Abstract
Fernández‐Montequín JI, Valenzuela‐Silva CM, González Díaz O, Savigne W, Sancho‐Soutelo N, Rivero‐Fernández F, Sánchez‐Penton P, Morejón‐Vega L, Artaza‐Sanz H, García‐Herrera A, González‐Benavides C, Hernández‐Cañete CM, Vázquez‐Proenza A, Berlanga‐Acosta J, López‐Saura PA, for the Cuban Diabetic Foot Study Group. Intra‐lesional injections of recombinant human epidermal growth factor promote granulation and healing in advanced diabetic foot ulcers: multicenter, randomised, placebo‐controlled, double‐blind study.
A multicenter, double‐blind, placebo‐controlled trial was carried out to evaluate the intra‐lesional infiltration of recombinant epidermal growth factor (EGF) in Wagner's grade 3 or 4 diabetic foot ulcers (DFUs). Subjects (149) were randomised to receive EGF (75 or 25 µg) or placebo, three times per week for 8 weeks and standard good wound care. The main endpoint was granulation tissue covering ≥ 50% of the ulcer at 2 weeks. It was achieved by 19/48 controls versus 44/53 in the 75 µg group [odds ratio (OR): 7·5; 95% confidence interval (CI): 2·9–18·9] and 34/48 in the 25 µg group (OR: 3·7; 1·6–8·7). Secondary outcome variables such as end‐of‐treatment complete granulation response (28/48 controls, 46/53 with 75 µg and 34/48 with 25 µg EGF), time‐to‐complete response (controls: 5 weeks; both EGF dose groups: 3 weeks), and wound closure after follow‐up (25/48 controls, 40/53 with 75 µg and 25/48 with 25 µg EGF) were also treatment dependent. Multivariate analyses yielded that they were significantly enhanced by 75 µg EGF treatment and neuropathic versus ischemic ulcers. Most adverse events were mild and no drug‐related severe adverse reactions were reported. It was concluded that recombinant human EGF (rhEGF) local injections offer a favourable risk–benefit balance in patients with advanced DFU.
Keywords: Diabetic foot ulcers, Epidermal growth factor, Wound healing
INTRODUCTION
Prevalence of diabetes mellitus is expected to rise to 366 million in 2030 (1). Around 15% of patients develop a diabetic foot ulcer (DFU), which precedes 85% of major amputations in this population (2). The annual incidence of DFU is more than 2% of diabetic patients (3) and increases if peripheral neuropathy is present (4). Up to 7–20% of the total expenditure on diabetes might be attributable to diabetes foot disease (5).
Metabolic control, wound care, debridement, pressure relief, moist dressings and antibiotics are basic interventions for DFU management. Revascularisation procedures are performed in cases with macroangiopathy‐related ischemia. New therapies are emerging to promote wound healing and to reduce amputations. These include recombinant human platelet‐derived growth factor 6, 7, low molecular weight heparin (8) and skin equivalents obtained by tissue engineering 9, 10. However, these products have been only studied in relatively small, neuropathic‐origin wounds. Amputation is still a foreseeable outcome in cases with large, advanced DFU, moreover if ischaemia is present.
Epidermal growth factor (EGF) exerts potent mitogenic activity through binding to a specific cell membrane receptor (11). Some clinical trials were conducted to evaluate EGF topical application on different indications, including DFU 12, 13, 14.
The availability of the growth factor at the wound deeper layers is an important issue to obtain an adequate efficacy. This can be a limitation with topical formulations because active agent diffusion is affected by necrotic tissue, sepsis, inflammation and wound proteases 15, 16, 17. Intra‐lesional injection can take the growth factor to the desired region.
A preliminary clinical study, where recombinant human EGF (rhEGF) (25 µg thrice weekly for 5 weeks) was injected intralesionally in advanced DFU, yielded encouraging results in terms of useful granulation tissue formation and major amputations prevention in more than 50% of the 29 patients treated (18). A second trial evaluated the efficacy and safety of this regime to promote granulation tissue formation in advanced DFU at two dose levels (25 and 75 µg) in a randomised, double‐blind design in 41 patients. Both doses yielded more than 60% granulation response (19).
However, these studies were small and not controlled. The possibility of a placebo effect and the action of endogenous growth factors, induced by the debridement and infiltration procedures could not be ruled out. The aim of this work was to evaluate the effect of intra‐lesional rhEGF infiltrations on advanced DFU healing in a multicentre, randomised, placebo‐controlled fashion to confirm the previous findings.
PATIENTS AND METHODS
Angiology departments from 20 centres throughout all Cuban provinces participated. Patients (type 1 or 2 diabetes) ≥ 18 years old were included if they had a Wagner's (2) grade 3 or 4 DFU, > 1 cm2, and signed their informed consent to participate. Exclusion criteria were revascularisation surgery possibility (for ischaemic ulcers), haemoglobin < 100 g/l, uncompensated chronic diseases such as heart failure signs, diabetic coma or ketoacidosis and renal failure (creatinine > 200 mg/dl), malignancies, psychiatric or neurological diseases that could impair proper reasoning for consent, immunosuppressor drugs or corticosteroids use, pregnancy and nursing. The protocol was approved by each institutional Ethics Committee and by the National Regulatory Authority.
Patients were randomised to receive rhEGF 75 µg (group I), 25 µg (group II) or placebo (group III), intralesionally, three times per week on alternate days. Randomisation was simple, central and stratified by investigation site. RhEGF was presented as a lyophilised powder containing 75 or 25 µg per vial (Heberprot‐P®, Heber Biotec, Havana). Both doses and placebo vials (containing all components of the formulation except EGF) were indistinguishable.
Products were dissolved with 5 ml of water for injection. In every visit, this volume was distributed throughout the lesion, in 0·5–1 ml injections, starting from the deeper zones. The treatment lasted 8 weeks or less if 100% granulation was achieved. After 2 weeks, if no response, the code was opened. Patients on placebo or 25 µg EGF were offered to continue treatment unblindly with 25 or 75 µg, respectively. This constraint was imposed by the Ethics Committees because it was considered that 2 weeks was enough to detect onset of response and it was unethical to continue non responders on placebo.
