 80–89 years old. Although the vessels re‐occluded in 85·2% of the patients, the majority enjoyed improved QOL during this year following this minimally invasive procedure. For palliative care patients, treatment or care options can be guided by the severity of the ischaemia, overall health of the patient and patient wishes. If the ischaemia is mild to moderate, ambulation or medication may be effective treatments (9).
An additional adjunctive therapy with limited research evidence to encourage endogenous wound healing is recombinant human platelet‐derived growth factor (rhPDGF). This growth factor has been used for treatment of the diabetic foot ulcer to stimulate cell proliferation 39, 47. In another systematic review of RCT studies of diabetic foot ulcers, Eldor et al. (24) reported significant wound healing in less time with rhPDGF treatment, especially with Becaplermin in wounds less than 5 cm. This review recommended this alternative therapy to trial to avoid amputation when the usual treatments for diabetic ulcers—off loading, debridement, antibiotics, glycaemic control and revascularisation‐fail. However, for the end‐of‐life patient, it is important to assess the likelihood of having the basic substrates in the wound bed to mobilise before considering rhPDGF treatment.
Given that lower extremity amputations have a profound effect on QOL, Margolis et al. (106)designed a cohort study that estimated effectiveness of rhPDGF for wound healing and prevention of amputation. Patients were selected from wound care clinics that had diabetic neuropathic foot ulcers. Although there were limitations to the study because of inability to control certain variables such as wound characteristics, grade, compliance, specialty care and unknown covariates, comparisons within each quintile estimated significant positive results (95% CI) (106).
System approach to chronic wound management
In an effort to improve the practice of palliative medicine for recalcitrant wounds and improve QOL in patients, system wide approaches are taking place to institute research for formulating clinical protocols or standards of practice 44, 107. Clinicians would be guided by standards that contribute to healing, control symptoms and are cost effective (108).
Wound experts at the palliative care unit at Calvary Hospital in New York have carried out studies on recalcitrant wounds and effective interventions aimed at comfort which have included original medication compounds that are cost effective (9). These interventions target components of an original mnemonic for palliative chronic wound management: S‐P‐E‐C‐I‐A‐L (S = stabilising the wound; P = preventing new wounds; E = eliminate odour; C = control pain; I = infection prophylaxis; A = advanced, absorbent wound dressings; L = lessen dressing changes) (9). Patients are assessed by a multi‐disciplinary team of experts guided by this tool in determining care strategies consistent with the patient's wishes and the prognosis for wound improvement.
At a Chicago hospital with a palliative care unit, Ennis and Meneses (44) have published data conveying the growth in numbers of chronic wound cases from 1999 to 2004 that have posed clinical, economical and ethical challenges when healing was unlikely for debilitated patients. This hospital has a subacute wound unit for wounds with care delivery provided by a multi‐disciplinary team. A prospective management study was performed over 6 months duration on 108 patients with 133 wounds with intent to heal approach for total healing and for significant reduction in wound size defined by greater than 50% volume reduction (44). If the patient showed partial healing or more, this patient would transition across the continuum for a different level of care such as home health. Wounds healed or improved in 68·4%; 16·1% were determined to be non healing, and these patients were enrolled in the palliative care program for non healing end points for symptom control (44). The goal for these patients was a stable, non healing wound after confirmation with healing rate data to avoid being labelled ‘too difficult or costly to heal’ (44, p. 103). It was also important to confer with the patient and family to decide whether palliative care goals and objectives were appropriate to support QOL as defined by the patient and for realistic expectations (44). The authors are in the process of ‘identifying a predictive profile’ of patients that will not heal to provide guidelines for evidence‐based decision‐making and practice (44, p. 104).
CONCLUSION
For all wound types, the clinician should identify the patient's understanding of disease and the effect of the chronic wound on his/her life. It is also important to identify the impact on the family or caregiver and coping ability. Symptom distress involves assessing QOL domains: physical, psycho‐social, cultural and spiritual. Once symptoms are identified, the clinician involves the patient in the wound management that will achieve realistic goals that improve QOL. A multi‐disciplinary team approach is optimal for care planning and cost‐effectiveness in the context of disease aetiology, life expectancy and patient perspective. Using clinical indicators and validated risk tools, realistic expectations can be communicated for prevention and treatment strategies based on best practice guidelines for correcting underlying causes, stabilising the wound, closing the wound, or controlling distressful symptoms. Best practice guidelines should be viewed through the lens of the patient and family for optimal comfort and not necessarily for intent to heal. Further quality designed research is needed for evidence‐based chronic wound care that supports QOL and which encourages a partnership with the patient and family.
RECOMMENDATIONS FOR PALLIATIVE MANAGEMENT OF CHRONIC WOUNDS
Based upon the information reviewed from this literature synthesis, the following recommendations for palliative management of chronic wounds are suggested for care practice. The clinician is encouraged to rate the articles for level and quality of evidence (Figure 1) and to keep in mind that palliative care research is sparse, often based on case study examples. Finally, the clinician is encouraged to adopt a holistic approach to chronic wound care for optimal patient outcomes.
-
1
Identify patients at risk for skin breakdown using the Braden risk scale and institute the hospital prevention skin care protocol. Engage and educate the patient and family in wound prevention, wound stabilisation, care options and choices.
