Abstract
Debridement of devitalised tissue is an essential component of the effective treatment of chronic wounds. The Versajet™ Hydrosurgery System is a new technology that simultaneously cuts and aspirates soft tissue. In this study we compared Versajet with conventional surgical techniques in the debridement of lower extremity ulcers to assess impact on time and resources for debridement. Forty‐one patients with a mean age of 68 years (range 33 to 95 years) underwent surgical debridement of a lower extremity ulcer. Operating room (OR) sessions were randomised to Versajet (n= 22) or conventional debridement (n= 19) with scalpel plus pulsed lavage. Procedure time and utilisation of consumables were recorded. Wound areas were monitored for 12 weeks. There was significant evidence (P < 0·008) of a shorter debridement time (10·8 min) using Versajet over conventional debridement (17·7 min); a mean saving of 6·9 minutes (39%). In addition, a significant reduction in use of pulsed lavage and saline (P < 0·001) was observed with Versajet. Overall, clinical efficacy of the shorter debridement procedure was similar: median time to wound closure 71 days (Versajet) vs. 74 days (conventional) (P= 0·733). We found Versajet to be quicker than conventional debridement in the debridement of lower extremity ulcers without compromising wound healing. Potential cost savings were identified from the use of VERSAJET through the shorter debridement time allowing more patients to be treated in the same operating schedule.
Keywords: Debridement, hydrosurgery, lower extremity ulcer, Versajet
Introduction
Debridement of devitalised, bacterially contaminated or senescent cells is an essential part of the treatment of wounds which are slow to heal (1). Schultz et al. 2, 3, 4 have reviewed the literature which identifies four components that should be addressed in order to maximise wound healing: T (tissue); I (Infection); M (moisture) and E (edge) ‐ the TIME principle of wound healing. Debridement has a role in three of these components through the removal of necrotic tissue (T), removal of infection or contamination (I), and treatment of non‐advancing or undermined wound edges (E). While passive autolytic or enzymatic debridement procedures are appropriate for patients who are unable to tolerate surgery, surgical debridement is able to rapidly remove necrotic, contaminated tissue and cellular debris and can offer many advantages in returning a wound to a healing trajectory 1, 5
Surgical debridement procedures have conventionally been performed with scalpels and other sharp instruments; however alternative techniques are now available. The Versajet™ Hydrosurgery System is a recent development in which a high‐pressure jet‐stream of sterile normal saline is pumped to a disposable hand‐held cutting/aspirating tool (Figure 1). Within the lower surface of the handpiece, the high velocity saline jet crosses an open chamber and cuts the tissue which is drawn up into the chamber due to the partial vacuum created by the saline jet (Venturi effect). Excess saline and tissue debris is evacuated away from the cutting field and pumped to waste.
Figure 1.
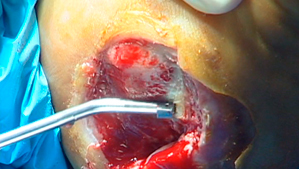
VERSAJET™ Hydrosurgery system in use. The VERSAJET (45° 14mm handpiece) in use on a lower extremity ulcer. The cutting chamber (14 mm) and the evacuation tube are on the lower surface of the instrument. The powered console and the hoses supplying high pressure fluid and removing fluid and waste are not visible.
This highly effective cutting tool leaves a clean, dry surgical field while removing minimum tissue volumes. Cutting efficiency is controlled through downward hand pressure, angle of orientation and saline velocity. Although the clinical efficacy of the Versajet system has been demonstrated, potential users will also be interested as to how the new technology compares in terms of the cost and time required to achieve effective debridement.
The purpose of this study was to measure the time required for wound debridement and major equipment used during a single debridement procedure on a range of lower extremity ulcers using either the Versajet system or conventional surgical debridement.