Study medication and placebo were administered together with standardised good wound care. Patients were hospitalised during treatment. Ulcers were sharply debrided, gangrenous and necrotic tissue removed (toe disarticulation or trans‐metatarsal amputation if necessary) and saline‐moistened gauze dressing used. The affected area was pressure off‐loaded by bed rest during the in‐hospital period and appropriate footwear afterwards. Metabolic control was strictly followed. Broad‐spectrum antibiotics were used if needed to clear infections before intra‐lesional injections started.
Evaluation consisted in baseline and weekly examination during treatment and at 3, 6 and 12 months follow‐up. Initial evaluation included ankle/brachial indexes and limb radiography. Ulcers were classified regarding their etiopathogeny (ischaemic or neuropathic) and in grades according to Wagner (2). Laboratory tests (at baseline, 3 weeks, end‐of‐treatment , 3, 6 and 12 months afterwards) included blood counts, haemoglobin, haematocrit, glycohaemoglobin (HbA1c), transaminases and creatinine. Blood glucose was measured more frequently for metabolic control. Wound infection was monitored by cultures before and during therapy.
Ulcer areas and percent granulation were measured by planimetry from a manual tracing on a transparent grid sheet, using a portable device (Visitrack™, Smith & Nephew, UK) (20). Sheets were kept and lesions were photographed before and after treatment for review.
The efficacy variable was the ulcer surface covered by granulation tissue defined as: ≤ 25% (no response); 26–50% (minimal response); 51–75% (partial response), and >75% (complete response). However, actually all complete responses obtained consisted in > 98% granulation. The main outcome was the proportion of patients with partial or complete response after 2 weeks of treatment, as this was the time interval when all the patients were blinded and in their originally allocated groups. Secondary outcomes were complete response rate at 8 weeks, time‐to‐complete response, complete wound closure, need for amputation and recurrences up to 1 year follow‐up. All evaluations were blinded. Patients whose codes were opened on week 2 were considered failures for their original groups, on intention‐to‐treat basis, for end‐of‐treatment response and wound closure.
Safety was monitored daily during treatment. Severity of adverse events was classified as (i) mild, if no therapy was necessary; (ii) moderate, if specific treatment was needed and (iii) severe, in case of death, life‐threatening, hospitalisation or its prolongation.
Sample size was estimated using the PASS software. Based on results from the previous studies with intra‐lesional rhEGF where 55% of the patients had at least 50% granulation at 2 weeks (19), the trial hypothesised that a 30% advantage would be obtained with either dose group, as compared with placebo, assuming from medical experience that 25% could achieve this result with standard good wound care alone. Considering a 5% alpha error and 80% power, sample size needed was 41 per group. A 20% excess was added to compensate for withdrawals and non adherence.
SPSS version 15 software was used for statistical analyses. Response rates comparison among groups was assessed by the chi‐squared test and odds ratios (ORs) with their 95% confidence interval (CI). The influence of different variables on response was tested in a multivariate analysis with a logistic regression model. Agreement between granulation response and closure rates was estimated using the kappa index. Sensitivity, specificity and predictive values were calculated using the Epidat software. Times to complete response were estimated by survival analyses (Kaplan–Meier) and compared with log‐rank test. A Cox regression model was used to determine the influence of baseline characteristics on this variable. The level of significance chosen was α = 0·05. All analyses were carried out on intention‐to‐treat basis. In addition, treatment dependence analyses of 8 weeks and follow‐up evaluations was repeated deleting patients whose treatment shifted at week 2 in order to evaluate the bias that could have been introduced by such design.
RESULTS
The flow chart of the trial is shown in Figure 1. Interruptions were because of wound progression in > 20 cm2, mostly ischaemic, grade 4 ulcers or to local infections that required amputation. One, five, and eight of the non responders at week 2 belonged to the 75 µg, 25 µg and control groups, respectively. Lesion progression during follow‐up occurred in patients that previously had complete (three cases) or partial (one) granulation response.
Figure 1.
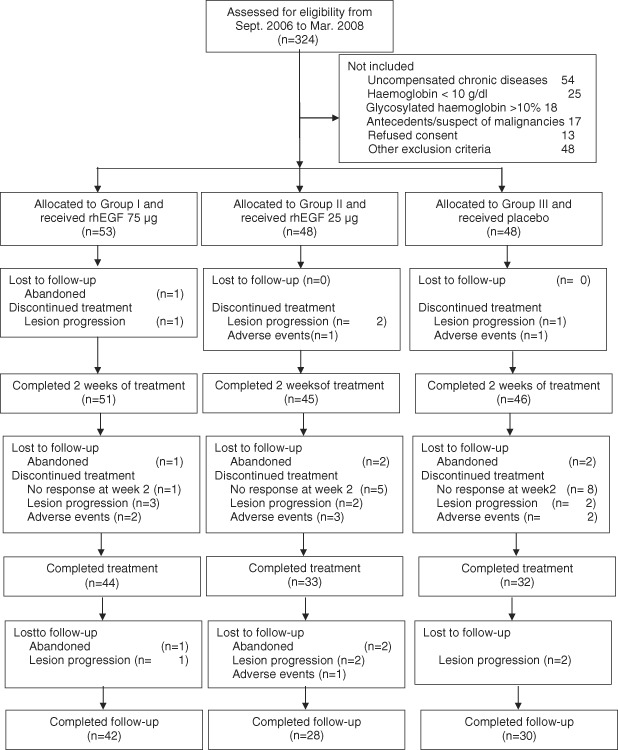
Flow chart of the trial.
Table 1 shows that the baseline characteristics of the treatment groups were similar. Groups also looked comparable regarding other baseline characteristics not shown in the table.
Table 1.