-
2
Correct the underlying cause of the tissue damage if possible.
-
3
Ensure adequate tissue perfusion.
-
4
Use the MEASURE mnemonic for wound bed preparation: tissue debridement, bacterial burden reduction and moisture balance.
-
5
Confer with patient and family to determine risks and benefits of various treatments in light of the overall goals and wishes to promote QOL. Refer to best practice guidelines for the type of wound for care planning.
-
6
Assess for clinical indicators for non healing wounds to guide patient‐centred discussions for goals of care in context of life expectancy, disease aetiology and patient wishes. Use the PUSH tool to assess wound healing.
-
7
Consider the aspects of best wound care practice that support the patient goals and focus the interventions on achieving outcomes in accordance with the patient's wishes to control distressful symptoms: pain, exudate, bleeding, infection, odour and dressing wear time and comfort that supports improved QOL. Assess and address the emotional aspects of living with a wound to help the patient and family cope. Use the CWIS to measure QOL with a wound.
-
8
Consider a 2‐ to 4‐week trial of ‘intent to heal’ and then discuss with the patient and family whether or not to pursue treatment or switch to S‐P‐E‐C‐I‐A‐L wound care if healing is unlikely and if in accordance with the patient's wishes.
-
9
Engage the patient and family in participation with the multi‐disciplinary care team through education, on‐going assessment and dressing choices for comfort, cost, wear time, appearance and wound characteristics. Provide psycho‐social and spiritual support and promote independence.
-
10
Recognise that comfort of the patient, especially at the end of life, may take precedence over ulcer prevention and should be discussed by the multi‐disciplinary team for goal reassessment and documented as supporting patient's wishes.
-
11
Skin breakdown is a visible sign to the patient that his/her health status has deteriorated. Offer hope and support that patient's comfort and care remain priority.
-
12
Encourage adequate nutrition or nutritional supplements as tolerated by the patient 5, 7, 9, 27, 42, 51, 52, 53.
-
13
Assess pain with VAS or FRS or FLACC (cognitively impaired) pain rating tools and provide adequate pain control. Pre‐medicate prior to dressing change. Assess for psycho‐social and cultural issues that impact pain measurement accuracy. Minimise and control pain during dressing removal and wound cleansing as defined previously. Consider non pharmacological pain interventions.
-
14
Refer to wound care experts for consult on recalcitrant wounds (44).
-
15
Consider a wound care clinic for uniform practice and system wide approach for palliative management of chronic wounds (44).
-
16
Support research in palliative care and end of life for standardised care practices that support QOL.
BEST PRACTICES AND PALLIATIVE CARE WOUND MANAGEMENT
Fungating wounds
Because there is a lack of standardised protocols based on research and evidence, treatment of fungating wounds is based on case studies or expert opinion. Below are listed common principles for palliative care of the fungating wound.
-
1
Cleanse wound gently with warm normal saline and keep wound moist.
-
2
Wound cares are directed to controlling the symptoms distressful to the patient with attention to comfort, anxiety, cosmetic appearance, dressing wear time and proper fit. Consider using the WoSSAC for patient self‐assessment of distressful symptoms. Anticipate bleeding for care strategies listed previously. Efforts are geared to stabilising the wound and preventing further deterioration. For other palliative measures to treat the underlying malignancy, discuss with the patient/family benefit versus burden at end of life.
-
3
Case studies promote the use of wound care products, such as polyurethane foam and non adhesive gelling foam dressings to reduce pain and handle leakage; activated charcoal dressings for malodour; and antimicrobial dressings with activated charcoal for infected, malodourous wounds for comfort. Consider topical metronidazole gel for odour control.
-
4
Other dressing brands to consider are ones that have a non adherent wound contact layer (soft silicone perforated sheet) for exudates to be absorbed and moisture evaporated from the second layer or alginates with a secondary retention layer of foam. Include the patient in choosing the product that is most comfortable with long wear time.
Diabetic wounds
Diabetic foot ulcers occur in 15% of diabetic patients and are a leading cause of amputations (24). Re‐occurrence rates are 8–59% (47). Best practices primarily include aetiology treatment, patient education for prevention, off loading, debridement of callous and necrotic tissue, glycaemic control, adequate nutrition, optimal tissue perfusion, infection control, wound bed preparation, appropriate dressings for moist environment, evaluation of arterial and venous status of the affected limb, surgery and adjunctive treatments such as platelet‐derived growth factors (PDGFs), NPWT, electrical stimulation, HBOT and revascularisation for ischaemic ulcers (47). Perform wound culture or tissue biopsy to identify infection for local or systemic antibiotic treatment. Treat if wound has bacterial load of 105 or more per gram of tissue or any tissue level of beta haemolytic streptococci 9, 38, 52, 53.
-
1
Educate patient on proper foot wear, lower extremity and foot inspection, glycaemic control, exercise, smoking cessation, weight management and adequate nutrition. Instruct on nutritional supplements if malnourished 24, 47, 109.
-
2
Check laboratory values as appropriate such as prealbumin, glucose, haemoglobin A1c, complete blood count, lipid profile, hepatic function profile, erythrocyte sedimentation rate, thyroid‐stimulating hormone level, urinary microalbumin, prothrombin time/international normalised ratio and basic metabolic panel 47, 109.