Methods
Clinical procedure
All surgeries took place at Clara Maass Medical Center during study operating room (OR) sessions (defined as all surgeries performed by the primary author on the same day). Full thickness debridement with sharp excision of fibronecrotic tissue was performed. For the majority of patients this involved the debridement of a single study wound, however 3 patients had bilateral wounds. While these bilateral patients underwent debridement of both wounds, only the largest wound was included in the study. Each OR session was prospectively randomised to debridement with VERSAJET™ Hydrosurgery System (Smith & Nephew Inc, Largo, USA) or conventional debridement, involving scalpel plus pulse lavage (Interpulse Powered Lavage System: Stryker Instruments, Kalamazoo, MI, USA). The randomisation schedule was concealed throughout the trial. Treatment allocation was revealed in writing to the primary author, two days prior to surgery. A Zimmer sagittal saw and Kirschner wire driver were used as required in either treatment group to remove bone and fenestrate bone cortex respectively. The end‐point of debridement was defined as the point when the margins of the debrided wound reached pin‐point bleeding. Wound debridement time was measured in 5 minute blocks and the volume of saline used with either Versajet or pulse lavage was recorded. Total wound areas were traced before surgery by means of transparent films laid onto the wound. Areas of black and yellow tissue were identified and the areas estimated by visual observation.
Prior to entry into the study, written consent and HIPAA authorisation was obtained for all patients. The Institutional Review Board approved the study. The primary author was responsible for patient recruitment and trial assessments. For the purposes of this study patients with clinical signs of infection in the study ulcer (e.g. erythema of surrounding skin, purulence, odour), together with those suffering from bacterial, viral or fungal skin diseases that may have interfered with wound healing, were excluded. Following surgical debridement, patients in both groups were treated similarly using absorbent dressings in a mixture of inpatient and outpatient settings. Venous leg ulcer patients additionally received Coban™ 2 Layer Compression System (3M™, Minnesota, USA). Diabetic foot ulcer patients received pressure redistribution as appropriate. The use of topical anti‐microbials was determined by clinical presentation at the time of dressing changes. Wound healing progress was documented by wound area tracing using transparent film and subsequent planimetry to calculate the surface area. All patients were followed for 12 weeks during the post‐debridement period to assess clinical outcomes.
Statistical analysis
For the primary analysis, a multiple regression model with covariate terms included for ulcer type, area of black necrotic tissue, area of yellow tissue and depth of ulcer was fitted to assess the difference in the wound debridement time between the two treatment groups. Treatment by covariate interactions were also individually assessed. Exact probability values were calculate and are reported in the text.
A Wilcoxon rank‐sum test was used to test for a difference in saline usage between the treatment groups. An accelerated failure time (AFT) model (6) was used to test for a difference in time to wound closure between treatment groups, with covariate terms included for ulcer type, area of black necrotic tissue, area of yellow tissue and ulcer duration. A further AFT model was fitted with a covariate term included for ulcer area. A Kaplan‐Meier analysis of the probability of wound closure over time for each treatment was also plotted.
Results
Patient characteristics
Forty‐one patients, 19 in the conventional debridement group and 22 in the Versajet group, underwent the first surgical debridement of a lower extremity ulcer (54% diabetic foot ulcers, 44% venous stasis ulcers). The patient and wound characteristics at baseline are shown in Table 1. The study ulcers were relatively small and fresh. At baseline, the median ulcer duration was 1·2 months in both groups (range 0·3 to 24·4 months and 0·4 to 36·1 months in the Versajet and conventional groups respectively). Ulcers in the Versajet group had a median surface area of 5·9 cm2 (range 0·5 to 62·4 cm2) and median area of devitalised tissue (black and yellow) of 5·3 cm2 (range 0·0 to 62·4 cm2). Ulcers in the conventional group had a median surface area of 3·9 cm2 (range 1·5 to 68·6 cm2) and a median area of devitalised tissue of 3·7 cm2 (range 1·3 to 68·6 cm2). The median area of black necrotic tissue in both groups was low; 0·2 cm2 (range 0·0 to 59·3 cm2) for ulcers in the Versajet group, 0·0 cm2 (range 0·0 to 18·3 cm2) in the conventional debridement group. Overall, there were no significant differences detected between the baseline characteristics of the two treatment groups.