Baseline characteristics of the study population
| Characteristic | Group I (N = 53) | Group II (N = 48) | Group III (N = 48) | |
|---|---|---|---|---|
| Age in years: median (25th–75th percentiles) | 63 (55–69) | 65·5 (56–72) | 64·0 (51–70) | |
| Gender: males/females (% males) | 28/25 (52·8%) | 21/27 (43·8%) | 27/21 (56·3%) | |
| Diabetes mellitus: type 1/type 2 (% type 1) | 10/43 (18·9%) | 11/37 (22·9%) | 11/37 (22·9%) | |
| Time with diabetes in years: median (25th–75th percentiles) | 19·5 (10–22) | 15·0 (11·8–26) | 15·0 (10–22) | |
| Ulcer duration in weeks: median (25th–75th percentiles) | 4·3 (2·9–10·3) | 4·3 (2·6–8·3) | 4·9 (3·3–12·9) | |
| Ulcer size after initial debridement (cm2): median (25th–75th percentiles) | 28·5 (10·4–42·8) | 20·1 (11·0–34·0) | 21·8 (8·8–34·6) | |
| Predominant etiopathogenic feature | Neuropathic | 24 (45·3%) | 17 (35·4%) | 26 (54·2%) |
| Ischemic | 29 (54·7%) | 31 (64·6%) | 22 (45·8%) | |
| Wagner's classification | Grade 3 | 38 (71·7%) | 29 (60·4%) | 37 (77·1%) |
| Grade 4 | 15 (28·3%) | 19 (39·6%) | 11 (22·9%) | |
| Ulcer location (more than one localisation in some patients) | ||||
| Toes | 26 (49·1%) | 26 (54·2%) | 20 (41·7%) | |
| Internal edge | 3 (5·7%) | 2 (4·2%) | 4 (8·3%) | |
| External edge | – | 2 (4·2%) | 3 (6·3%) | |
| Dorsum | 4 (7·5%) | 6 (12·5%) | 7 (14·6%) | |
| Sole | 16 (30·2%) | 8 (16·7%) | 15 (31·3%) | |
| Transmetatarsal | 6 (11·3%) | 3 (6·3%) | 5 (10·4%) | |
| Heel | 7 (13·2%) | 13 (27·1%) | 3 (6·3%) | |
Table 2 shows the granulation response rates. Both dose levels fulfilled the > 30% threshold advantage over the control group for the 2 weeks main outcome. Besides, the higher dose resulted in significant end‐of‐treatment complete response advantage. Results were homogenous among sites, as yielded by a multilevel analysis where the ‘worst’ and ‘best’ centres differed < 4% in response rate (result not shown). A multivariate logistic regression analysis of the influence of different variables on end‐of‐treatment complete response rate showed significant effect (OR; 95% CI) for neuropathic ulcers versus ischaemic (3·7; 1·6–8·6) and higher EGF dose versus placebo (5·9; 2·1–16·6). The 25 µg EGF group did not reach significance (2·3; 0·93–5·8). The other baseline variables had no significant influence on granulation response rate.
Table 2.
Granulation response to treatment with intra‐lesional rhEGF
| Group I (N = 53) | Group II (N = 48) | Group III (N = 48) | P (χ2 test) | |
|---|---|---|---|---|
| After 2 weeks of treatment | ||||
| Complete + partial response (≥ 50% granulation) | 44 (83·1%) | 34 (70·8%) | 19 (39·6%) | 0·000015 |
| Difference versus control group (95% CI) | 43·8 (24·3;62·6) | 31·2 (10·3;52·2) | ||
| Odds ratio (95% CI) | 7·5 (2·9;18·9) | 3·7 (1·6;8·7) | ||
| After the end of treatment | ||||
| Complete response (> 75% granulation) | 46 (86·8%) | 34 (70·8%) | 28 (58·3%) | 0·005 |
| Difference versus control group (95% CI) | 28·5 (9·8;47·1) | 12·5 (− 8·6;33·6) | ||
| Odds ratio (95% CI) | 4·7 (1·8;12·5) | 1·7 (0·7;4·0) | ||
| Weeks to complete response (median; 95% CI) P versus group III (log rank test) | 3 (2·6–3·4) P = 0·006 | 3 (2·3–3·7) P = 0·031 | 5 (3·2–6·8) | |
Median time‐to‐complete response was shorter for both EGF‐treated groups (Table 2). Cox regression analysis yielded (OR; 95% CI) that neuropathic lesion (1·8; 1·2–2·7), 75 µg EGF (2·1; 1·3–3·4) and 25 µg EGF (1·8; 1·1–3·0) had significant shortening effect on this variable. Figure 2 illustrates two of the responses obtained.
Figure 2.
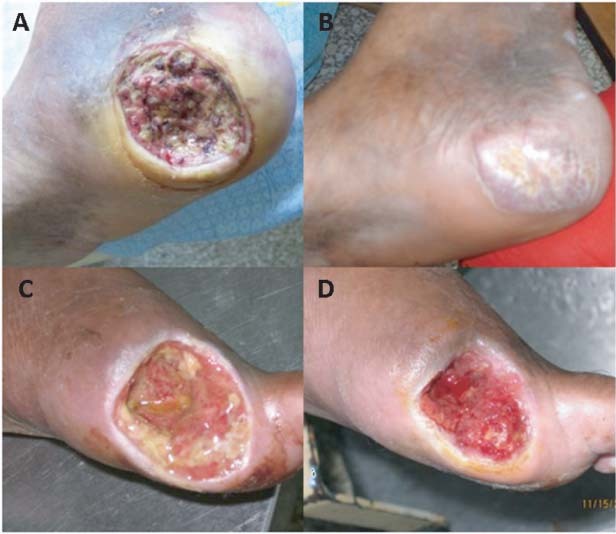
Examples of lesions treated with intra‐lesional EGF. (A) calcaneus, Wagner's 3, 21·4 cm2 ischaemic ulcer in a 52‐year‐old man. Note the epithelial hypertrophic edges and necrotic bed before treatment. After 16 instillations (5 weeks), the patient reached complete granulation. (B) Healing was further completed in 20 weeks. (C) First toe disarticulation residual base in a 70‐year‐old neuropathic man, refractory to heal for 12 months. (D) Complete productive granulation, evidence of contour contraction and incipient epithelial migration were achieved after six instillations (2 weeks). This patient finally healed completely after 19 weeks.
Ulcer closure occurred in 41 (77·4%), 25 (52·1%) and 27 (56·2%) patients from groups I, II and III, respectively (chi‐squared test: P = 0·018), but one of the healed patients in group I died as a result of pulmonary thromboembolism and two placebos relapsed so the actual rates for healed, relapse‐free, living patients after 1 year follow‐up are those shown in Table 3 (chi‐squared test: P = 0·020). A logistic regression model resulted that closure was significantly favoured (OR; 95 CI) by neuropathic versus ischaemic ulcer (5·5; 2·3–13·5); smaller wound area (0·98; 0·96–0·99); and treatment with 75 µg EGF (3·6; 1·4–9·5). Time‐to‐closure during follow‐up was significantly shorter in the 75 µg group (Table 3).