-
3
Assess for risks and clinical indicators of a non healing wound: failure to show reduction in ulcer size of 40% after 4 weeks and use of the SAD classification system. Re‐assess treatment strategy and goals of care with the patient (49).
-
4
Use a multi‐disciplinary specialist team approach for care (109).
-
5
Consider assessing QOL with use of the CWIS to minimise suffering and address distressful symptoms. Prevent amputation if possible.
-
6
Assess pain with a valid pain tool and manage pain for comfort. Consider gabapentin for neuropathic pain. Suggest capsaicin cream (0·25–0·075%) applied thinly three to four times daily to affected areas (110).
Venous ulcers
The primary treatment for uncomplicated venous leg ulcers (ankle–brachial oressure index of >0·8) is compression bandaging (53). Bandaging reduces hypertension in veins, reduces oedema, improves venous return and blood flow (111). Compression should be applied correctly by a trained practioner (53).
-
1
Assess aetiology, comfort and tolerance to determine level of compression 53, 54, 111.
-
2
Instruct to elevate legs if tolerated by patient (53).
-
3
Use MEASURE for wound bed preparation.
-
4
Treat and manage infection after debridement.
-
5
No specific dressing is recommended to use under compression bandaging, but dressing should be comfortable, stay in place and effective in managing the wound exudates. For venous ulcers with a high bioburden consider using silver or cadeoxomer iodine dressings to control bacterial levels.
-
6
Assess for non healing ulcer if no reduction in size after 4 weeks or use the Rule of 6: ulcer >6cm2in size, duration >6 months, and unlikely to heal with compression in 6 months (111). Re‐assess treatment plan and goals with patient.
-
7
Instruct on adequate nutrition and exercise if tolerated 53, 54.
-
8
Assess pain with a valid pain tool and manage pain for comfort as described previously with attention to minimising pain with dressing removal and cleansing. Consider non pharmacological modalities for pain control.
-
9
Consider prescribing oral pentoxifylline 400 mg three times daily to influence microcirculatory blood flow and tissue oxygenation to ischaemic tissues.
-
10
Consider using the CWIS to measure QOL and areas of patient distress for goals of care discussions.
Pressure ulcers
The most common ulcer for palliative care patients is the pressure ulcer that results from pressure that damages underlying tissue 9, 52. Total national cost of pressure ulcer treatment in the USA exceeds $1·33 billion (52). The best practice guidelines are from the NPUAP and the AHCPR 52, 56.
Follow hospital pressure ulcer prevention protocols but always consider the patient's definition of comfort such as customising position comfort or use of air mattress and other off loading devices. Consult physical therapy for immobility issues.
-
1
Use the Braden risk factors but consider also risk factors identified in the Hunters Hill tool for hospice patients relative to the underlying medical condition to appreciate the need for flexibility with care strategies. Reduce risk by identifying reversible causes of incontinence and/or control conditions short‐term with indwelling urine catheter if comfortable for the patient or stoma pouches for drainage to protect skin integrity 3, 9, 40, 52, 56.
-
2
Reduce friction and shear by using lift sheet, trapeze and head of bed (HOB) no more than 30 degrees unless there are medical contraindications such as difficulty breathing, mechanical ventilator pneumonia prevention, or aspiration precautions 9, 52, 59.
-
3
Provide frequent turning per hospital protocol (at least every 2 hours) to stay off existing pressure ulcers and to prevent new ulcers. Patient and family may opt against scheduled turning if not comfortable. Offer analgesic prior to turning and re‐evaluate support surface to maximise comfort. Keep linens dry and wrinkle free 3, 7, 9, 15, 27, 31, 40.
-
4
Provide good skin care, perineal cares and regular skin inspection. Use barrier cream and re‐apply after each incontinence episode or cleansing. Avoid use of adult diapers while in bed or leave open to air if possible. Change underpads promptly when soiled 3, 9, 56.
-
5
Recognise and prepare the patient and family that the skin fails also at end of life and wound closure may not be a realistic goal. Discuss with the patient as an interdisciplinary team realistic goals such as control of troublesome symptoms.
-
6
For palliative care patients that have pre‐existing pressure ulcers, discuss the benefits versus the burdens of an ‘intent to heal’ 2‐ to 4‐week treatment trial. If there is no progress towards healing during this time period, the patient, the ulcer, and the treatment plan should be re‐evaluated. Patient/family wishes will be respected for goals of care.
-
7
Assess for fears and address coping ability. As the skin fails, this may signal to the patient that his condition is worsening and facing his/her mortality. Be attentive and listen to validate feelings and provide support to patient and family.
-
8
Assess pain with a valid pain tool and treat to acceptable level of intensity identified by the patient. Practice gentle dressing removal and cleansing as outlined previously. Consider non pharmacological modalities for pain management. Reduce pressure over ulcer site.
-
9
For end‐of‐life patients or the frail elderly, prepare the patient and family that the swallowing reflex will weaken, which can result in poor nutrition or aspiration. Offer care conferences to discuss benefit versus burden of enteral nutrition. Provide oral nutritional supplements for the malnourished patient 9, 15, 27, 51, 52.