Table 1.
Patient and wound characteristics
| Versajet | Conventional | Total | |
|---|---|---|---|
| Sex | |||
| Male | 15 (78·9%) | 11 (50%) | 26 (63·4%) |
| Age (Years) | |||
| Mean (Range) | 68·5 (37·0 – 95·0) | 67·6 (33·0 – 91·0) | 68·0 (33·0 – 95·0) |
| Ulcer type | |||
| Venous stasis | 7 (36·8%) | 11 (50%) | 18 (43·9%) |
| Diabetic foot | 11 (57·9%) | 11 (50%) | 22 (53·7%) |
| Decubitus | 1 (5·3%) | 0 | 1 (2·4%) |
| Ulcer location | |||
| Ankle | 8 (42·1%) | 10 (45·5%) | 18 (43·9%) |
| Foot | 10 (52·6%) | 11 (50%) | 21 (51·2%) |
| Leg | 0 | 1 (4·5%) | 1 (2·4%) |
| Ankle & Foot | 1 (5·3%) | 0 | 1 (2·4%) |
| Duration of Ulcer up to first debridement (months) | |||
| Median (Range) | 1·2 (0·3 – 24·4) | 1·2 (0·4 – 36·1) | 1·2 (0·3 – 36·1) |
| Area of ulcer (cm2) | |||
| Median (Range) | 5·9 (0·5 – 62·4) | 3·9 (1·5 – 68·6) | 4·3 (0·5 – 68·6) |
| Depth of ulcer (mm) | |||
| Median (Range) | 4·0 (1·0 – 35·0) | 6·5 (2·0 – 40·0) | 5·0 (1·0 – 40·0) |
| Area of black necrotic tissue (cm2) | |||
| Median (Range) | 0·2 (0·0 – 59·3) | 0·0 (0·0 – 18·3) | 0·1 (0·0 – 59·3) |
| Area of yellow tissue (cm2) | |||
| Median (Range) | 3·5 (0·0 – 17·0) | 2·5 (0·4 – 65·2) | 2·5 (0·0 – 65·2) |
| Area of devitalised (black + yellow) tissue (cm2) | |||
| Median (Range) | 5·3 (0·0 – 62·4) | 3·7 (1·3 – 68·6) | 4·0 (0·0 – 68·6) |
| N | 19 | 22 | 41 |
Wound debridement time
The time taken to debride the wounds until they were considered clean enough to allow treatment by absorbent dressings was measured in the operating room. The mean wound debridement time for Versajet patients was 10·8 minutes compared to 17·7 minutes for patients in the conventional debridement group (Table 2). Debridement using Versajet was significantly quicker (P= 0·008) than by conventional surgical methods, with a mean saving of 6·9 minutes (39%) per procedure.
Table 2.
Analysis of times of first surgical debridement
| Parameter | 95% confidence interval | |||
|---|---|---|---|---|
| P‐value | Difference | Lower | Upper | |
| Conventional ‐ Versajet | 0·008 | 6·9 | 1·9 | 11·91 |
| Adjusted means | |||
|---|---|---|---|
| Time (Min) | Lower | Upper | |
| Conventional | 17·7 | 11·5 | 23·8 |
| Versajet | 10·8 | 5·3 | 16·2 |
There was marginal statistical evidence (P= 0·098) that the difference between the treatment groups in wound debridement time depended on the area of black necrotic tissue. This is illustrated in Figure 2, which shows that the mean saving in the wound debridement time for Versajet was greater for ulcers with black necrotic tissue (10·6 minutes) than for ulcers without black necrotic tissue (5·5 minutes).
Figure 2.