Table 3.
Final outcome of the patients (including treatment and follow‐up periods)
| Endpoint | Group I (N = 53) | Group II (N = 48) | Group III (N = 48) |
|---|---|---|---|
| Complete closure without recurrences | 40 * (75·5%) | 25 (52·1%) | 25 (52·1%) |
| Weeks to complete closure (95% CI); P versus control group (log rank test) | 14 (11–17) P = 0·040 | 12 (9–14) P = 0·200 | 20 (14–25) |
| Failures: | |||
| Healed but recurred | 0 | 0 | 2 |
| Lesion persisted at the end of follow‐up | 1 | 3 | 2 |
| Amputations (pure neuropathic) | 7 (1) | 10 (0) † | 12 (5) ‡ |
| Days from inclusion to amputation: median (95% CI) | 27 (17–45) | 16·5 (8–45) | 24 (15–43) |
| Abandoned | 3 | 6 | 3 |
| Switched group (no response at week 2) All (healed) | 0 | 4 (2) | 5 (3) |
| Deceased | 2 | 2 | 2 |
*In one case closure was reached after skin graft.
†Includes one deceased and one group switcher.
‡Includes two group switchers and one relapser.
If the patients who shifted treatment at week 2 are deleted from the 8‐week granulation and follow‐up closure analyses, treatment‐dependence of outcomes remains (logistic regression: P = 0·018 for effect of treatment on 8‐week complete granulation response; OR, 95% CI for 75 µg EGF treatment: 4·7, 1·6–14·0; P = 0·048 for effect of treatment on wound closure during follow‐up; OR, 95% CI for 75 µg EGF treatment: 2·7, 1·01–7·4).
Amputations registered were not enough for any statistical analysis. Interestingly, except for one case, all amputations in the EGF‐treated groups were ischaemic patients, whereas five neuropathic patients with placebo suffered them (Table 3).
Both 2 weeks > 50% granulation and end‐of‐treatment complete granulation predicted final wound closure well, as shown by the highly significant measure of agreement statistics (Table 4).
Table 4.
Agreement between granulation response and final closure in advanced diabetic foot ulcer patients
| Wound closure during follow‐up | + predictive value (%) | Sensitivity (%) | |||
|---|---|---|---|---|---|
| Lesion closed | Lesion not closed | –predictive value (%) | Specificity (%) | ||
| Two weeks granulation response | ≥50% | 76 | 21 | 78·4% | 81·7% |
| <50% | 17 | 35 | 67·3% | 62·5% | |
| End‐of‐treatment granulation response | Complete | 90 | 18 | 83·3% | 96·8% |
| Not complete | 3 | 38 | 92·7% | 67·9% | |
Kappa statistics were significant (P < 0·001) for both correlation analyses. All percentages have 95% confidence limits ≤ 1%.
Table 5 shows the adverse events. They were mostly mild or moderate. Serious adverse events appeared in 19 patients; in 9 of them caused treatment interruption. Only mild or moderate shivering and chills occurred more frequently, and in a dose‐related fashion, in the EGF‐treated patients.
Table 5.
Adverse events frequency
| Events | Group I n = 53 | Group II n = 48 | Group III n = 48 |
|---|---|---|---|
| Subjects with any adverse event | 37 (69·8%) | 28 (58·3%) | 31 (64·5%) |
| Severe | |||
| Infection | 4 | 4 | 2 |
| Other | Renal failure Cellulitis | Renal failure (lethal) Myocardial infarct Pneumonia | Acute pulmonary oedema (2; 1 lethal) Cellulitis Knee abscess |
| Mild or moderate (occurring in 10 patients or more) | |||
| Pain at the administration site | 13 (24·5%) | 13 (27·1%) | 20 (41·7%) |
| Burning sensation | 12 (22·6%) | 10 (20·8%) | 14 (29·2%) |
| Shivering | 17 (32·1%) | 8 (16·7%) | 2 (4·2%) |
| Local infection | 3 (5·7%) | 4 (8·3%) | 7 (18·8%) |
| Chills | 11 (20·8%) | 4 (8·3%) | 1 (2·1%) |
| Anaemia | 4 (7·5%) | 3 (6·3%) | 5 (10·4%) |
| Fever | 2 (3·8%) | 4 (8·3%) | 6 (12·5%) |
| Nauseas | 4 (7·5%) | 1 (2·1%) | 2 (4·2%) |
| Vomits | 3 (5·7%) | 2 (4·2%) | 1 (2·1%) |
DISCUSSION
This study confirms that treatment with intra‐lesional rhEGF, associated with good wound care measures, can benefit patients with advanced DFU for which otherwise there is no available specific therapy. The multicentre, randomised and placebo‐controlled features adds higher level of evidence to the previous, non controlled trials reported with this procedure 18, 19, and improves its external validity because the population studied was more representative.
The trial performed well. Randomisation and blindness were strictly followed and patient adherence was satisfactory. Patients lost from endpoints evaluation were much less than the 20% previewed for sample size calculation. The rest of the withdrawals were actual treatment failures. Groups were comparable according to demographic and baseline characteristics.
The results fulfilled the hypothesis to obtain at least 30% difference with respect to the control group after 2 weeks of treatment. Treatment‐dependency was also found for complete granulation, which was additionally accelerated by EGF, and for complete closure, despite being reached during follow‐up, as outpatients, only under general wound care measures. Time‐to‐closure was also shortened in the higher dose group. This apparent ‘EGF‐memory’ effect can be explained by the granulation tissue stimulation, which was highly predictive of closure.
That both granulation variables agreed with final healing is an interesting finding that could be useful as a decision point for future clinical trial designs and to identify non responders that would require other management strategies. Previous studies have identified partial wound closure as predictive of complete healing for Wagner's grade 1 or 2 DFU (21), and other ulcers 22, 23, but this is the first report of an early surrogate endpoint in Wagner's grade 3 or 4 DFU. Ischaemia appeared as a significant bad prognosis factor in multivariate analyses, for both granulation and closure, which agrees with previous reports on DFU management and evolution (24). Most amputations occurred in ischemic patients as well.