-
10
Use the MEASURE or SPECIAL mnemonic tools to assess the wound and target care strategies. The ideal dressing is comfortable, requires minimal changing, controls wound characteristics and provides moisture. Encourage the patient in choice of dressing for comfort.
-
11
May leave heel eschars alone if intact (3). For other sites, debridement as an option needs to be considered in the context of patient's wishes, goals and benefits 9, 52.
REFERENCES
- 1. National Consensus Project for Quality Palliative Care . Clinical practice guidelines for quality palliative care, 2nd edn, 2009. National Consensus Project, Pittsburgh, PA, USA. URL http://www.nationalconsensusproject.org/Guidelines_download.asp‐8k‐cached [accessed 29 June 2009].
- 2. The World Health Organization . The World Health Organization quality of life (WHOQOL)‐BREF. 2004, 1–5. URL http://www.who.int/substance_abuse/research/tools/whoqolbref/en [access ed 29 June 2009].
- 3. Institute for Clinical Systems Improvement . Health care order set: palliative care. November 2007. URL http://www.icsi.org [accessed 20 May 2009].
- 4. Alvarez OM, Meehan M, Ennis W, Thomas DR, Ferris FD, Kennedy KL, Rogers R, Bradley M, Baker JJ, Fernandez‐Obregon A, Rodeheaver G. Chronic wounds: palliative management for the frail population. Wounds 2002;14(8 Suppl): 4S–27S. [Google Scholar]
- 5. Brown G. Long‐term outcomes of full‐thickness pressure ulcers: healing and mortality. Ostomy Wound Manage 2003;49(10):42–50. [PubMed] [Google Scholar]
- 6. Galvin J. An audit of pressure ulcer incidence in a palliative care setting. Int J Palliat Nurs 2002;8(5):214–21. [DOI] [PubMed] [Google Scholar]
- 7. Henoch I, Gustafsson M. Pressure ulcers in palliative care: development of a hospice pressure ulcer risk assessment scale. Int J Palliat Nurs 2003;9(11):474–84. [DOI] [PubMed] [Google Scholar]
- 8. Lund‐Nielsen B, Müller K, Adamsen L. Malignant wounds in women with breast cancer: feminine and sexual perspectives. J Clin Nurs 2005;14(1):56–64. [DOI] [PubMed] [Google Scholar]
- 9. Alvarez OM, Kalinski C, Nusbaum J, Hernandez L, Pappous E, Kyriannis C, Parker R, Chrzanowski G, Comfort CP. Incorporating wound healing strategies to improve palliation (symptom management) in patients with chronic wounds. J Palliat Med 2007;10(5):1161–89. [DOI] [PubMed] [Google Scholar]
- 10. De Laat E, Scholte OP, Reimer WJ, Van Achterberg T. Pressure ulcers: diagnostics and interventions aimed at wound‐related complaints: a review of the literature. J Clin Nurs 2005;14(4):464–72. [DOI] [PubMed] [Google Scholar]
- 11. Grocott P. Care of patients with fungating malignant wounds. Nurs Stand 2007;21(24): 57–66. [PubMed] [Google Scholar]
- 12. Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients' quality of life. Health Qual Life Outcomes 2007;5(4):44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones JE, Robinson J, Barr W, Carlisle C. Impact of exudate and odour from chronic venous leg ulceration. Nurs Stand 2008;22(45):53. [DOI] [PubMed] [Google Scholar]
- 14. Langemo DK. When the goal is palliative care. Adv Skin Wound Care 2006;19(3):148–54. [DOI] [PubMed] [Google Scholar]
- 15. McDonald A, Lesage P. Palliative management of pressure ulcers and malignant wounds in patients with advanced illness. J Palliat Med 2006;9(2):285–95. [DOI] [PubMed] [Google Scholar]
- 16. Naylor, W. A guide to wound management in palliative care. Int J Palliat Nurs 2005;11(11): 572–9. [DOI] [PubMed] [Google Scholar]
- 17. Persoon A, Heinen MM, Van Der Vleuten CJM, De Rooij MJ, van de Kerkhof PCM, van Achterberg T. Leg ulcers: a review of their impact on daily life. J Clin Nurs 2004;13(3): 341–54. [DOI] [PubMed] [Google Scholar]
- 18. Reddy M, Kohr R, Queen D, Keast D, Sibbald RG. Practical treatment of wound pain and trauma: a patient‐centered approach. an overview [corrected] [published erratum appears in OSTOMY WOUND MANAGE 2003 May; 49(5):8]. Ostomy Wound Manage 2003;49(4):2. [PubMed] [Google Scholar]
- 19. Spilsbury K, Nelson A, Cullum N, Iglesias C, Nixon J, Mason S. Pressure ulcers and their treatment and effects on quality of life: hospital inpatient perspectives. J Adv Nurs 2007;57(5):494–504. [DOI] [PubMed] [Google Scholar]
- 20. Walshe C. Living with a venous leg ulcer: a descriptive study of patients' experiences. J Adv Nurs 1995;22(6):1092–100. [DOI] [PubMed] [Google Scholar]
- 21. White R, Morris C. Mepitel: a non‐adherent wound dressing with safetac technology. Br J Nurs 2009;18(1):58–64. [DOI] [PubMed] [Google Scholar]
- 22. Enoch S, Price P. Should alternative endpoints be considered to evaluate outcomes in chronic recalcitrant wounds? WORLD WIDE WOUNDS 2004. [WWW document]. URL http://www.worldwidewounds.com/2004/october/Enoch‐Part2/Alternative‐Enpoints‐To‐Healing.html [accessed 7 March 2009].