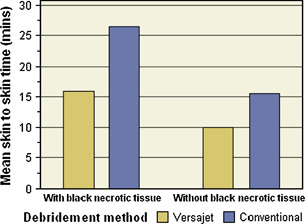
Wound debridement times of first surgical debridement. The debridement times (skin to skin) were recorded in surgical debridement procedures that were randomised to either conventional scalpels and pulsed lavage or VERSAJET as described in Materials and Methods. The data from wounds were subsequently analysed in accordance with whether or not they contained black necrotic tissue. There was marginal statistical evidence (P= 0·098) that the difference between the treatment groups in wound debridement time depended on the area of black necrotic tissue.
Instrument sets and saline
Table 3 summarises the instrument sets used during a debridement procedure for each treatment group. In the conventional group a debridement tray comprising 100 instruments was opened for each patient. Additionally, an Interpulse device was used to irrigate the wound and to remove cellular debris after conventional surgical excision.
Table 3.
Instrument sets and saline used in Versajet and conventional debridement
| Instrument sets used | Versajet | Conventional | P‐value |
|---|---|---|---|
| Debridement trays | 0 | 22 (100%) | |
| Interpulse | 0 | 22 (100%) | |
| Jetpack | 19 (100%) | 0 | |
| Zimmer sagittal saws | 4 (21·1%) | 5 (22·7%) | |
| Kischner wire drivers | 15 (78·9%) | 20 (90·9%) | |
| Electrocautery | 0 | 1 (4·5%) | |
| Volume of Saline used (ml) | |||
| Mean | 431·6 | 3000·00 | |
| Median | 300 | 3000·00 | <0·001 |
For each patient in the Versajet group, a ‘Jetpack’ (a customised debridement tray comprising 13 instruments) was used along with a Versajet handpiece. An Interpulse device was not required since Versajet cleans contaminants from the wound simultaneously with the surgical cutting action.
There was significantly less saline use in the Versajet group (P < 0·001), with a mean saving of 2568·4 ml; 3000ml of saline was used for each patient that underwent a conventional debridement, in contrast with a mean of 431·6 ml for Versajet patients.
Wound closure
The median time to wound closure was 71 days for patients in the Versajet group and 74 days for those in the conventional group (Figure 3). There was no statistically significant difference in time to wound closure between patients treated with Versajet and those undergoing conventional debridement (P= 0·733). After 12 weeks, the wounds were closed in 52·6% of patients in the Versajet group and 47·4% of patients in the conventional group.
Figure 3.
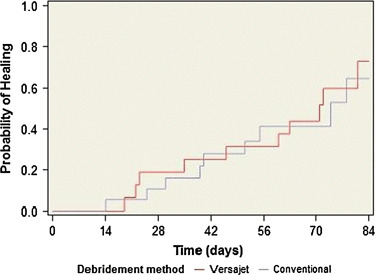
Kaplan Meier plot of the probability of wound closure. Following debridement the wounds were followed with area tracing for 12 weeks if they had not subsequently healed. The probability of closure was calculated for VERSAJET (red line) and Conventional (blue line) as described in Materials and Methods.
Adverse events
The occurrence of any adverse events and whether they were related or unrelated to the Versajet device was recorded. The nature of the events is shown in Table 4. No device‐related adverse events were reported. Five (25%) patients in the Versajet group reported serious adverse events two of which involved the study wound; below the knee amputation secondary to peripheral vascular disease; bleeding resulting in split‐thickness skin graft. Two (9·5%) patients in the conventional group reported 3 serious adverse events, of which one (gangrene secondary to severe peripheral vascular disease) involved the study wound.
Table 4.