Nine patients in the lower EGF dose and placebo groups switched treatment at week 2 and are defined as non healers in further analysis. This design could have some impact on outcome regarding granulation rates at the week 8 visit and closure rates at 1 year follow‐up. Even if this kind of analyses may introduce bias in the interpretation of the results, the opposite also does. The ‘intention‐to‐treat' evaluation principle has been usually preferred in randomised clinical trial data reading. In this work, the interpretation is further validated by the fact that analyses of secondary variables after deleting the group‐shifting patients yielded similar treatment‐dependence of outcomes.
Most of the adverse events were mild and easily manageable. Only shivering and chills appeared more frequently in the EGF‐treated groups, apparently dose‐dependent. The severe adverse events, including deaths, do not seem to be EGF treatment‐related. One of the major concerns of exogenous EGF use at concentrations higher than physiological is that it could promote development of neoplasia. An accurate assessment of this event was included in the follow‐up of this study. It was not observed in any of the subjects. However, this time interval is too short for this purpose so additional observations are necessary with a larger number of patients as long as the use of this product is extended.
Another concern with the intra‐lesional route of administration is the risk of spreading bacterial infection. It was minimised by the concomitant good wound care practices, broad‐spectrum antibiotic coverage, and adequate aseptic injection procedures. However, local infections accounted for most of the therapeutic failures. Infection control remains a critical problem in such advanced DFU.
The study was not powered to determine differences between the two dose levels, but to compare each of them versus placebo. However, dose‐effect was suggested by the tendency to a better response in the main outcome with the 75 µg dose. Besides, only this higher dose yielded significant difference with placebo for some secondary variables. More studies are required to further elucidate this aspect as well as its interaction with baseline variables such as ulcer etiopathogeny (pure neuropathic or with ischemia), and severity, in order to reach optimal treatment schedules.
Other growth factors such as becaplermin have been used topically in neuropathic and smaller lesions 6, 7. A meta‐analysis of those studies concluded that treatment with becaplermin gel increases complete closure rate (25). However, 95% of the patients included in those trials had ulcers ≤ 10 cm2 and an adequate blood supply. On the contrary, the present study treated more advanced, larger (median > 20 cm2) and both neuropathic and ischaemic wounds.
Topical use of EGF on DFU has been reported. Closure rate was significantly enhanced in a randomised, double‐blind, controlled trial (12). But this study also included only Wagner's grades 1 and 2, ≤ 4 cm2, neuropathic ulcers. A non controlled trial with topical rhEGF obtained 76% closure in patients with grade 2–3, average 4·8 cm2, neuropathic, ulcers (13). Another placebo‐controlled study in 60 patients with Wagner's grade 1 or 2 ulcers report closure rate improvement from 10 to 60% by 10 weeks (14). A post marketing pharmacosurveillance report from the same group confirmed that result (26).
A limitation to the efficacy of topical formulations is that the growth factor cannot reach the deeper wound layers. Diffusion is affected by necrotic tissue, infection, inflammation, and by the action of wound proteases (16). Chronic wounds have elevated pro‐inflammatory cytokines, high protease activity, decreased levels of natural metalloproteinase inhibitors and diminished growth factor activity 17, 27, 28. The still active factor may be unavailable for biologic activity because of trapping or binding to molecules such as fibrinogen, macroglobulin, or albumin 29, 30. In addition, wound bacteria produce proteases and other metalloproteinases that further degrade the growth factors and their receptors (31). These facts can contribute to explain the lack of efficacy of topical EGF and PDGF at lower doses 6, 12.
Intra‐lesional injection of the growth factor can take the active agent into the desired region and avoid the inactivating agents. All results obtained with this intervention (ref. 18,19,32 and this study) have been in advanced ulcers (Wagner's grade 3 or 4, mostly > 20 cm2, including ischaemic) with higher risk of amputation. Recurrence rate has been very low. The findings can be considered clinically relevant, because they offer an alternative to wounds otherwise difficult to manage that constitute an important burden to medical care systems and where amputation is not a seldom outcome. Further clinical research is encouraged to extend these results, extrapolate them to other populations, precise the effects on subgroups and evaluate its impact on amputation rates and health care economy.
ACKNOWLEDGEMENTS
The authors thank Heber Biotec S.A. for the supply of the investigational product. The Ministry of Public Health of Cuba supported the work. Authors C.V.S. and P.A.L.S. are employees of the Centre for Biological Research, which is part of the Centre for Genetic Engineering and Biotechnology (CIGB), Havana network, where rhEGF is produced and the new formulation was developed and produced; J.B.A. works at CIGB itself and is author of the patent that sustains the project. J.I.F.M. is also coauthor of the patent. The rest of the authors have no conflict of interests.
Cuban Diabetic Foot Study Group.