- 23. Jones KR, Fennie K, Lenihan A. Chronic wounds: factors influencing healing within 3 months and nonhealing after 5‐6 months of care. Wounds 2007;19(3):51–63. [PubMed] [Google Scholar]
- 24. Eldor R, Raz I, Ben Yehuda A, Boulton AJM. New and experimental approaches to treatment of diabetic foot ulcers: a comprehensive review of emerging treatment strategies. Diabet Med 2004;21(11):1161–73. [DOI] [PubMed] [Google Scholar]
- 25. European Wound Management Association . Position document: hard‐to‐heal wounds: a holistic approach. London: MEP Ltd, 2008. [Google Scholar]
- 26. Moffatt CJ, McCullagh L, O’Connor T, Doherty DC, Hourican C, Stevens J, Mole T, Franks PJ. Randomized trial of four‐layer and two‐layer bandage systems in the management of chronic venous ulceration. Wound Repair Regen 2003;11(3):166–71. [DOI] [PubMed] [Google Scholar]
- 27. Whitney J, Phillips L, Aslam R, Barbul A, Gottrup F, Gould L, Robson MC, Rodeheaver G, Thomas D, Stotts N. Guidelines for the treatment of pressure ulcers. Wound Repair Regen 2006;14(6):663–79. [DOI] [PubMed] [Google Scholar]
- 28. Anand SC, Dean C, Nettleton R, Praburaj DV. Health‐related quality of life tools for venous‐ulcerated patients. Br J Nurs 2003;12(1): 48–59. [DOI] [PubMed] [Google Scholar]
- 29. Harding KG, Moore K, Phillips TJ. Wound chronicity and fibroblast senescence–implications for treatment. Int Wound J 2005;2(4):364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaufman MW. The WOC nurse: economic, quality of life, and legal benefits. Dermatol Nur 2001;13(3):215–9. [PubMed] [Google Scholar]
- 31. Chaplin J. Wound management in palliative care. Nurs Stand 2004;19(1):39–42. [DOI] [PubMed] [Google Scholar]
- 32. Hollinworth H. Sharing the burden: the complex practice of wound care in the community. Br J Community Nurs 2004;9(1):5–10. [DOI] [PubMed] [Google Scholar]
- 33. Price P, Harding K. Cardiff wound impact schedule: the development of a condition‐specific questionaire to assess health‐related quality of life in patients with chronic wounds of the lower limb. Int Wound J 2004;1(1):10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jocham HR, Dassen T, Widdershoven G, Halfens R. Quality of life in palliative care cancer patients: a literature review. J Clin Nurs 2006;15(9):1188–95. [DOI] [PubMed] [Google Scholar]
- 35. The World Health Organization . WHO definition of palliative care. 2009a. URL http://www.who.int/cancer/palliative/en [accessed 13 May 2009].
- 36. Ferrans CE, Powers MJ. Psychometric assessment of the quality of life index. Res Nurs Health 1992;15(1):29–38. [DOI] [PubMed] [Google Scholar]
- 37. Mudge EJ, Meaume S, Woo K, Sibbald RG, Price PE. Patients' experience of wound‐related pain: an international perspective. EWMA J 2008;8(2):19. [Google Scholar]
- 38. Fonder M, Lazarus G, Cowan D, Aronson‐Cook B, Kohli A, Mamelak A. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008;58:185–206. [DOI] [PubMed] [Google Scholar]
- 39. Schultz GS, Barillo DJ, Mozingo DW, Chin GA. Wound bed preparation and a brief history of TIME. Int Wound J 2004;1(1):19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chaplin J. Pressure sore risk assessment in palliative care. J Tissue Viability 2000;10(1):27–3. [DOI] [PubMed] [Google Scholar]
- 41. Jørgensen B, Friis GJ, Gottrup F. Pain and quality of life for patients with venous leg ulcers: proof of concept of the efficacy of biatain‐ibu, a new pain reducing wound dressing. Wound Repair Regen 2006;14(3):233–9. [DOI] [PubMed] [Google Scholar]
- 42. Soriano LF, Vázquez M, Maristany CP, Graupera J, Wouters‐Wesseling W, Wagenaar L. The effectiveness of oral nutritional supplementation in the healing of pressure ulcers. J Wound Care 2004;13(8):319–22. [DOI] [PubMed] [Google Scholar]
- 43. Stephens P, Wall IB, Wilson MJ, Hill KE, Davies CE, Hill CM, Harding KG, Thomas DW. Anaerobic cocci populating the deep tissues of chronic wounds impair cellular wound healing responses in vitro. Br J Dermatol 2003;148(3):456–66. [DOI] [PubMed] [Google Scholar]
- 44. Ennis W, Meneses P. Palliative care and wound care: 2 emerging fields with similar needs for outcome data. Wounds 2005;17(4):99–104. [Google Scholar]