Adverse events recorded during the study. There were no device related adverse events
| VERSAJET | Conventional |
|---|---|
| Non‐serious non‐device related | |
| 1. Hemoglobin/hemotosis | 0 |
| Serious non‐device related | |
| 1. Amputation of the study leg below the knee (contributory factor: peripheral vascular disease) | 1. Lower abdominal pain, constipation and decreased urinary output |
| 2. Aspiration pneumonia and renal failure | 2. Gangrene at study wound, secondary to severe peripheral vascular disease |
| 3. Uncontrolled bleeding from study wound ‐ required debridement & a split‐thickness skin graft | 3. Death due to cardiac arrest & respiratory failure |
| 4. Acute renal insufficiency (progressed into renal failure) ‐ surgery delayed until renal function improved | |
| 5. Death due to respiratory arrest (contributory factor: hypertension) ‐ following discharge against medical advice. | |
Discussion
The quality of debridement using the Versajet hydrosurgery system in burns 7, 8, venous leg ulcers 9, 10 and pressure ulcers (11) has already been described. Precise and controlled eschar removal, allowing maximal preservation of viable tissue whilst avoiding collateral damage has been reported by Cubison (8), Rennekampff (7), and Mosti (9). Versajet debridement has also been found to reduce bacterial burden in the wound (9).
Regarding the impact on resources, Granick et al. (12) found that Versajet reduced the number of surgical procedures required to achieve a clean wound bed, in a range of acute and chronic wounds (mean of 1·2 procedures per patient with Versajet compared with 1·9 procedures with conventional debridement (P < 0·005)). The reduction in procedures was estimated to be equivalent to an overall saving in the cost of debridement of $1,900 per patient.
Mosti et al. (10) compared the time to debridement of hard‐to‐heal leg ulcers in hospital in‐patients using Versajet and non‐surgical moist dressing techniques. In the majority of cases (76%), a single debridement procedure with Versajet was sufficient to achieve an adequately debrided wound bed with a mean debridement time of 5·9 ± 3·6 minutes per procedure. The mean time required to achieve a clean wound bed was 1·3 ± 0·6 days in the Versajet group, compared with 4·3 ± 3·9 days in the control group, leading to a reduction in hospital length of stay.
This study is the first to measure prospectively the time taken in the operating room to achieve debridement of a group of lower extremity ulcers, randomised to either conventional surgery or Versajet hydrosurgery, and to follow wound healing to closure in order to confirm whether debridement was effective. We show that operating room debridement is significantly faster with Versajet hydrosurgery, taking a mean of 10·8 minutes compared to 17·7 minutes for conventional surgery (P= 0·008). Our findings suggest that the time saving may be dependent on the area of black necrotic tissue within the wound (P= 0·098). In wounds with black necrotic tissue, which typically take longer to debride, we experienced a greater saving of 10·6 minutes (15·9 minutes vs 26·5 minutes) through the use of Versajet hydrosurgery, than for wounds without black necrotic tissue, where we saw a time saving of 5·5 minutes (10 minutes vs 15·5 minutes).
In this study the debrided ulcers were followed for 12 weeks to see whether the more rapid procedure led to any compromise in it’s clinical effectiveness. On the contrary, the overall time to closure for Versajet hydrosurgery and for conventional surgery were similar (71 days Versajet vs 74 days conventional). After 12 weeks approximately 50% of patients were closed in both groups (52·4% Versajet, 47·4% conventional).
The Versajet console is a capital item and the per case cost depends on the volume of cases and on the expected life of the investment. The disposable handpiece costs up to $500 per patient. In our practice, the protocol for conventional debridement always includes mechanical irrigation with pulse lavage. The wound is flushed with 3000ml of saline to simultaneously remove dead and/or contaminated tissue and dilute the bacterial load. In contrast, we have not found it necessary to use pulse lavage with Versajet. The cost of the handpiece was therefore mainly offset by savings in the cost of pulse lavage ($240) and a reduction in the amount of saline required ($10).
We have also identified other savings which are difficult to quantify on a per patient basis. In a previous study it was found that only a small disposable incision and drainage tray was needed to perform debridement with Versajet, and there is no need to open a major instrument tray (12). We have assembled a set of 13 re‐usable instruments for use with the Versajet (‘Jetpack’) which is much smaller than the standard instrument tray opened in all conventional debridement cases, regardless of the number of instruments actually used. Sterilization and re‐packing costs are similar, but the lower acquisition cost of the Versajet instrument set means that less capital is tied up in equipment: the replacement cost of a debridement tray is $21,000 compared with $500 for a ‘Jetpack’.