Patient recruitment, treatment and follow‐up (number of patients included at each site): National Institute for Angiology and Vascular Surgery, Havana (18), José Fernández‐Montequin, Neobalis Franco‐Vega, William O Savigne‐Gutiérrez, Calixto Valdés‐Pérez, Nelson Chirino‐Carreño, Héctor T Álvarez‐Duarte, Agustín Llanes, Alfredo Aldama‐Figueroa, Daniel Reynaldo, Milagros Garcia , José G Hernández, Maria E Triana‐Mantilla, Martha González.González, Mireya Alonso‐Rios, Olga Morejon‐Reinoso, Julia Zapata‐Vinent, Ines Pilar Gonzalez, Martina Garcia‐Insua, Dulce M Armenteros‐Herrera, Alicia Rodriguez‐Perez, Amada A Fernandez‐Boloña; ‘Abel Santamaría’ Hospital, Pinar del Río (18): Laureano Peña‐Bazart, Ana L Hernandez‐Rojas, Antonio J Díaz‐Díaz, Marta M Moreira‐Martinez, Aidé Hernández‐Hernández, Odalys Rivera‐Martinez, Martha L Gutiérrez‐Gutiérrez, Yudexy Martinez‐Pino, Martha Hernandez‐Miranda, Ilsa Dopico‐Reyes, Marlen Millar‐Rojas; ‘Agostinho Neto’ Hospital, Guantánamo (14): Georgina Greaves‐Turro, Arturo Pérez‐Chamber, Alina Carrió‐Berenguer, Orquídea Rodríguez‐Imbert, Ángel Cuza‐Rodríguez, Sandra Ferrer‐Fernández, Mylen Correa‐Colomé, Nielbis Frómeta‐Rodríguez, Yadileidi Elías‐Oquendo; ‘Saturnino Lora’ Hospital, Santiago de Cuba (13): Natacha Sancho‐Soutelo, Sergio Álvarez‐González, Yirsa Luna‐Negret, Sandra Durañones‐Góngora, Mercedes Guilart‐Pérez, Martha Fernández‐Hechevarria, Mirta Domínguez‐Salgueras, Ledis Garbey‐Delas, Vivian Soto‐Infante, Maria Elena Pardo‐Portuondo; ‘Manuel Ascunce Domenech’ Hospital, Camag&uey (10): Fidel Rivero‐Fernández, Ariel Hernández‐Varela, Jorge L Valdés‐Nápoles, Odalys Escalante‐Padrón, Víctor Alfonso‐Lanz, Nicolás Socarrás‐Olivera, Melsa Vázquez‐Miranda, Idalín Conde‐Guerra, Daliana Somonte‐Sánchez, Tomás Jiménez‐Leyva, Yaneydis Lores‐Méndez, Judith Ojeda‐de Pedro; ‘Camilo Cienfuegos Hospital’, Sancti Spíritus (9): Pablo Sánchez‐Penton, Ana L Tabio‐Reyes, Irelio Borroto‐Carpio, Diosnelkis Vera‐Candelario, Deisy Piña‐López, Maria de La C Hernández‐Morgado, Carmen Landa‐Quintero, Marielis Hernández‐Oria, Martha E Armas‐Rivadeneira, Belquis Herrera‐Lopez, Nancy Machin‐González, Herminia Rodriguez‐Catañeda; ‘Joaquín Albarrán’ Hospital, Havana (7): Lourdes Morejón‐Vega, Edgar George‐Laffita, Luis Mayor‐González, Alberto Valerio‐Mendiondo, Luis Thaureaux‐Montes de Oca, Raquel Ramón‐Brizuela; ‘Manuel Fajardo’ Hospital, Havana (6): Máximo Sandez‐López, Miladys Rodríguez‐Caballero, Wilfredo Suárez‐del Castillo, Liana Padrón‐Menéndez, José L Alonso‐González, Dulce M. Garcia‐Esplugas, Aida Lopez, Rosa Diaz; ‘Enrique Cabrera’ Hospital, Havana (6): Heriberto Artaza‐Sanz, Pedro Goicochea‐Díaz, Angela Blanco‐Díaz, Natalia Pool‐Marrón, Dinorah González‐Viñales, Evaristo Vargas‐Machiran, Eduardo Atencio‐Sariol, Alexis Janero‐Valdés, Marta Pérez‐Linares, Cherezada Vidal‐Ramírez, Teresa Alvarez‐Padrón, Delfina Costales‐Elizarde, Gerardo Maza; ‘José R. López Tabranes' Hospital, Matanzas (6): Arístides García‐Herrera, Edel Fleitas‐Pérez, Raúl Rodríguez‐Fernández, Vania María Peña‐Ruiz, Ridel Febles‐Sanabria, Zoraida Gómez‐Sotolongo, Ana Gloria Alfonso‐León, Rosa Medina‐Quesada, Pedro Castillo‐Herrera, Vivian Ramos‐Rguez, Andrés Lamas‐Acevedo, Dayamí Montes de Oca‐Fernández, Frank Aguirre‐Rdguez; ‘Arnaldo Milián Castro’ Hospital, Santa Clara (6): Cecilio González‐Benavides, Teresita Feito‐Costex, Juan M García‐Velásquez, Felicia García‐Seco, Amel Alfonso‐Simón, Yasmin Rodríguez‐Ríos, Omar Hernández‐González, Magalys de la Barca‐Barrera, Ileana Wong‐Romero, Manuel Cruz‐Sosa, Yaritsy Abreu‐Gómez, Galia Averhoff, Maria Margarita Ríos‐Cabrera; ‘Gustavo Aldereguía Lima’ Hospital, Cienfuegos (6): Jose R Ferra‐Gonzalez, Ivonne Marrero‐Rodríguez, Javier de J Borrego‐Acosta, Nancy Ramírez‐Martínez, Belkis Calaña‐González‐Posada, Ivette García‐Álvarez, Yadira González‐Suárez, Dayamí Villaveirán‐Fernández, Yanet Rabassa‐Martínez, Brandy Viera‐Valdés, Maydel de la C Armas‐Ramírez, Caridad M Guerrero, Gredsy Escandón‐López; ‘Antonio Luaces Iraola’ Hospital, Ciego de Ávila (5): Carlos Manuel Hernández‐Cañete, Jesús Tejidor‐Fernández, Jorge Luís Morales‐Florá, Misleny Álvarez Hernández, Jorge Herrera‐Zamora, Rolando Vegas‐García, María Elena Sánchez‐Montiel, María Esther Morgado‐Carbonell, Odanay Fernández‐Castillo, Élida Menocal‐Cabrera, Luisa María Boizant‐Crombet; ‘Carlos Manuel Céspedes' Hospital, Bayamo (5): Alberto Vázquez‐Proenza, Francisco A Vázquez‐Milanes, Santiago Luna‐Hernández, Héctor Rivera‐Ortiz, Caridad Guerra‐Vázquez, Enrique Menéndez‐Saborit, Julio Cesar López‐González, Roberto T Pelegrino‐Reyes, Martha Corpas‐Paneque; ‘Ernesto Guevara’ Hospital, Las Tunas (5): José L Solís‐Licea, José Pablo Pose‐Levive, Zulema Elliot‐Perez, Lidia Sosa‐Ramos, Estela Zaragoza‐Alvarez, Dulce Báez‐Escobar, Yusimi Trista‐Área, Magnelia Vásquez‐Ruiz; ‘Luis Díaz Soto’ Hospital, Havana (4): José E Sauri‐Chávez, Maricela Rodríguez‐Betancourt, Pilar Herrera‐Marturell, Azalia