- 45. Keast DH, Bowering CK, Evans AW, MacKean GL, Burrows C, D’Souza L. MEASURE: a proposed assessment framework for developing best practice recommendations for wound assessment. Wound Repair Regen 2004;12(3):S1–17S. [DOI] [PubMed] [Google Scholar]
- 46. Sheehan P, Jones P, Giurini JM, Caselli A, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Plast Reconstr Surg 2003;117(7):239S–44S. [DOI] [PubMed] [Google Scholar]
- 47. Steed DL, Attinger C, Colaizzi T, Crossland M, Franz M, Harkless L, Johnson A, Moosa H, Robson M, Serena T, Sheehan P, Veves A, Wiersma‐Bryant L. Guidelines for the treatment of diabetic ulcers. Wound Repair Regen 2006;14(6):680–92. [DOI] [PubMed] [Google Scholar]
- 48. Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers:tThe association of wound size, wound duration, and wound grade on healing. Diabetes Care 2002;25(10):1835–9. [DOI] [PubMed] [Google Scholar]
- 49. Treece KA, Macfarlane RM, Pound N, Game FL, Jeffcoate WJ. Validation of a system of foot ulcer classification in diabetes mellitus. Diabet Med 2004;21(9):987–91. [DOI] [PubMed] [Google Scholar]
- 50. Van Rijswijk L. Full‐thickness pressure ulcers: patient and wound healing characteristics. Decubitus 1993;6(1):16–21. [PubMed] [Google Scholar]
- 51. Van Rijswijk, L. & Polansky, M. Predictors of time to healing deep pressure ulcers. Ostomy Wound Manage 1994;40(8):40–51. [PubMed] [Google Scholar]
- 52. Agency for Healthcare Policy and Research . Pressure ulcer treatment quick reference guide for clinician. Washington, D.C.: Government Printing Office, 1994. Report No.: AHCPR95‐0653. [Google Scholar]
- 53. Registered Nurses’Association of Ontario . Nursing best practice guidelines: assessment and management of venous leg ulcers, 2002:1–8. URL http://www.rnao.org/page.asp?/pageId=924& [accessed 19 April 2009].
- 54. Robson MC, Cooper DM, Aslam R, Gould LJ, Harding KG, Margolis DJ, Ochs DE, Serena TE, Snyder RJ, Steed DL, Thomas DR, Wiersma‐Bryant L. Guidelines for the treatment of venous ulcers. Wound Repair Regen 2006;14(6): 649–62. [DOI] [PubMed] [Google Scholar]
- 55. Langemo DK, Brown G. Skin fails too: acute, chronic, and end‐stage skin failure. Adv Skin Wound Care 2006;19(4):206–11. [DOI] [PubMed] [Google Scholar]
- 56. National Pressure Ulcer Advisory Panel . Pressure ulcer prevention points. Washington, D.C.: National Pressure Ulcer Advisory Panel, 2007. Report No.: Revised 2007. URL http://www.npuap.org [accessed 19 April 2009]. [Google Scholar]
- 57. Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11(1 Suppl): S1–28S. [DOI] [PubMed] [Google Scholar]
- 58. Pancorbo‐Hidalgo P, Garcia‐Fernandez FP, Lopez‐Medina IM, Alvarez‐Nieto C. Risk assessment scales for pressure ulcer prevention: a systematic review. J Adv Nurs 2006;54(1):94–110. [DOI] [PubMed] [Google Scholar]
- 59. Findlay D. Practical management of pressure ulcers. Am Fam Physician 1996;54(5):1519–28. [PubMed] [Google Scholar]
- 60. Bergstrom N, Braden B, Kemp M, Champagne M, Ruby E. Predicting pressure ulcer risk: a multisite study of the predictive validity of the braden scale. Nurs Res 1998;47(5):261–9. [DOI] [PubMed] [Google Scholar]
- 61. European Wound Management Association . Position document: wound bed preparation in practice. London: MEP Ltd, 2004:1–17. [Google Scholar]
- 62. Kalinski C, Schnepf M, Laboy D, Hernandez L, Nusbaum J, McGrinder B, Comfort C, Alvarez OM. Effectiveness of a topical formulation containing metronidazole for wound odor and exudate control. Wounds 2005;17(4):84–90. [Google Scholar]
- 63. Gardner SE, Frantz RA, Bergquist S, Shin CD. A prospective study of the pressure ulcer scale for healing (PUSH). J Gerontol A Biol Sci Med Sci 2005;60A(1):93–7. [DOI] [PubMed] [Google Scholar]
- 64. European Pressure Ulcer Advisory Panel . Pressure ulcer prevention guidelines. EPUAP, Oxford, UK 1998;1‐4[EPUAP document] URL http://www.epuap.org/glprevention.html [accessed 7 March 2009]. [Google Scholar]
- 65. Grocott P. Developing a tool for researching fungating wounds. World Wide Wounds 2001(July): 1–17 [WWW document]. URL http://www.worldwidewounds.com/2001/july/Grocott/Fungating‐Wounds.html [accessed 14 March 2009].
- 66. Naylor W. Part 2: symptom self‐assessment in the management of fungating wounds.[World Wide Wounds Document] 2002(July): 1–13.URL http://www.worldwidewounds.com/2002/july/Naylor‐Part2/Wound‐Assessment‐Tool.html [accessed 14 March 2009].