This study demonstrates that the Versajet Hydrosurgery system is a quick and effective means of debriding lower extremity ulcers. In this application debridement time can be shortened by nearly 40% leading to savings in operating room time which make it possible to treat more patients in a scheduled operating session. For the primary author, who conducts an average of 12 debridement procedures per week and runs more than one OR per operating schedule, minimising the effects of OR turnaround between cases, the saving due to VERSAJET enables a theoretical time saving of 82·8 minutes per week, translating into an additional 7·7 VERSAJET™ procedures per week, based upon debridement time alone. In addition, there are savings to be made in the cost of pulse lavage as the Versajet system does not require a separate irrigation procedure. Finally, a relatively small instrument set is all that is needed to debride with Versajet thereby avoiding the need to open an expensive debridement set.
Acknowledgements
Data were analysed by the Smith & Nephew Wound Management Health Economics & Outcomes Research Department, Hull, UK. Smith & Nephew Inc, 11775 Starkey Road, PO Box 1970, Largo, Florida 33779, USA provided the funding for this study. WJ Caputo, DJ Beggs and JJ DeFede have no commercial associations or financial disclosures to be declared. L Simm and H Dharma are employees of Smith & Nephew Medical Ltd. We thank Robin Martin (Smith & Nephew) for help with the manuscript.
References
- 1. Attinger CE, Janis JE, Steinberg J, Schwartz J, Al‐Attar A, Couch K. Clinical approach to wounds: débridement and wound bed preparation including the use of dressings and wound‐healing adjuvants. Plast Reconstr Surg 2006;117:72S–109S. [DOI] [PubMed] [Google Scholar]
- 2. Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11:S1–S28. [DOI] [PubMed] [Google Scholar]
- 3. Schultz GS, Barillo DJ, Mozingo DW, Chin GA; Wound Bed Advisory Board Members. Wound bed preparation and a brief history of TIME. Int Wound J 2004;1:19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schultz G, Mozingo D, Romanelli M, Claxton K. Wound healing and TIME; new concepts and scientific applications. Wound Repair Regen 2005;13:S1–11. [DOI] [PubMed] [Google Scholar]
- 5. Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. J Am Coll Surg 1996;183:61–4. [PubMed] [Google Scholar]
- 6. Collett, D . Modelling survival data in medical research, 2nd edition Ed: Chapman and Hall/CRC Press, 2003. [Google Scholar]
- 7. Rennekampff HO, Schaller HE, Wisser D, Tenenhaus M. Debridement of burn wounds with a water jet surgical tool. Burns 2006;32:64–9. [DOI] [PubMed] [Google Scholar]
- 8. Cubison TC, Pape SA, Jeffery SL. Dermal preservation using the Versajet hydrosurgery system for debridement of paediatric burns. Burns 2006;32:714–20. [DOI] [PubMed] [Google Scholar]
- 9. Mosti G, Iabichella ML, Picerni P, Magliaro A, Mattaliano V. The debridement of hard to heal leg ulcers by means of a new device based on Fluidjet technology. Int Wound J 2005;2:307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mosti, G. , Mattaliano, V . The debridement of chronic leg ulcers by means of a new fluidjet‐based device. Wounds 2006;18:227–237. [Google Scholar]
- 11. Gurunluoglu R. Experiences with waterjet hydrosurgery system in wound debridement. World J Emerg Surg 2007;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Granick MS, Posnett J, Jacoby M, Noruthun S, Ganchi PA, Datiashvili RO. Efficacy and cost‐effectiveness of a high‐powered parallel waterjet for wound debridement. Wound Repair Regen 2006;14:394–7. [DOI] [PubMed] [Google Scholar]