Díaz‐Gonzalez, Ofelia Bounet‐Jiménez, Jesús Rodríguez‐Escribano; ‘Lucia Iñiguez’ Hospital, Holguín (4): Armando González‐Expósito, Esther Peña‐Guillen, Envida Carballosa‐Peña, Idania Hidalgos‐Batista, Yanelis Silva‐Ricardo, Anaísi Hernández‐Borges, Elio Lozano‐Álvarez; ‘Joaquín Castillo Duany’ Hospital, Santiago de Cuba (3): Celso Suárez‐Lescay, Bencay Joa‐Liranza, Inalvis Alfonseca‐Miranda, María Cristina Smith‐Machú, Yolanda Rosabal‐Poig; ‘Calixto García’ Hospital’, Havana (2): Julio Núñez‐Vasquez, Amelie Esteva‐Guas, Jorge A Rodriguez‐Mayoral, Aimee Rodríguez‐Hernández, Osmel Castillo‐Luque, Remberto García‐González, Susana Martinez, Edita B Thomas‐Hay, Mirta Teresa Rodríguez‐Gutierrez, Lorena‐Cambert, ‘Miguel Enríquez’ Hospital, Havana (2): Justa Peñalver‐Castillo, Luís Herrrera‐Báez, Enf Nixia Salas‐Ge, Michelin Buscaron‐Torres, Antonio Padua, Mileydis Stable‐Walcott, Myrna García‐Meneses, Ileana Fernández‐Hidalgo, Miriam Avila‐Núñez; Trial performance and monitoring: National Center for Coordination of Clinical Trials, Havana: Odalys M González‐Díaz, Débora Monterrey‐Cao, Grisel Soto‐Arguelles, Clara Ballagas‐Flores, Maria Amparo Pascual; ‘Ernesto Guevara’ Faculty of Medicine, Pinar del Río: Karina Miranda‐Hernández, Yoryana Ramírez‐Sanchez, Yurka Prieto‐Ferro; Faculty of Medicine, Matanzas: Sandra A dela Naranjo‐Rodriguez , Marisel Negret‐Hernández; Faculty of Medicine, Cienfuegos: Ana M Ramos‐Cedeño, Leslie Pérez‐Ruiz, Diana Rosa Fernández‐Ruiz; High Institute for Medical Sciences, Santa Clara: Maykel Pérez‐Machin, Miriam Cid‐Rios; Faculty of Medicine, Sancti Spiritus: Liliana Ramos‐Torres, Hector Ruiz‐Calabuch; Faculty of Medicine, Ciego de Ávila: Giselle Veguilla‐Alomar, Thayde Trujillo‐Tirado; High Institute for Medical Sciences, Camag&uey: Iliana Pérez‐Chong, Ana R. Valls‐Hung, Janet Batista‐Lorenzo; Faculty of Medicine, Las Tunas: Norma Montes de Oca‐Escobar; Faculty of Medicine, Holguín: Zaimar Rodríguez‐Feria, Dulce Mariño‐Cruz; Faculty of Medicine, Granma: Heriberto Martínez‐Suárez; High Institute for Medical Sciences, Santiago de Cuba: Doris Perdomo‐Leyva; Faculty of Medicine, Guantánamo: Elizabeth Pereira‐Relis, Jorge A. Silva‐Valido, Rider Hernández‐Martínez; Data Management, Statistical Design and Analyses: Centre for Biological Research: Carmen Valenzuela‐Silva, Elizeth García‐Iglesias, Leovaldo Álvarez‐Falcón; Trial Protocol Design, Monitoring, Analysis of Results: Centre for Biological Research: B. Y. Batancourt, Amaurys del Río‐Martín, Yaleysis Rosales‐Pantoja, Hugo Nodarse‐Cuní, Cimara Bermúdez‐Badell, Grettel Melo‐Suárez, Ketty Cruz‐Chirino, Pedro A López‐Saura; Centre for Genetic Engineering and Biotechnology: Jorge Berlanga‐Acosta, Luis Herrera‐Martínez, Ernesto López‐Mola, Ricardo Silva‐Rodríguez, Marianela García.
See Appendix for study Group details.
REFERENCES
- 1. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–53. [DOI] [PubMed] [Google Scholar]
- 2. Frykberg RG, Armstrong DG, Giurini J, Edwards A, Kravette M, Kravitz S, Ross C, Stavosky J, Stuck R, Vanore J. Diabetic foot disorders. A clinical practice guideline. J Foot Ankle Surg 2000; 39(5 Suppl): S1–S60. [PubMed] [Google Scholar]
- 3. Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, Hann AW, Hussein A, Jackson N, Johnson KE, Ryder CH, Torkington R, Van Ross ER, Whalley AM, Widdows P, Williamson S, Boulton AJ; North‐West Diabetes Foot Care Study. The North‐West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community‐based patient cohort. Diabetic Med 2002; 19: 377–384. [DOI] [PubMed] [Google Scholar]
- 4. Abbott CA, Vileikyte L, Williamson S, Carrington AL, Boulton AJM. Multicenter study of the incidence of and predictive risk factors for diabetic neuropathic foot ulceration. Diabetes Care 1998; 21: 1071–5. [DOI] [PubMed] [Google Scholar]
- 5. Boulton AJ, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005; 366: 1719–24. [DOI] [PubMed] [Google Scholar]
- 6. Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet‐derived growth factor‐BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo‐controlled double‐blind study. Diabetes Care 1998; 21: 822–7. [DOI] [PubMed] [Google Scholar]
- 7. Margolis DJ, Bartus C, Hoffstad O, Malay S, Berlin JA. Effectiveness of recombinant human platelet‐derived growth factor for the treatment of diabetic neuropathic foot ulcers. Wound Repair Regen 2005; 13: 531–6. [DOI] [PubMed] [Google Scholar]
- 8. Rullan M, Cerdà L, Frontera G, Masmiquel L, Llobera J. Complications treatment of chronic diabetic foot ulcers with bemiparin: a randomized, triple‐blind, placebo‐controlled, clinical trial. Diabetic Med 2008; 25: 1090–5. [DOI] [PubMed] [Google Scholar]
- 9. Veves A, Falanga V, Armstrong DG, Sabolinski ML. Apligraf Diabetic Foot Ulcer Study. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001; 24: 290–5. [DOI] [PubMed] [Google Scholar]