- 67. Browne N, Grocott P, Cowley S, Cameron J, Dealey C, Keogh A, Lovatt A, Vowden K, Vowden P. Woundcare research for appropriate products (WRAP): validation of the TELER method involving users. Int J Nurs Stud 2004;41(5):559–71. [DOI] [PubMed] [Google Scholar]
- 68. Szor JK, Bourguignon C. Description of pressure ulcer pain at rest and at dressing change. J WOCN 1999;26(3):115–20. [DOI] [PubMed] [Google Scholar]
- 69. Evans E, Gray M. Do topical analgesics reduce pain associated with wound dressing changes or debridement of chronic wounds? J Wound Ostomy Continence Nurs 2005;32(5):287–90. [DOI] [PubMed] [Google Scholar]
- 70. Krasner D. The chronic wound pain experience: a conceptual model. Ostomy Wound Manage 1995;41(3):20–5. [PubMed] [Google Scholar]
- 71. Price P, Fogh K, Glynn C, Krasner DL, Osterbrink J, Sibbald RG. Managing painful chronic wounds: the wound pain management model. Int Wound J 2007;(4 Suppl)1:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Malviya S, Voepel‐Lewis T, Burke C, Merkel S, Tait AR. The revised FLACC observational pain tool: improved reliability and validity for pain assessment in children with cognitive impairment. Paediatr Anaesth 2006;16(3):258–65. [DOI] [PubMed] [Google Scholar]
- 73. Roth RS, Lowery JC, Hamill JB. Assessing persistent pain and its relation to affective distress, depressive symptoms, and pain catastrophizing in patients with chronic wounds: a pilot study. Am J Phys Med Rehabil 2004;83(11):827–34. [DOI] [PubMed] [Google Scholar]
- 74. European Wound Management Association . Position document: pain at wound dressing changes. London: MEP Ltd, 2002, 1–15. [Google Scholar]
- 75. Briggs M, Nelson EA. Topical agents or dressings for pain in venous leg ulcers (Review). Cochrane Database Syst Rev 2003;(1):1–20. [DOI] [PubMed] [Google Scholar]
- 76. Queen D, Woo K, Schulz VN, Sibbald RG. Chronic wound pain and palliative cancer care. Ostomy Wound Manage 2003;49(10):16–8. [PubMed] [Google Scholar]
- 77. World Unionof Wound Healing Societies (WUWHS) . Principles of best practice: wound exudate and the role of dressings. A consensus document. London: MEP Ltd, 2007:1–10. [Google Scholar]
- 78. Schuren J, Becker A, Gary Sibbald R. A liquid film‐forming acrylate for peri‐wound protection: a systematic review and meta‐analysis (3M cavilon no‐sting barrier film). Int Wound J 2005;2(3):230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Draper C. The management of malodour and exudate in fungating wounds. Br J Nurs 2005;14(11):S4–12S. [DOI] [PubMed] [Google Scholar]
- 80. Daniels S, Sibbald RG, Ennis W, Eager CA. Evaluation of a new composite dressing for the management of chronic leg ulcer wounds. J Wound Care 2002;11(8):290–4. [DOI] [PubMed] [Google Scholar]
- 81. Palfreyman S, Nelson EA, Michaels JA. Dressings for venous leg ulcers: systematic review and meta‐analysis. BMJ 2007; 335(244):1–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hollinworth H. The management of patients' pain in wound care. Nurs Stand 2005;20(7):65. [DOI] [PubMed] [Google Scholar]
- 83. Medical Education Partnership Ltd . Wound infection in clinical practice: an international consensus. Int Wound J 2008;5(3):1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Williams RL. Cadexomer iodine: an effective palliative dressing in chronic critical limb ischemia. Wounds 2009;21(1):15–28. [PubMed] [Google Scholar]
- 85. Williams D, Enoch S, Miller D, Harris K, Price P, Harding KG. Effect of sharp debridement using curette on recalcitrant nonhealing venous leg ulcers: a concurrently controlled, prospective cohort study. Wound Repair Regen 2005;13:131–7. [DOI] [PubMed] [Google Scholar]
- 86. Heinen MM, Van Achterberg T, Reimer W, Van De Kerkhof PCM, De Laat E. Venous leg ulcer patients: a review of the literature on lifestyle and pain‐related interventions. J Clin Nurs 2004;13(3):355–66. [DOI] [PubMed] [Google Scholar]
- 87. Vernassiere C, Cornet C, Trechot P, Alla F, Truchetet F, Cuny JF, Commun N, Granel Brocard F, Barbaud A, Schmutz JL. Study to determine the efficacy of topical morphine on painful chronic skin ulcers. J Wound Care 2005;14(6):289–93. [DOI] [PubMed] [Google Scholar]
- 88. Zeppetella G, Paul J, Ribeiro MDC. Analgesic efficacy of morphine applied topically to painful ulcers. J Pain Symptom Manage 2003;25(6):555–8. [DOI] [PubMed] [Google Scholar]
- 89. The World Health Organization . The world health organization pain ladder 2009b. URL http://www.who.int/cancer/palliative/painladder/en/index.html [accessed 29 June 2009].
- 90. Langemo D, Anderson J, Hanson D, Thompson P, Hunter S. Wound and skin care: understanding palliative wound care. Nurs 2007;37(1):65–6. [DOI] [PubMed] [Google Scholar]
- 91. Bale S, Tebble N, Price P. A topical metronidazole gel used to treat malodorous wounds. Br J Nurs 2004;13(11):S4. [DOI] [PubMed] [Google Scholar]
- 92. Vollstedt C. Symptom management: management of malignant malodorous wounds. J Hosp Palliat Nurs 2000;2(1):28–30. [Google Scholar]
- 93. National Pressure Ulcer Advisory Panel . NPUAP PUSH tool. Washington, D.C.: National Pressure Ulcer Advisory Panel, 1998. URL http://www.npuap.org/push3‐0htm [accessed 19 April 2009]. [Google Scholar]
- 94. Bates‐Jensen BM, Vredevoe DL, Brecht ML. Validity and reliability of the pressure sore status tool. Decubitus 1992;5(6):20–8. [PubMed] [Google Scholar]
- 95. Adderley UJ, Smith R. Topical agents and dressings for fungating wounds (review). Cochrane Database Syst Rev 2007;2:1–15. [DOI] [PubMed] [Google Scholar]
- 96. O’Meara S., Al‐Kurdi D, Ovington L. Antibiotics and antiiseptics for venous leg ulcers. Cochrane Database Syst Rev abstract 2008(1):1–3. [DOI] [PubMed] [Google Scholar]
- 97. Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum‐assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care 2008;31(4):631–6. [DOI] [PubMed] [Google Scholar]
- 98. Ubbink DT, Westerbos SJ, Evans D, Land L, Vermeulen H. Topical negative pressure for treating chronic wounds (Review). Cochrane Database Syst Rev 2008(3):1–32. [DOI] [PubMed] [Google Scholar]
- 99. Vermeulen H, Van Hattem JM, Storm‐Versloot MN, Ubbink DT. Topical silver for treating infected wounds. Cochrane Database Syst Rev 2007(1):1–43. [DOI] [PubMed] [Google Scholar]
- 100. Bergin S, Wraight, P. Silver based wound dressings and topical agents for treating diabetic foot ulcers (Review). Cochrane Database Syst Rev 2006(1):1–17. [DOI] [PubMed] [Google Scholar]
- 101. Jull AB, Rodgers A, Walker N. Honey as a topical treatment for wounds. Cochrane Database Syst Rev 2008(4):1–47. [DOI] [PubMed] [Google Scholar]
- 102. Jull AB, Arroll B, Parag V, Waters J. Pentoxifylline for treating venous leg ulcers. Cochrane Database Syst Rev 2007(3):1–49. [DOI] [PubMed] [Google Scholar]
- 103. Kranke P, Bennett MH, Debus SE, Roeckl‐Wiedmann I, Schnabel A. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev 2004(1):1–37. [DOI] [PubMed] [Google Scholar]
- 104. Al‐Kurdi D, Syer S‐B, Flemming K. Therapeutic ultrasound for venous leg ulcers (Review). Cochrane Database Syst Rev 2008;1:1–26. [DOI] [PubMed] [Google Scholar]
- 105. Amato B, Iuliano GP, Markabauoi AK, Piscitelli V, Masone S, Compagna R, Esposito G, Piscione F. Endovascular procedures in critical leg ischemia of elderly patients. Acta Biomed 2005;1(76 Suppl): 11–5. [PubMed] [Google Scholar]
- 106. Margolis DJ, Bartus C, Hoffstad O, Malay S, Berlin JA. Effectiveness of recombinant human platelet‐derived growth factor for the treatment of diabetic neuropathic foot ulcers. Wound Repair Regen 2005;13(6):531–6. [DOI] [PubMed] [Google Scholar]
- 107. Hughes RG, Bakos AD, O’Mara A, Kovner CT. Palliative wound care at the end of life. Home Health Care Management & Practice 2005;17(3):196–202. [Google Scholar]
- 108. Keen D, James J. A tool to aid nurses' decision making in relation to dressing selection. Br J Nurs 2004;13(15):S6. [DOI] [PubMed] [Google Scholar]
- 109. Brem H, Sheehan P, Rosenberg HJ, Schneider JS, Boulton AJ. Evidence‐based protocol for diabetic foot ulcers. Plast Reconstr Surg 2006; 117(7):193S. [DOI] [PubMed] [Google Scholar]
- 110. Wound, Ostomy, and Continence Nurses Society . Guideline for management of wounds in patients with lower‐extremity neuropathic disease, Vol. 3 (WOCN clinical practice guideline), WOCN: 2004, 1–6. URL http://www.guideline.gov [accessed 08 March 2009]. [Google Scholar]
- 111. Smith & Nephew Ltd ., Dublin, Ireland. Grace P. (Eds). Leg ulcer guidelines: a pocket guide for practice, Vol. 39, 2006, 1–13. URL http://www.guideline.gov [accessed 08 March 2009]. [Google Scholar]
- 112. Naylor W. Using a new foam dressing in the care of fungating wounds. Br J Nurs 2001; (supplement) 10(6):524–530. [DOI] [PubMed] [Google Scholar]