- 10. Marston WA, Hanft J, Norwood P, Pollak R. Dermagraft diabetic foot ulcer study group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003; 26: 1701–5. [DOI] [PubMed] [Google Scholar]
- 11. Bazley LA, Gullick WJ. The epidermal growth factor receptor family. Endocr Relat Cancer 2005; 12: S17–S27. [DOI] [PubMed] [Google Scholar]
- 12. Tsang MW, Wong WK, Hung CS, Lai KM, Tang W, Cheung EY, Kam G, Leung L, Chan CW, Chu CM, Lam EK. Human Epidermal Growth Factor Enhances Healing of Diabetic Foot Ulcers. Diabetes Care 2003; 26: 1856–1861. [DOI] [PubMed] [Google Scholar]
- 13. Hong JP, Jung HD, Kim YW. Recombinant human epidermal growth factor (EGF) to enhance healing for diabetic foot ulcers. Ann Plast Surg 2006; 56: 394–8. [DOI] [PubMed] [Google Scholar]
- 14. Viswanathan V, Pendsey S, Sekar N, Murthy GSR. A Phase III study to evaluate the safety and efficacy of recombinant human epidermal growth factor (REGEN‐D™150) in healing diabetic foot ulcers. Wounds 2006; 18: 186–96. [Google Scholar]
- 15. Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen 1996; 4: 411–20. [DOI] [PubMed] [Google Scholar]
- 16. Berlanga J, Lodos J, Reyes O, Infante JF, Caballero E, López‐Saura P. Epidermal growth factor stimulated re‐epithelialization in pigs. The possible role of acute‐wound proteases. Biotecnología Aplicada 1998; 15: 83–7. [Google Scholar]
- 17. Medina A, Scott PG, Ghahary A, Tredget EE. Pathophysiology of chronic nonhealing wounds. J Burn Care Rehabil 2005; 26: 306–19. [DOI] [PubMed] [Google Scholar]
- 18. Berlanga J, Savigne W, Valdez C, Franco N, Alba JS, Del Rio A, et al. Epidermal Growth Factor intra‐lesional infiltrations can prevent amputation in diabetic patients with advanced foot ulcers. Int Wound J 2006; 3: 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fernández‐Montequín JI, Infante‐Cristiá E, Valenzuela‐Silva C, Franco‐Pérez N, Savigne‐Gutiérrez W, Artaza‐Sanz H, Morejón‐Vega L, González‐Benavides C, Eliseo‐Musenden O, García‐lglesias E, Berlanga‐Acosta J, Silva‐Rodriguez R, Betancourt BY, López‐Saura PA, for the Cuban Citoprot‐P Study Group. Intra‐lesional injections of Citoprot‐P® (recombinant human Epidermal Growth Factor) in advanced diabetic foot ulcers with risk of amputation. Int. Wound J. 2007; 4: 333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sugama J, Matsui Y, Sanada H, Konya C, Okuwa M, Kitagawa A. A study of the efficiency and convenience of an advanced portable Wound Measurement System (VISITRAK ™). J Clin Nurs 2007; 16: 1265–9. [DOI] [PubMed] [Google Scholar]
- 21. Sheehan P, Jones P, Giurini JM, Caselli A, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Plast Reconstr Surg 2006; 117(Suppl. 6): 239S–44S. [DOI] [PubMed] [Google Scholar]
- 22. Van Rijswijk L, Polansky M. Predictors of time to healing deep pressure ulcers. Ostomy Wound Manage 1994; 40: 40–8. [PubMed] [Google Scholar]
- 23. Margolis D, Kantor J, Margolis DJ. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br J Dermatol 2000; 142: 960–4. [DOI] [PubMed] [Google Scholar]
- 24. Prompers L, Schaper N, Apelqvist J, Edmonds M, Jude E, Mauricio D, Uccioli L, Urbancic V, Bakker K, Holstein P, Jirkovska A, Piaggesi A, Ragnarson‐Tennvall G, Reike H, Spraul M, Van Acker K, Van Baal J, Van Merode F, Ferreira I, Huijberts M. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia 2008;51: 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smiell JM, Wieman TJ, Steed DL, Perry BH, Sampson AR, Schwab BH. Efficacy and safety of becaplermin (recombinant human platelet‐derived growth factor‐BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen 1999; 7: 335–46. [DOI] [PubMed] [Google Scholar]
- 26. Krishna Mohan V. Recombinant human epidermal growth factor (REGEN‐DTM 150): effect on healing of diabetic foot ulcers. Diabetes Res Clin Practice 2007; 78: 405–11. [DOI] [PubMed] [Google Scholar]
- 27. Nwomeh BC, Yager DR, Cohen IK. Physiology of chronic wounds. Clin Plast Surg 1998; 25: 341–56. [PubMed] [Google Scholar]
- 28. Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen 1996; 4: 321–5. [DOI] [PubMed] [Google Scholar]
- 29. Falanga V, Eaglstein WH. The trap hypothesis of venous ulceration. Lancet 1993; 341: 1006–8. [DOI] [PubMed] [Google Scholar]
- 30. Robson MC, Smith PD. Topical use of growth factors to enhance healing. In: Falanga V, editor. Cutaneous wound healing. London: Martin Dunitz Limited, 2001; 379–98. [Google Scholar]
- 31. Robson MC, Stenberg BD, Heggers JP. Wound healing alterations caused by infection. Clin Plast Surg 1990; 7: 485–92. [PubMed] [Google Scholar]
- 32. Fernández‐Montequín JI, Betancourt BY, Leyva‐Gonzalez G, López Mola E, Galán‐Naranjo K, Ramírez‐Navas M, Bermúdez‐Rojas S, Rosales F, Garcialglesias E, Berlanga‐Acosta J, Silva‐Rodriguez R, Garcia‐Siverio M, Herrera Martinez L. lntralesional administration of epidermal growth factor‐based formulation (Heberprot‐P) in advanced diabetic foot ulcer: Treatment up to complete wound closure. Int Wound J 2009; 6: 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


